Synthesis of high purity Li2CO3 and MgCO3·3H2O in a homogeneous-like organic phase☆
Lang Li*,Jinsong Sui,Wei Qin
Department of Chemical Engineering,State Key Laboratory of Chemical Engineering,Tsinghua University,Beijing 100084,China
Keywords:Li2CO3 MgCO3·3H2O Morphology Crystallization mechanism
ABSTRACT Herein,three kinds of Li2CO3 and two kinds of MgCO3·3H2O crystals are easily synthesized in a homogeneouslike organic phase.The morphology and size of synthesized crystals are controllable and adjustable in the single organic phase,with the morphology of Li2CO3 ranging from micro-flaky,flower to nanobranch,MgCO3·3H2O ranging from nanosphere to nanorod. Compared with coupled reaction and solvent extraction process, of which the crystallization process occurred in the interface of two phase,our proposed method made it possible that the crystallization process occurred in the single organic phase,which resulted in better crystal morphology.Moreover,the formation mechanism of different crystal morphologies is discussed,the results showed that the crystals in micron size and nano size are involved in two crystallization mechanism,the micron particles in the form of flake and flower-like is a typical radial growth,which means that the growth occurs by diffusion around a nucleus as starting point,while the reaction model for small particles should be similar to a water-in-oil structure.As the reaction carried out,the crystal should be restricted in a constrained organic structure.
1.Introduction
As the lightest metal,lithium and its compounds are widely used in alloy[1],nuclear power,glass,medicine fields[2,3]and CO2capture[4],especially in lithium ion batteries [5-11].The fabrication of valuable chemicals from brines has recently attracted intensive interest because of its relatively low-cost,high-purity and large scale production as compared to traditional methods.Due to the high ratio of Mg/Li in brine,the recovery of lithium is always coupled with the utilization of magnesium[12].Our previous work where LiCl was the main product has described in detail about the extraction of lithium from salt lakes [13-14].However,massive magnesium is ignored.High purity of MgCO3·3H2O and Li2CO3are important industrial products,which also serve as the additive of drugs and several materials,such as the cathode materials[15-18]and electrolyte salt.
Many literatures have focused on the integrated utilization of brines.Among these studies,the coupled reaction and solvent extraction are attracting more and more attentions; CaCO3[19-22], MgCO3·3H2O[23],Li2CO3[24]and SrCO3[25]are the byproducts.With a rapid increase in demand for lithium ion secondary batteries,the fabrication of battery grade Li2CO3has also been increasingly demanded.Various approaches have widely investigated the preparation of battery grade lithium carbonate.These approaches include microreaction[26],ionexchange[27],gas-liquid crystallization[28]and the spray pyrolysis method[29].In our previous work,the synthesized crystals are in the form of the ellipsoid and flaky together;the crystal morphologies are hard to control because of the complex reaction process. Moreover,the extraction and reaction process reacted in the interface of aqueous and organic phase,which made the reaction difficult to carry out.It is necessary to conduct the experiments in a single phase to better control the crystal morphologies and make the reaction process simpler.
In this paper,the coupled reaction and solvent extraction process is divided into two separate single operations.Firstly,the cationic(Li or Mg) is extracted into the alcohol phase, then the organic phase is added to the alcohol phase,finally the reaction is carried out in the organic phase,where Tri-n-octyl amine(Alamine 336)was selected as the extracting agent[30-33].N-butanol was served as the extracting agent of LiCl and the diluents for the HCl extraction.
2.Experimental
2.1.Chemicals
Analytical reagents used were as follows:LiCl(>97%,Beijing Yili,Fine, Chemical, Co., Beijing, China), MgCl2·6H2O (>98%, Sinopharm Chemical Reagent Co.,Ltd),absolute ethyl alcohol(>97%,Beijing,Chemical factory,Beijing,China),n-butanol(>97%,Beijing,Chemical factory,Beijing,China).CO2gas(>95%,Tsinghua University,Beijing,China)and Alamine 336 industrial reagent(Sinopec,Beijing,China)were used.

