Neural regeneration by regionally induced stem cells within poststroke brains:Novel therapy perspectives for stroke patients
Takayuki Nakagomi,Toshinori Takagi,Mikiya Beppu,Shinichi Yoshimura,Tomohiro Matsuyama
Takayuki Nakagomi,Institute for Advanced Medical Sciences,Hyogo College of Medicine,Nishinomiya,Hyogo 663-8501,Japan
Takayuki Nakagomi,Tomohiro Matsuyama,Department of Therapeutic Progress in Brain Diseases,Hyogo College of Medicine,Nishinomiya,Hyogo 663-8501,Japan
Toshinori Takagi,Mikiya Beppu,Shinichi Yoshimura,Department of Neurosurgery,Hyogo College of Medicine,Nishinomiya,Hyogo 663-8501,Japan
Abstract
Key words:Ischemic stroke; Stroke patients; Central nervous system; Neural stem/progenitor cells; Multipotent stem cells; Stem-cell-based therapies
INTRODUCTION
Cerebrovascular diseases,including stroke,are a leading cause of death worldwide.Owing to recent therapeutic advances such as reperfusion therapies by intravenous administration of recombinant tissue plasminogen activator (IV t-PA) and neuroendovascular treatment,including mechanical thrombectomy[1-3],some patients can recover from stroke without sequelae.With the increased implementation of these therapies,it is speculated that more stroke patients can benefit from them.In addition,the therapeutic time window of IV t-PA was extended to 4.5 h[4].Moreover,there is a possibility,when guided by imaging,for the IV t-PA indication to be expanded in patients with acute ischemic stroke of unknown onset[5].As for mechanical thrombectomy,the therapeutic time window was expanded up to 16 h from onset or to 24 h if the acute stroke patients had a mismatch between the ischemic core and hypoperfusion area[6,7].However,many patients with stroke are not eligible for these therapies because of excluding factors (e.g.,time after onset and portion of vascular obstruction).Currently,approximately 13%-20% of acute ischemic stroke patients are potentially eligible for mechanical thrombectomy[7,8].In patients who had mechanical thrombectomy,the rate of good clinical outcome was below 50%[3].Alternatively,patients receive rehabilitation,but many continue to suffer from various sequelae such as paresis.
Thus,more attention is paid to reparative medicines,particularly to those based on stem cell therapies.Various types of stem cells,including neural stem/progenitor cells (NSPCs)[9-12],mesenchymal stem cells (MSCs)[13,14](e.g.,bone marrow-derived MSCs,adipose-derived MSCs[15,16]),embryonic stem (ES) cell-derived NSPCs[17],and induced pluripotent stem (iPS) cell-derived NSPCs[18],are considered as candidates for cell transplantation following ischemic stroke.
Although the central nervous system (CNS),brain and spinal cord,was long considered not to have regeneration potential after injury,accumulating evidence indicate that the adult CNS contains NSPCs[19,20].Therefore,CNS repair might be achieved through endogenous stem cells.However,no concrete evidence showing that stem-cell-based therapies by NSPCs are clinically useful for patients with various CNS diseases,including stroke,was reported.Although the reason remains unclear,increasing evidence shows that the traits of not only stem cells themselves but also a stem-cell niche surrounding stem cells (e.g.,endothelial cells) alter after ischemia/hypoxia and differ among the developing ages of mice in the CNS[21-24].Thus,the lack of data may be due to the NSPCs being derived not from pathological but from normal conditions (e.g.,developmental fetal NSPCs)[9,10]and investigation having focused on the reparative mechanism not emerging from the pathological CNS.
