Fabrication and Combustion Properties of TEGN/RDX Based Microcellular Combustible Objects
DING Ya-jun, ZHANG Shuo, YING San-jiu, XIAO Zhong-liang
(Key Laboratory of Special Energy Materials (Nanjing University of Science and Technology), Ministry of Education,Nanjing 210094, China )
Abstract:In order to improve the combustion properties of combustible objects, the microcellular combustible objects were fabricated by the supercritical carbon dioxide (SC-CO2) foaming technology. Scanning electron microscope (SEM) and the closed vessel tests were applied to characterize the cell structure and combustion properties of microcellular combustible objects. The results demonstrated that there were numerous micron-scale cells in the microcellular combustible objects fabricated by the SC-CO2 foaming technology, which enhanced the specific surface area. Both the foaming temperature of 90°C and the foaming time of 15min led to the increase of the cell density and diameter. The closed vessel tests indicated that the microcellular combustible objects performed the shorter burning time (14.66ms) than that of original sample (16.49ms), and the decrement was 11%. The higher foaming temperature, the longer foaming time and the higher RDX mass fraction promoted the spread rate of burning gas of the microcellular combustible objects in burning process because of the higher specific surface area and oxygen balance. Therefore, the microcellular combustible objects foamed by SC-CO2 had a very good application foreground in weapons.
Keywords:material science; microcellular combustible objects; SC-CO2; cell structure; combustion property
Introduction
The combustible objects of polymer bonded nitramine propellants have several advantages like high energy mass fraction, high burning rate, variable burning characteristics and low vulnerability, and they can be used in the combustible cases and the caseless ammunition[1-2]. Compared with the conventional metal cases, the presence of microporous can lighten the quality of ammunition, which enhances the bomb load for the soldiers. In addition, the combustion and mechanical properties of microcellular combustible objects can be adjusted by changing the energetic filler and controlling the structure of microporousmicropores.
However, as the conventional combustible objects cannot burn completely, many researchers explore the methods to improve the oxygen balance and the combustion process in aspects of the formulation and the internal structure of combustible objects. Increasing the energetic material mass fraction and the specific surface area are the effective ways to promote the combustion properties. Wang B[3]has investigated the effects of particle size and morphology of nitroguanidine (NQ) on the combustion properties of triple-base propellants. He found that the triple-base propellant with smaller NQ particle size exhibited significantly higher burning rate, as the smaller particle size provided the larger specific surface. In recent years, some researchers investigated the ways to break through a barrier of energy utilization ratio from the structure of propellants themselves. The reaction injection moulding processes have been applied to produce RDX-based foamed propellants, and the burning rate of this gun propellant with porous structure was farther than that of the conventional gun propellants[4-6]. The partial high-pressure gases produced by the burning of gun propellant penetrate into the porous solid, and then it ignites the matrix around the pores.
Supercritical carbon dioxide (SC-CO2) foaming technology is another method to produce the microcellular combustible objects with cells in the level of 1μm[7]. Meanwhile, the conventional foaming agents stay in the foamed propellants as the non-energetic component, while SC-CO2escapes from the microcellular combustible objects completely, which makes no difference to the combustion properties of propellants. Yang[8]produces the microcellular combustible objects with hexogen/poly (methyl methacrylate) (RDX/PMMA) and hexogen/cellulose acetate (RDX/CA) formula system, and the microcellular combustible objects perform higher burning rate than the original samples, but the oxygen balance are not ideal for applications resulting from the non-energetic polymer binder.
In order to solve the current problems above, the energetic mass fraction of RDX (37.5%) and triethylene glycol dinitrate (TEGN) propellant (59.0%) is as high as 92.5% to improve the oxygen balance of combustible objects. TEGN propellant is the mixture of nitrocellulose and TEGN, and it acts as not only the energetic binder but also the matrix to absorb some amounts of CO2for cell nucleation in this work. The thermoplastic monomer, methyl methacrylate (MMA), is blended with the matrix to prepare the novel semi-interpenetrating network structure, promoting the mechanical strength of samples.The microcellular combustible objects with high energetic mass fraction have been fabricated by the two-step foaming process with SC-CO2under different processing conditions. The cell structure and combustion properties of microcellular combustible objects are characterized by scanning electron microscope (SEM) and the closed vessel tests.