Fig. 1. Schematic diagram of reaction process: (1) CO2 gas cylinder, (2) valve,(3)flowmeter,(4)reactor,(5)water bath.
2.2.Synthesis procedur e
The synthesis schematic diagram of Li2CO3or MgCO3·3H2O is showed in Fig.1.Firstly,the LiCl(MgCl2)solution with the concentration of 2-5 mol·L-1was mixed with n-butanol in a separating funnel which was shaken for 10 min by hand,while n-butanol was served as the extraction agent.Then,LiCl and coupled water were extracted into the n-butanol phase.The upper phase for n-butanol was removed and added to a three-necked flask, and Alamine 336 was added at the same time with the ratio of organic phase to alcohol phase 2/1. CO2gas was dispersed into the reactor with the stirrer speed at 150 r·min-1. The white powder appears in several minutes, after 45 min reaction, the sediment precipitated around the flask, and it was filtered and washed by ethanol,the Li2CO3and MgCO3·3H2O crystals were obtained.The reaction equation is described as follows:

2.3.Characterization
The samples were analyzed using X-ray diffraction (XRD; D8-Advance,Bruker,Germany)operating at 40 kV and 40 mA using Cu Kαradiation at a scanning rate of 5(°)·min-1.Particle-size distribution was determined using a Malvern particle size analyzer (Mastersizer2000,Malvern,England)with ethanol as dispersant and morphology by a scanning electron microscope(SEM,JSM7401,JEOL,Japan),a Tabletop scanning electron microscope(TM3000,Hitachi).The Li ion concentration was determined using optical atomic absorption spectrometry(Z-5000-AAS,Hitachi,Japan).The absorption peak of the organic phase was tested by a Fourier transform infrared(FT-IR)spectra were measured on a Tensor 27 spectrometer(Bruker,Germany).
3.Result and Discussion
To give a glimpse view of the morphology differences which were obtained in a single organic phase(this work)and two phase(coupled reaction and solvent extraction process),the contrast of crystals synthesized by the two methods are illustrated in Figs.2 and 3.As shown in Fig.2a,b and Fig.3a,b,c,two kinds of MgCO3·3H2O and three kinds of Li2CO3morphologies at various loading concentration lithium(magnesium)ion in organic phase were prepared.Compared with our previous research(Figs.2c and 3d),where the reaction process occurred in the interface of aqueous and organic phase,it is difficult to control the uniform morphologies of crystals.In this work,the whole reaction process occurred in a homogeneous-like organic phase,which means the crystallization behavior is the same, the obtained crystals are of the same size and morphology. The contrast of crystal morphology and crystallization condition is showed in Table 1.
The X-ray diffraction pattern(XRD)shows that the synthesized crystals are highly crystalline MgCO3·3H2O and Li2CO3without additional impurity peaks(Fig.4).The XRD patterns of every crystal are presented in the Supporting Information Figs.S1 and S2.
3.1.Effect of crystal morphologies