INSPCS/ISCS DERIVED FROM MICE ISCHEMIC BRAINS
In our laboratory,we aimed to develop a method to isolate and utilize endogenous NSPCs specifically induced by brain injury such as ischemic stroke (injury/ischemiainduced NSPC; iNSPC).We used a mouse model of cerebral infarction whose postischemic areas were highly reproducible[25,26].As a result,we demonstrated for the first time that,although mature neural cells such as neurons,astrocytes,and oligodendrocytes underwent cell death within ischemic regions,iNSPCs that had the potential to differentiate into these cells developed within the same areas[27].In addition,we have shown that activation of iNSPCs promoted neural repair and functional recovery following ischemic stroke[22,28].
BRAIN PERICYTES FOLLOWING ISCHEMIA:DO THEY FUNCTION AS NSPCS?
Many types of cells,including astrocytes in the subventricular zone (SVZ)[29,30],reactive astrocytes[31],resident glia[32],oligodendrocyte precursor cells (OPCs)[33,34],and ependymal cells[35,36],have been reported as NSPC candidates.Although the origin of iNSPCs remains unclear,previous studies showed that several types of NSPCs such as SVZ astrocytes[37,38]and OPCs[39,40]reside near blood vessels,in close association with endothelial cells.We have previously shown that nestin+iNSPCs within ischemic areas express various pericyte markers such as platelet-derived growth factor receptor beta (PDGFRβ),neuronal/glial 2 (NG2),and alpha smooth muscle actin (αSMA)[21,24,41].Importantly,nestin+cells were absent from non-ischemic areas in the cortex of adult mice,indicating that normal pericytes in the adult brain do not express nestin.Thus,we proposed that brain pericytes,localized near blood vessels,are potentially giving rise to iNSPCs after injuries such as ischemic stroke[24,42].
Pericytes are localized near blood vessels and form a neurovascular unit (NVU)together with endothelial cells and neural lineage cells (neurons and astrocytes).Pericytes are heterogeneous cells:although PDGFRβ,NG2,nestin,αSMA,CD146,Glast,Tbx18,and regulator of G protein signaling 5[24,43-51]are expressed on pericytes,none of those are specific markers.Birbrairet al[44]divided skeletal-muscle-derived pericytes into two subtypes (nestin-/NG2+type-1 pericytes and nestin+/NG2+type-2 pericytes).Using their proposed categorization,iNSPCs would be classified as type-2 pericytes as they express both nestin and NG2.In addition,Birbrairet al[52]reported that nestin+/NG2+type-2 pericytes have NG2+glia-like traits.However,NG2+glia is identical to OPCs[53],and both pericytes and OPCs express common markers,including NG2 and PDGFRα[54].Thus,the precise connection between iNSPCs and resident glia should be determined in further studies (Figure 1).
BRAIN PERICYTES FOLLOWING ISCHEMIA:DO THEY FUNCTION AS MULTIPOTENT STEM CELLS?
Brain pericytes are a key component of the NVU and play an important role in maintaining this unit[55].Even after severe stress such as ischemic stroke,cells forming the NVU,including pericytes[42]and endothelial cells[23],survive,suggesting that these cells play an essential role under pathological conditions as well as under normal conditions.
Besides endothelial cells[56-59],pericytes possess plasticity[54,60]and function as multipotent stem cells as well[43,44,47,61-67].Therefore,we investigated whether iNSPCs maintain their multipotency under pathological conditions.We found out that iNSPCs can differentiate into not only neural but also mesenchymal lineages,including osteoblasts,adipocytes,and chondrocytes[21,41].Thus,under ischemic conditions following stroke,brain pericytes might convert into injury/ischemiainduced multipotent stem cells (iSCs) by acquiring the stemness,thereby producing iNSPCs (Figure 1).Consistent with our previous reports[21,41],using a mouse model of cerebral infarction,other groups have also shown that brain pericytes following ischemia display the potential to differentiate into multilineage cells[68].We also showed that iSCs share angioblast features and give rise to hematopoietic cell lineages such as microglia[21,41].Consistent with these reports,a recent study showed that brain pericytes and endothelial cells share certain traits[69].Interestingly,a subtype of pericytes was reported to be derived from hematopoietic lineages,including microglia[70-72].Thus,the relationship among iSCs,pericytes,and hematopoietic lineages remains to be elucidated in future studies.