1 Experimental
1.1 Materials
TEGN and RDX (10μm) were provided by Luzhou North Chemical Industries Co., LTD. The thermoplastic monomer MMA was purchased from Nanjing Zhanyi Co., LTD. Ethanol (analytically pure, AR) and ethyl acetate (AR) were purchased from Sinopharm Chemical Reagent Co., LTD. Industrial CO2(purity≥99.9%) was supplied as the physical blowing agent from Nanjing Wenda Special Gas Co., LTD.
1.2 Fabrication of combustible objects
A kneading machine was employed to kneading MMA, TEGN and RDX preliminarily with the mixed solvents of ethanol and ethyl acetate at 35 ℃ for 60min. The dough of composite propellant was extruded into sheets through the hydraulic machine and molding mould, and the length, width and thickness of the samples were 12, 12 and 2mm, respectively. Samples were dried in the oven to drive out the mixed solvents for 72h at 60℃ ,until the volatile mass fraction less than 0.5%.
1.3 Fabrication of microcellular combustible objects
The microcellular combustible objects were foamed by the two-step foaming process as shown in Figure 1. Samples were placed into the high-pressure vessel, and some amounts of CO2was injected into the vessel to reach a certain pressure (saturation pressure, ps) by the CO2injection pump. Then, the vessel was put into the water bath at a set temperature (saturation temperature,Ts). After being saturated for 10h, samples were carried out from the vessel and transported into the hot water (foaming temperature,Tf) for a certain time (foaming time,tf) immediately. The samples were taken out and dried for 48h to remove the residual water in the microcellular combustible objects.
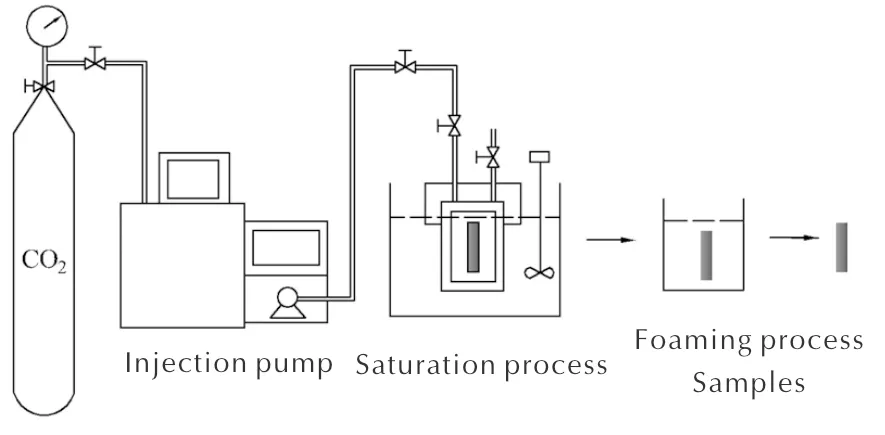
Fig.1 Schematic diagram of the two-stage batch foaming process
1.4 Characterization
The cell structure of microcellular combustible objects was observed by SEM (QUANTA FEG 250, FEI). The foamed samples were immediately fractured after being immersed in the liquid nitrogen for about 10min, and then the fractured surfaces were sputtered with gold. The cell structure was recorded with SEM at an acceleration voltage of 10kV, magnified by 40000 times.
Besides, the apparent density of microcellular combustible objects was measured by the method of volumetric flask as our previous work[89].
The combustion properties were studied by the closed vessel test with volume of 100mL, while the loading density was 0.20g/mL. All the samples were fired by 2# nitrocellulose under the same ignition pressure (10.98MPa) and ambient temperature. The pressure gradient—time (dp/dt—t) curves and the dynamic vivacity—relative pressure (L—B) curves of the microcellular combustible objects were calculated from the pressure (p) and rise time (t) recorded.
2 Results and Discussion
2.1 Cell structure of microcellular combustible objects
As the combustion properties of microcellular combustible objects are adjusted by the cell structure, the nucleation and growth of cells are significant to improve the specific surface area, and the foaming temperature and time are the crucial factors affecting the density and diameter of cells.
Table 1 and Figure 2 are the apparent densities, cell diameters, cell densities and cell structure of the foamed samples at different foaming temperatures (80, 85 and 90℃,ps=15MPa,Ts=40℃,ts=10h,tf=5min), respectively. The free swelling rate is the apparent density ratio between the original sample and the foamed sample, and porosity is the rate of volume change. The results demonstrate that the porosity and cell diameter of the foamed samples increase with increasing the foaming temperature, while the apparent densities of samples (ρs) decrease at the same time. Since the CO2dissolution process is an exothermic process, the higher foaming temperature improves the gas diffusion coefficient, which not only generates a large number of nucleation sites effectively but also provides the energy and power for cell growth. In addition, due to the classic nucleation theory, the temperature difference between saturation and foaming temperature is the main factor for the driving force of cell nucleation under the condition of the same amount of SC-CO2, and the higher foaming temperature leads to the higher nucleation rate[10]. Therefore, the cell diameter and cell density increase with the higher foaming temperature. Meanwhile, compared with the original sample, the microcellular combustible objects display numerous cells with mean diameter of about 0.4μm, which demonstrates that the SC-CO2foaming technology is ideal for fabricating microcellular combustible objects.

Fig.2 Cell structures of microcellular combustible objects under different foaming temperatures
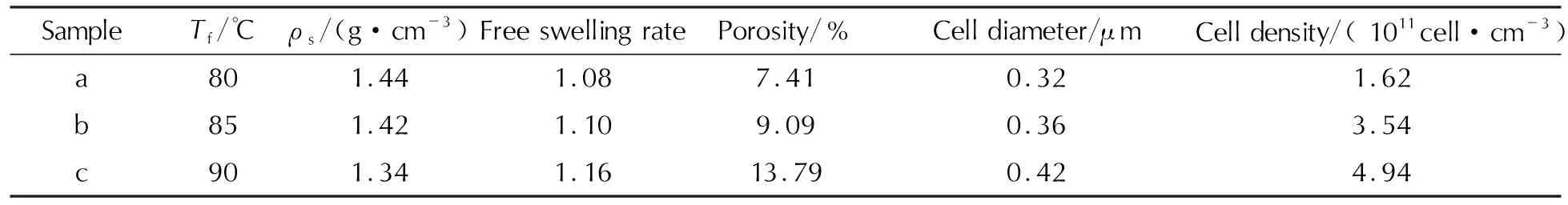
Table 1 Apparent density, cell diameter and cell density of microcellular combustible objects at different foaming temperatures
Table 2 and Figure 3 are the apparent densities, cell diameter, cell densities and cell structures of the foamed samples for different foaming time (5,10 and 15min,ps=15MPa,Ts=40℃,ts=10h,Tf=90℃), respectively. As the longer foaming time extends the growth time for cells, it is obvious that the porosity and cell diameter of microcellular combustible objects increase with the foaming time, while the apparent densities of samples decrease. However, when the foaming time is as long as 15min, the foaming process is extremely severe, and it comes to the phenomenon of collapse and coalescence among the cells. At the same time, the apparent density of microcellular combustible objects is 1.24g/cm3when the foaming time is 15min, resulting in the low charge mass and charge density for the weapons.