It remains unclear whether brain pericytes behave as multipotent stem cellsin vivo.Ideally,this should be clarified in mice using pericyte markers.A recent study using genetic mapping by the Cre-loxP system failed to demonstrate that Tbx18+brain pericytes function as multipotent stem cellsin vivofollowing mild injury,although they behave as multipotent stem cellsin vitro[50].However,phenotypes of cells expressing certain genes (e.g.,nestin) in transgenic mice differ depending on the intron regions in which a tag (e.g.,green fluorescent protein) is inserted[73-75].Accumulating evidence also shows that genetic mapping techniques by the Cre-loxP system present several pitfalls[76-78].For example,gene expression patterns and localizations of certain genes (e.g.,nestin) are different depending on the reporter mice used for crossbreeding[78].Additionally,recombination efficiency following tamoxifen treatment differs among the developing stages of mice[77].Furthermore,we have previously demonstrated that induction of iNSPCs/iSCs varies with the degree of ischemic stimuli and that a severe injury is essential for inducing iNSPCs/iSCs[42].Therefore,whether brain pericytes function as multipotent stem cells following injuryin vivoshould be carefully investigated in further studies.
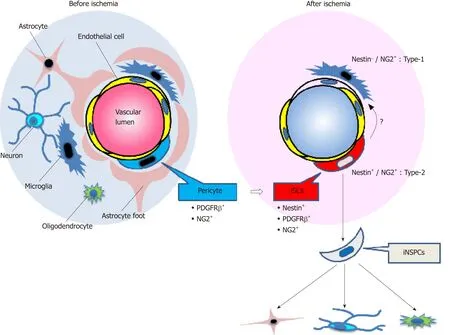
Figure1 Schematic representation of the fate of injury/ischemia-induced multipotent stem cells and injury/ischemia-induced neural stem/progenitor cells following ischemic stroke.
Moreover,to confirm that iSCs are multipotent,it is necessary to show that iSCs derived from a single-cell type can differentiate into multiple cell types.We previously proposed that iSCs might be composed of subpopulations each specifically differentiating into neural or mesenchymal lineages[79].If so,these subpopulations once isolated could be useful for clinical applications.For example,the subpopulation that can predominantly differentiate into neuronal lineages would be used for neural repair following CNS injuries.However,the precise relations between iNSPCs and iSCs should be clarified in further studies (Figure 1).
BRAIN PERICYTES FOLLOWING ISCHEMIA:HOW DO THEY ACQUIRE THE STEMNESS?
Although the mechanism by which brain pericytes acquire multipotency under ischemic conditions remains unclear,we have previously demonstrated that brain pericytes display up-regulated expression of various stem cell and undifferentiated cell markers when they are incubated under oxygen-glucose deprivation (OGD) that mimics ischemia/hypoxia[21,41].In general,pericytes have the characteristics of mesenchymal lineages,and NSPCs have traits of epithelial lineages.Following OGD stimuli,we showed that the mesenchymal-epithelial transition (MET) was facilitated in brain pericytes as demonstrated by the up-regulated expression of theSox2gene[21,41].
These findings suggest that iNSPCs/iSCs are derived from brain PCs having developed stemness through cellular reprogramming and MET.In support of this viewpoint,accumulating evidence shows that brain PCs reprogrammed by gene transduction (e.g.,Sox2gene) acquire neural lineage traits,including NSPC and neuron phenotypes[48,80].
In addition to the NSPC marker nestin,iNSPCs/iSCs express various stem cell and undifferentiated cell markers,including Sox2,Nanog,c-myc,and Klf4.However,iNSPCs/iSCs lackOct 3/4gene expression,which is essential in producing iPS cells[21,24,81],even though iNSPCs/iSCs can differentiate into neural and mesenchymal lineages.Therefore,iNSPCs/iSCs differ from pluripotent stem cells such as iPS cells and ES cells.We also found out that it is not easy for somatic adult pericytes to be reprogrammed into a pluripotent state even when subjected to severe stress such as ischemia[21].However,a recent study showed that an injury stimulus did convert skeletal muscle cells into a pluripotent state[82].Thus,whether injury stimuli can induce somatic cells to become pluripotent cells should be carefully investigated in future studies.