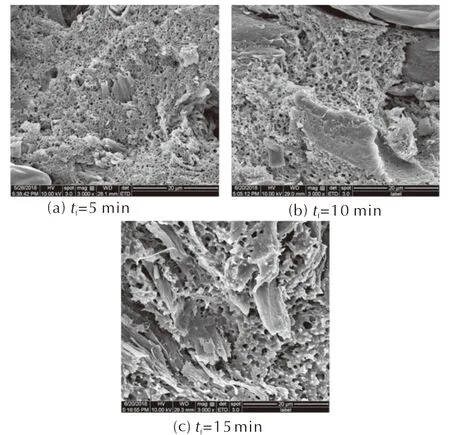
Fig.3 Cell structures of microcellular combustible objects for different foaming time
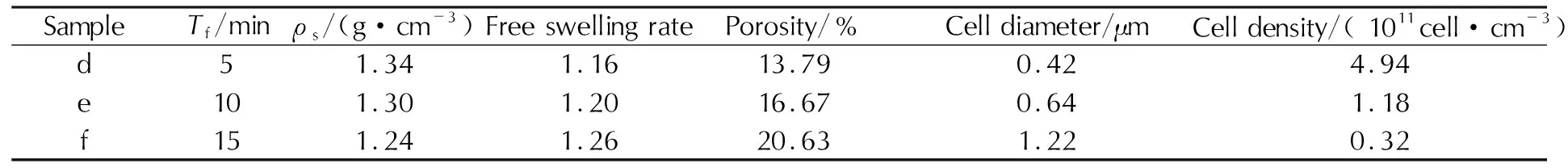
Table 2 Apparent density, cell diameter and cell density of microcellular combustible objects for different foaming time
2.2 Effect of foaming temperature on combustion property
Figure 4 (a), (b) and (c) are thep—t, dp/dt—tandL—Bcurves of the microcellular combustible objects at different foaming temperatures, respectively.
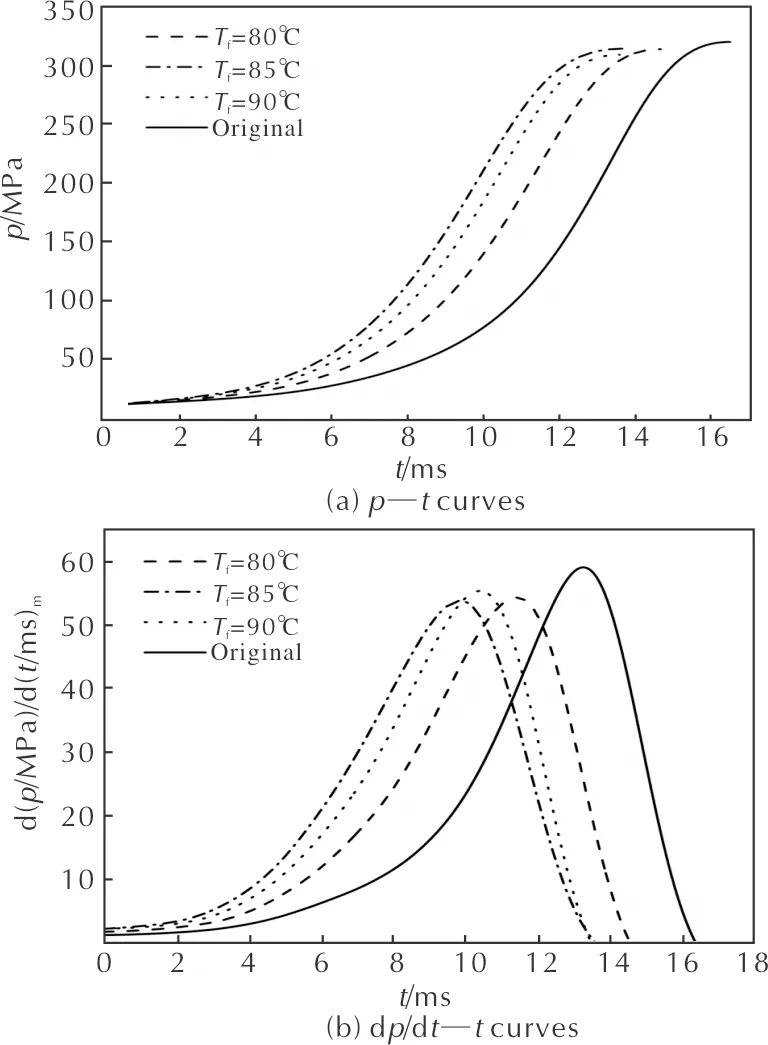

Fig. 4 Combustion curves of samples at various foaming temperatures
Table 3 is the combustion parameters obtained from the curves when the saturation temperature, saturation pressure, saturation time, foaming time and RDX mass fraction are 40℃, 15MPa, 10h, 5min and 35%, respectively. The burning time (tk) of microcellular combustible object is 14.66 ms when the foaming temperature is 80℃, while the burning time of original sample is 16.49ms, and the decrement is 11%. And the highest gas pressure (pm) has a little fluctuation. The presence of cell structure enhanced the specific surface area, which promotes the combustion property of gun propellants. In the burning process of the microcellular combustible objects, the high-temperature and high-pressure gas generates into the cells of samples rapidly, accelerating the burning rate. Thus, the microcellular combustible object is suitable to improve the combustion properties by the SC-CO2foaming technology. The figures and table also show that the samples burn slightly faster when the foaming temperature is higher, and the value of dp/dt—treaches the peak with less burning time. Whereas, theL—Bcurve indicates that the initial dynamic vivacity of microcellular combustible objects foamed at 80℃ is lower than that of samples at 85℃ or 90℃. Based on the investigation of cell structure above, the microcellular combustible objects foamed at higher temperature possess the higher cell density, promoting the burning rate of samples, so the burning time is shorter and the initial dynamic vivacity is higher when the foaming temperature is 85℃ or 90℃.
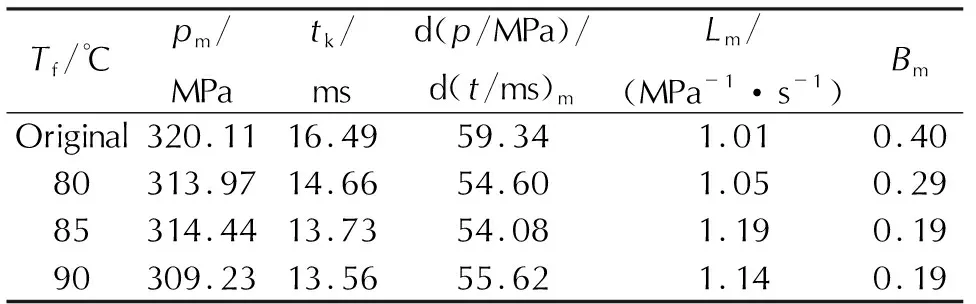
Table 3 Combustion parameters of samples at various foaming temperatures
2.3 Effect of foaming time on combustion property
In order to study the effects of foaming time on the combustion performance of microcellular combustible objects, the foaming time was set for 5, 10 and 15min, when the saturation temperature, saturation pressure, saturation time, foaming temperature and RDX mass fraction were 40℃, 15MPa, 10h, 90℃ and 35%, respectively. Figure 5 (a)-(c) are the combustion curves of microcellular combustible objects under different foaming times, respectively. Table 4 is the combustion parameters obtained from the curves, and the subscript m means the maximum value. As both the cell density and diameter of samples increase with the increasing foaming time, the burning rate is increased resulting from the higher specific surface area. Thus the burning time decreases from 13.56ms (5min) to 9.71ms (15min). Besides, the maximum dynamic vivacity (Lm) increases from 1.14(MPa·s)-1to 1.88(MPa·s)-1. TheL—Bcurve indicates that the microcellular combustible object performed the better burning progressivity when the foaming time is 5min or 10min, which exhibites the lower initial dynamic vivacity and the longer platform.