BRAIN PERICYTES FOLLOWING ISCHEMIA:ARE THEY IDENTICAL TO OTHER TYPES OF MULTIPOTENT STEM CELLS THAT RESIDE NEAR BLOOD VESSELS?
Akin to pericytes,previous studies showed that multipotent stem cells such as MSCs[83-87]and neural crest stem cells (NCSCs)[88]reside in the perivascular regions of multiple organs.These cells also differentiate into various lineages,including neural and mesenchymal lineages,consistent with the traits of iNSPCs/iSCs.
Comparing iNSPCs/iSCs with other types of multipotent stem cells such as bonemarrow-derived MSCs,iNSPCs/iSCs differentiate into mesenchymal lineages,including osteoblasts and adipocytes as well as MSCs.Using multi-electrode arrays[89],we recently reported that iNSPCs/iSCs,but not MSCs,have the potential to differentiate into electrophysiologic-functional neurons[90].On the basis of their developmental origin in multiple organs,the majority of non-CNS pericytes originate from the mesoderm.However,brain pericytes are likely neural crest derivatives[91,92].
The cells of the neural crest originate from the neural tube through the epithelialmesenchymal transition.The cells of the neural crest are multipotent stem cells(NSCs) that share both neural and mesenchymal traits[79,93,94].
Considering their origin,iNSPCs/iSCs have a stronger neural phenotype than MSCs.Thus,it is likely that iNSPCs/iSCs are stem cells which differ from previously reported ones.However,recent studies show that the traits of MSCs vary among organs[87].Thus,brain MSCs might have features differing from those of MSCs derived from other organs (e.g.,bone-marrow-derived MSCs)[95],and further investigations are necessary regarding the relations among iNSPCs/iSCs,brain pericytes,and brain MSCs.
INSPCS/ISCS DERIVED FROM HUMAN ISCHEMIC BRAINS
To translate the non-clinical findings obtained in mouse iNSPCs/iSCs into clinical applications,it is essential to understand the traits of human iNSPCs/iSCs obtained from patients with stroke.
Using brain samples obtained from stroke patients who needed both decompressive craniectomy and partial lobectomy as a life-saving therapy for diffuse cerebral infarction,we attempted to isolate human iNSPCs/iSCs.We detected iNSPCs/iSCs within post-stroke areas of the human brains,consistent with those of mouse brains[21,24,41,90].
Isolation and characterization of human iNSPCs/iSCs from stroke patients
Recently,we have reported the traits of iNSPCs/iSCs obtained from two patients with cerebral infarction[96].The samples obtained from two elderly patients displayed gross necrosis and histological cell death.Immunohistochemical analysis showed that,although mature neural cells disappear within post-stroke areas,nestin+cells were present within these areas.The nestin+cells localized near blood cells and expressed pericyte markers such as NG2 and αSMA.After the cells isolated from post-ischemic human tissues were incubated in medium with basic fibroblast growth factor (bFGF)and epidermal growth factor (EGF),many proliferative cells emerged,and they expressed the dividing cell marker Ki67.The cells isolated from post-ischemic human tissues expressed not only nestin but also the pericyte markers NG2,PDGFRβ,and αSMA.However,these nestin+cells did not express endothelial cells and astrocytes markers.These findings indicate that brain pericytes convert into nestin+iNSPCs/iSCs within post-stroke human brains,consistent with mouse brains[21].