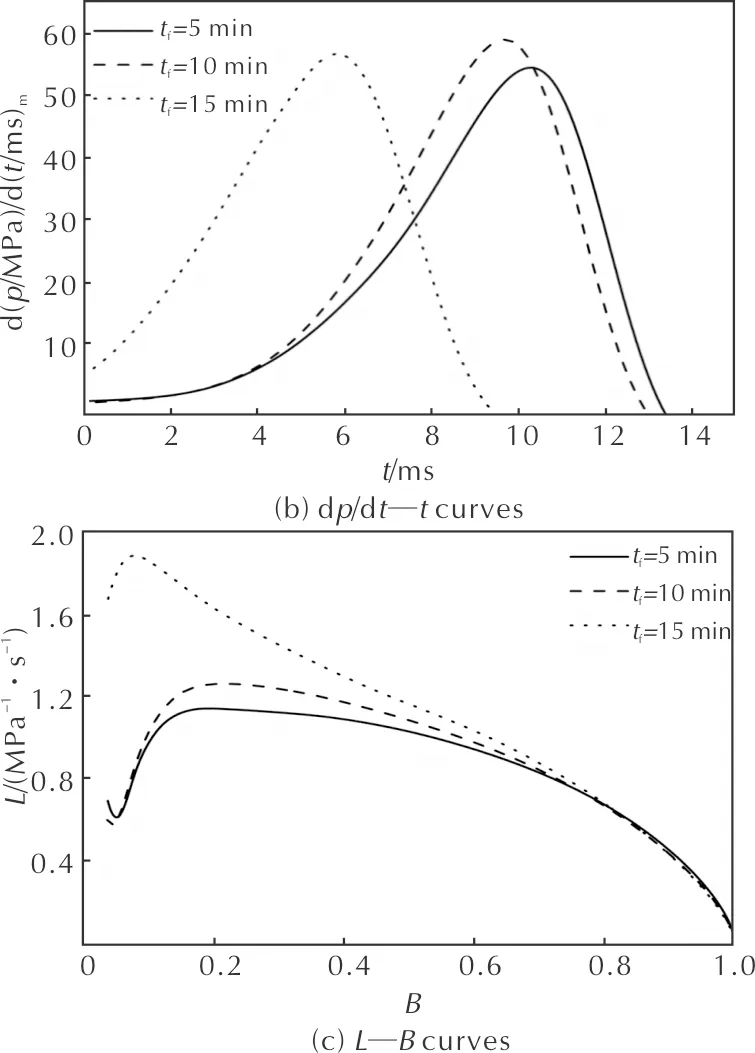
Fig. 5 Combustion curves of samples for various foaming times

tf/minpm/MPatk/msd(p/MPa)/d(t/ms)mLm/(MPa-1·s-1)Bm5309.2313.5655.621.140.1910318.0013.1760.111.260.2215304.469.7157.801.880.07
2.4 Effect of RDX mass fraction on combustion property
The addition of a large amount of high energy explosive compounds is an effective method to promote the formula energy and the combustion completeness of propellants. RDX and TEGN acted as the energetic components in the microcellular combustible objects in this work. Figure 6 (a)-(c) are thep—t, dp/dt—tandL—Bcurves of the microcellular combustible objects with various RDX mass fractions, respectively. Table 5 is the combustion parameters obtained from the curves. Thep—tcurve shows that the burning time of microcellular combustible objects decreases from 16.53ms to 11.93ms, with the RDX mass fraction increassing from 30% to 40%. The dp/dt—tandL—Bcurves mean that the higher RDX mass fraction accelerates the releasing rate of burning gas. It can be seen from theL—Bcurve that the dynamic vivacity and platform with 30% and 35% RDX mass fraction perform are better than those with 40% RDX. As CO2cannot dissolved into the RDX matrix, the higher RDX mass fraction has negative influences on the nucleation, growth and adjustment of cells. What’s worse, the presence of RDX goes against the mechanical property of microcellular combustible objects[11], so the RDX mass fraction needs to be controlled in a suitable range to meet the requirements of the combustion and mechanical property. Besides, the impact strength of the microcellular combustible objects is around 5kJ/m2which is 2.5 times higher than that of PMMA/RDX based combsutible objects.