Next,we examined the multipotency of human iNSPCs/iSCs.Even after several passages,nestin+iNSPCs/iSCs retained the expression of various stem cell and undifferentiated cell markers,including Sox2,c-myc,and Klf4.When they were incubated under conditions to promote the differentiation into mesoderm lineages such as osteoblasts,adipocytes,and chondrocytes,they differentiated into these cells,respectively.They also formed neurosphere-like cells under floating cultures and differentiated into Tuj-1+and MAP2+neuronal cells.These findings demonstrate that iNSPCs/iSCs are present within post-stroke human brains as well as in post-stroke mouse brains.
However,more precise traits of human iNSPCs/iSCs remain unclear,including their multipotency potential to differentiate into functional neurons.To address this question,we are now investigating the features of human iNSPCs/iSCs obtained from additional post-ischemic cerebral samples.Our preliminary study shows that human iNSPCs/iSCs expanded from a single-cell lineage mainly differentiated into Tuj1+neurons under neuronal differentiation conditions,and they differentiated into fatty acid binding protein 4 (FABP4)+adipocytes under adipogenic differentiation conditions.Our recent study also reveals that human iNSPCs/iSCs have the potential to differentiate into functional neurons[97].These results indicate that iNSPCs/iSCs (at least a sub-population) function as multipotent stem cells that differentiate into neuronal cells.Therefore,these cells should be renamed iSCs rather than iNSPCs because they can differentiate into various cell lineages other than neural.
Other questions remain.For example,the traits of iNSPCs/iSCs may differ from the time of injury onset to surgery.Also,iNSPC/iSC features may vary among CNS regions (e.g.,cerebrum,cerebellum,brainstem,spinal cord).Regarding the latter question,our recent study demonstrated that iNSPCs/iSCs could be isolated from the cerebellum[97]as well as the cerebrum[96].Comparative gene expression profiles showed that although the cerebellar iNSPCs/iSCs resembled cerebral iNSPCs/iSCs,they expressed certain cerebellum-specific genes[97].Thus,further studies are needed using additional samples to identify comprehensively the traits of iNSPCs/iSCs.
THE PROSPECTS OF REGENERATIVE THERAPIES USING INSPCS/ISCS
Evidence showing that iNSPCs/iSCs are present within post-stroke human brains suggests that stem-cell-based therapies using iNSPCs/iSCs could contribute to neural repair in patients with stroke in the future.Two strategies for clinical applications using iNSPCs/iSCs could be implemented as follows.
A strategy targeting exogenously transplanted NSPCs/iSCs
The first strategy implies to transplant exogenous iNSPCs/iSCs within or near postischemic areas (Figure 2A).iNSPCs/iSCs isolated from ischemic areas exhibit high proliferative activities in a medium containing bFGF and EGF[96].Thus,after a satisfactory expansion of iNSPCs/iSCs,the autologous transplantation of iNSPCs/iSCs could be performed during subacute and chronic periods.This therapy presents the advantage to repeatedly transplant iNSPCs/iSCs that satisfy certain cell profiles.Another advantage is that the cell number (e.g.,low dose of cells and high dose of cells) and the transplant location (e.g.,within ischemic areas,around ischemic areas,and non-ischemic areas) can be chosen.
On the other hand,there are several disadvantages.For example,several weeks are required to prepare enough iNSPCs/iSCsin vitro,not allowing iNSPC/iSC transplantation in stroke patients during acute phases.Furthermore,iNSPCs/iSCs cannot be obtained from any stroke patients.Currently,iNSPCs/iSCs can only be obtained from patients who needed both decompressive craniectomy and partial lobectomy as a life-saving therapy for diffuse cerebral infarction.It is ethically impossible to get iNSPCs/iSCs from patients with small infarcted areas (e.g.,lacunar infarction).Therefore,only a small portion of stroke patients would be eligible for this treatment in the future.
Currently,we are investigating the safety (e.g.,tumorigenesis onset and formation)and efficiency (e.g.,cell survival,neuronal differentiation,and functional improvement) upon transplantation of human iNSPCs/iSCs in mice post-stroke.Theoretically,the above-mentioned problems would be solved if iNSPCs/iSCs are expandable in allograft and autograft transplantations.However,we have to carefully evaluate whether iNSPCs/iSCs can be utilized as an allograft because iNSPCs/iSCs are stem cells that originated from brains that differ from stem cells derived from non-CNS (e.g.,bone marrow-derived MCS).
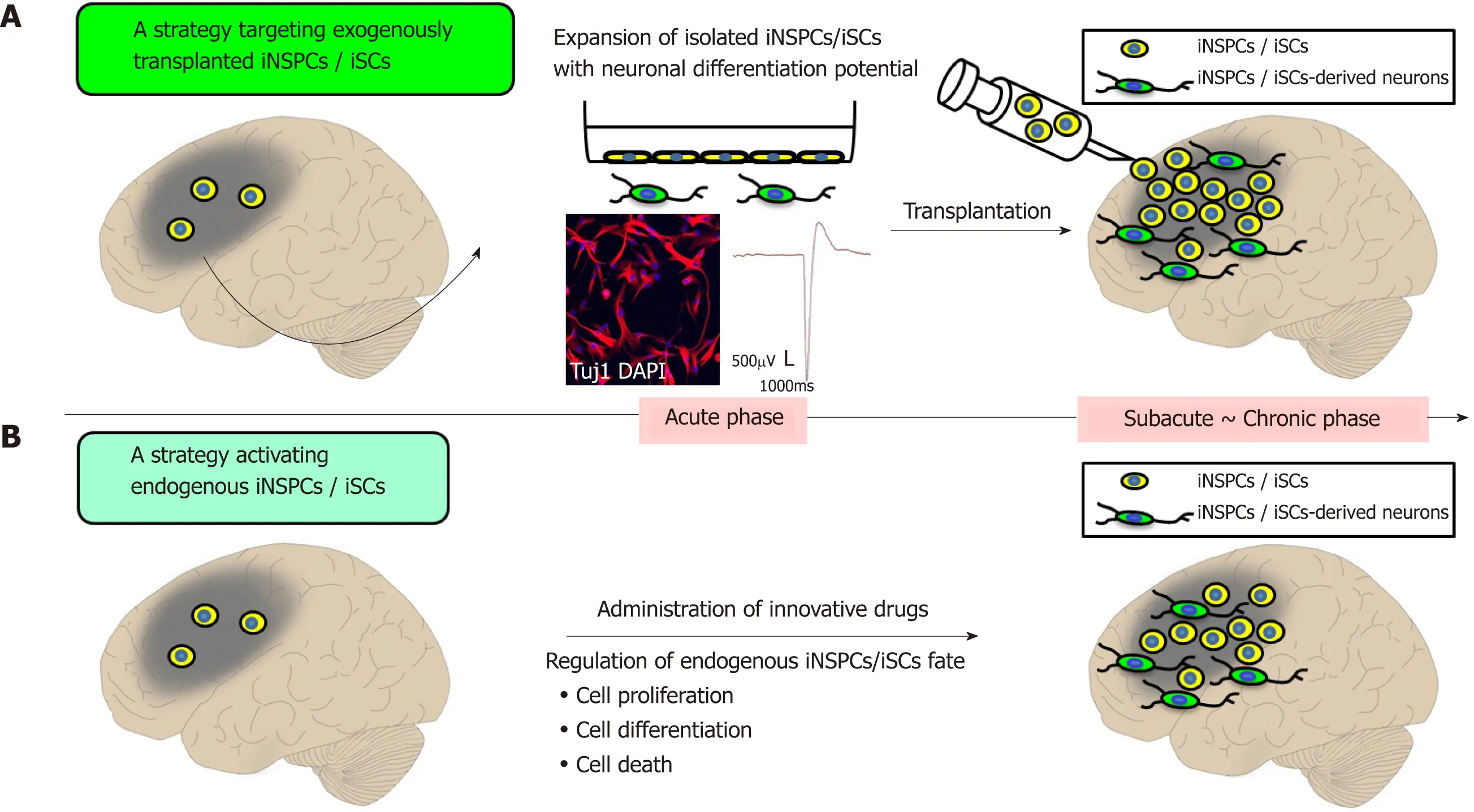
Figure2 Prospects of regenerative therapy using injury/ischemia-induced neural stem/progenitor cells and injury/ischemia-induced multipotent stem cells.