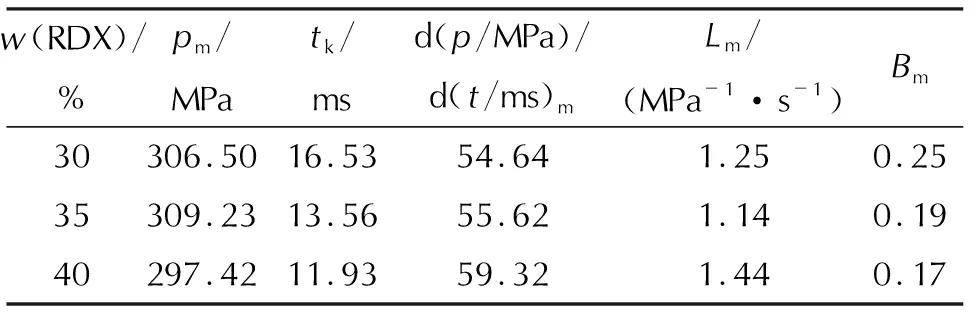
Table 5 Combustion parameters of samples with various RDX mass fraction

Fig. 6 Combustion curves of samples with various RDX mass fraction
2.5 Propellant force content
The force content of propellant is the important reference to evaluate the muzzle energy of weapons, and it is related to the ability of the burning gas to do work. Table 6 is the propellant force content of the original and microcellular combustible objects under different process conditions. It displays that the propellant force content modified by the SC-CO2foaming technology is higher than that of the original sample. As the microcellular combustible objects possess the higher specific surface area, they perform the higher efficiency rate of energy. Therefore, the microcellular combustible objects produce more burning gas in the burning process fastly, leading the higher propellant force. Corresponding to the effects of foaming temperature on the cell structure and combustion properties, the propellant force content increases with the foaming temperature. The foaming time has few effects on the propellant force. In addition, the propellant force with 35% RDX mass fraction (Tf=90℃,tf=5min) exhibits the highest value (1246kJ/kg) while the propellant force with 30% and 40% RDX mass fraction is 1161kJ/kg and 1146kJ/kg, respectively. As the combustion property with various RDX mass fraction analyzed above, the excess of RDX causes the decrease of cell density, so the ability of the burning gas to do work with 40% RDX is worse than that with 35% RDX.
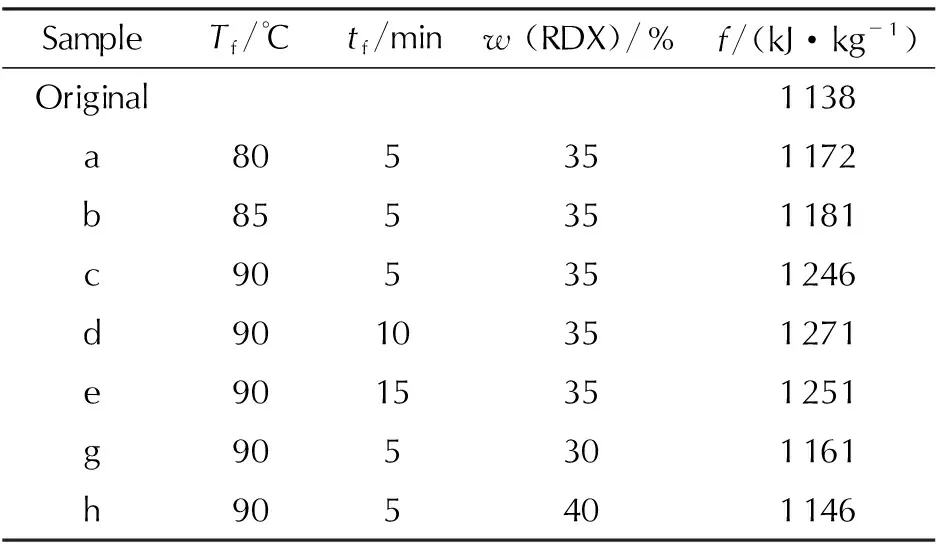
Table 6 Propellant force of samples
3 Conclusions
(1) The results demonstrate that the microcellular combustible objects posess numerous cells in the level of 1μm by the SC-CO2foaming technology, which enhances the specific surface area. Both the higher foaming temperature and the longer foaming time lead to the increase of the cell density and diameter.
(2) The closed vessel tests indicate that the microcellular combustible objects perform the faster burning time. The higher foaming temperature, the longer foaming time and the higher RDX mass fraction promote the burning process of the microcellular combustible objects because of the higher specific surface area or oxygen balance. The microcellular combustible object performs the better burning progressivity when the foaming time is 5min or 10min, exhibiting the lower initial dynamic vivacity and the longer platform.
(3) The propellant force of the microcellular combustible objects is promoted by the higher energy efficiency rate. The microcellular combustible objects performing excellent combustion properties are significant to the application of combustible cartridge case in the future.