These problems may be solved using iNSPCs/iSCs derived from iPS cells.For example,using iPS-cell-derived iNSPCs/iSCs obtained from skin fibroblasts of stroke patients,patients may receive an autologous transplantation therapy using iNSPCs/iSCs.However,when making iPS cells,new problems could emerge,such as tumor formation.
A strategy activating endogenous iNSPCs/iSCs
The second strategy involves identifying the factors regulating the fate of iNSPCs/iSCs (e.g.,factors promoting cell proliferation and differentiation,and factors inhibiting cell death) and to develop those as innovative drugs (Figure 2B).
Using a mouse model of cerebral infarction,we previously showed that iNSPCs/iSCs isolated from ischemic areas differentiated into electrophysiologicfunctional neurons and did express mature neuronal markers[27].In vivo,the number of nestin+iNSPCs/iSCs peaked around post-stroke day 3 and then gradually decreased.In addition,immature newly born neurons were identified within and near ischemic areas at post-stroke day 3,and their numbers decreased thereafter as well[24,42,49].
This suggests that,although iNSPCs/iSCs are present within ischemic areas,several factors regulate their survival,proliferation,and differentiation.In support of this viewpoint,we have previously demonstrated that the endothelial cells residing around iNSPCs/iSCs promote their survival,proliferation,and neuronal differentiation[22,28].This suggests that endothelial-derived trophic factors exhibit a positive effect on iNSPCs/iSCs.Alternatively,endothelial cells and/or the extracellular matrix produced by endothelial cells[98]may function as a niche for iNSPCs/iSCs,as it is the case with NSPCs[99].
Further investigations are needed to understand the factors involved in the regulation of iNSPCs/iSCs.However,our previous studies indicated that a subset of lymphocytes that infiltrated into ischemic areas during acute phases inhibited the survival of iNSPCs/iSCs[100,101].In addition,our preliminary study showed that inflammatory cells such as microglia/macrophages rapidly increase at the time when nestin+iNSPCs/iSCs disappear.These findings indicate that iNSPC/iSC regulation also relies on environmental factors surrounding them (e.g.,inflammatory cells),and both intrinsic and extrinsic factors play an essential role in neural regeneration.
CONCLUSION
Our studies showed that iNSPCs/iSCs are present within post-stroke areas of mouse and human brains.Further studies are needed to identify the traits,fate,proliferation,and differentiation factors of iNSPCs/iSCs for their clinical applications.However,iNSPCs/iSCs represent a cornerstone in contributing to CNS repair because they are stem cells that develop within ischemic areas following CNS injuries.Evidence of the presence of iNSPCs/iSCs within post-ischemic human brains is encouraging for the development of new stem-cell-based therapies for stroke patients.
ACKNOWLEDGEMENTS
We would like to thank members of Institute for Advanced Medical Sciences and Department of Neurosurgery at Hyogo College of Medicine for helpful assistance.
 World Journal of Stem Cells2019年8期
World Journal of Stem Cells2019年8期
- World Journal of Stem Cells的其它文章
- Moving forward on the pathway of cell-based therapies in ischemic heart disease and heart failure - time for new recommendations?
- Orchestrating stem cell fate:Novel tools for regenerative medicine
- Bone marrow microenvironment:The guardian of leukemia stem cells
- Tonsil-derived stem cells as a new source of adult stem cells
- Linking stemness with colorectal cancer initiation,progression,and therapy
- Derivation and applications of human hepatocyte-like cells