Fig.2.SEM images of MgCO3·3H2O.(a)Nanosphere with c(MgCl2)=1.5 mol·L-1,phase=1,(b)nanorods with c(MgCl2)=3 mol·L-1 phase=1,(c)crystal of two phase crystallization with c(MgCl2)=3mol·L-1 phase=2.
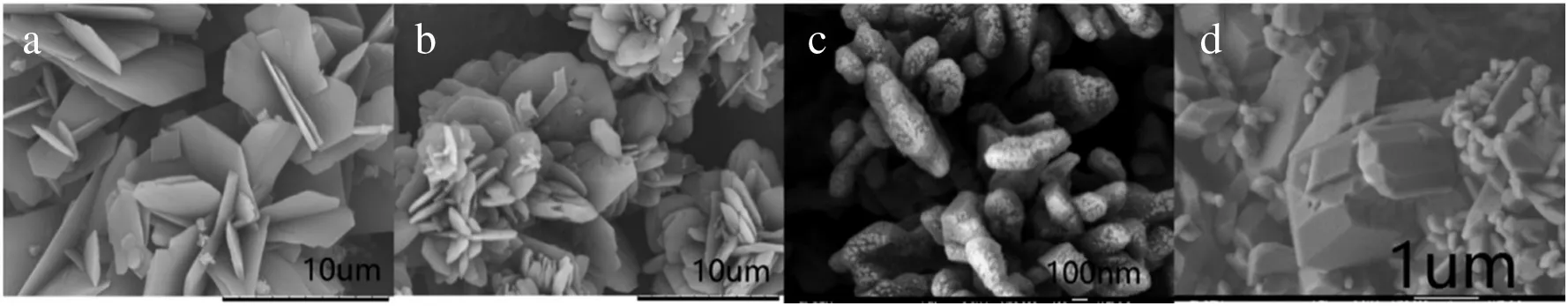
Fig.3.SEM images of Li2CO3.(a)flaky with c(LiCl)=2 mol·L-1 phase=1,(b)flower with c(LiCl)=3 mol·L-1 phase=1,(c)nanobranch with c(LiCl)=4 mol·L-1 phase=1,(d)crystal of two phase crystallization with c(LiCl)=3 mol·L-1 phase=2.
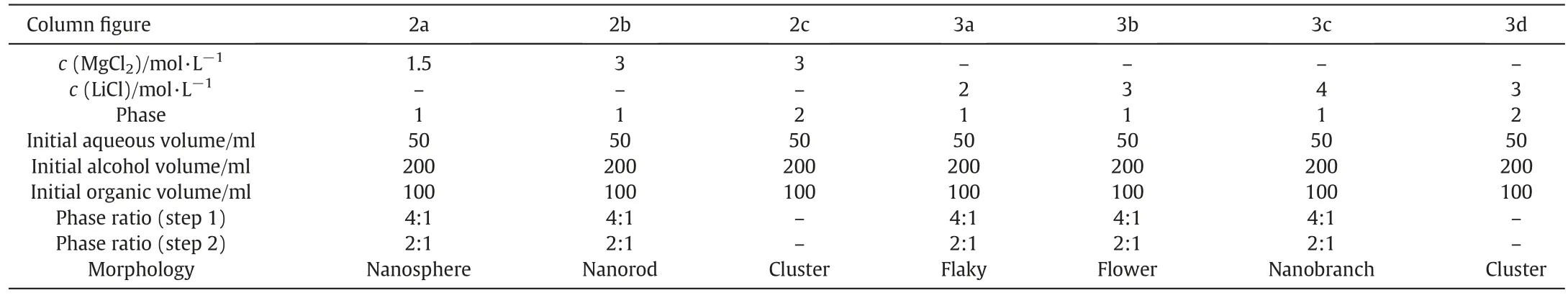
Table 1 Contrast of crystal morphology and crystallization condition

Fig.4.XRD patterns of synthesized crystals.
To analyze the reason for different crystal morphologies by tuning the concentration of LiCl or MgCl2, the lithium ion concentration in organic phase was tested.LiCl loaded in n-butanol phase from the aqueous phase was conducted by extraction process with n-butanol as the extractant.The initial concentration of LiCl in the aqueous phase(cin)was 2,3,4 and 5 mol·L-1,and the volume ratio of n-butanol phase to the aqueous phase (R) was 2:1, 3:1, 4:1 and 5:1, respectively. As shown in Fig. 5, the LiCl loaded concentration in n-butanol phase(corg)increased with cinand R.It is seen that with the higher concentration of lithium ion in organic phase,the smaller the particle size.Table 2 shows the lithium ion concentration in organic phase with initial LiCl concentration and volume ratio of alcohol phase to aqueous phase.
Particle-size distribution was determined using a Malvern particle size analyzer,the results showed that there exist two kinds of crystal particle-size regions in the prepared Li2CO3(Fig.6a,b,c,d and e)with the lithium ion concentration in organic phase ranges from 0.05-0.42 mol·L-1.The thermodynamics of this reaction has been analyzed with enough calculations which shows that the Gibbs free energy is negative,ensuring that the overall reaction is spontaneous;however little literature has revealed the microscopic mechanism of crystal formation.In this paper,the formation mechanism of particles in two ranges was studied,the reaction model which can explain the formation mechanism in detail was set up with the help of FT-IR spectroscopy.

Fig.5.Lithium ion concentration in organic phase.
3.2.Mechanism for nanoparticle formation
The large particles are in the form of flake and flower-like with an average size of 10 μm. Li2CO3growth is of typical radial form [34],which means that the growth occurs by diffusion around a nucleus as starting point.Radial growth can occur again with a new grown raised point.Thus,large particles can be obtained by this type of growth.The flaky Li2CO3is gathered together due to the presence of stirring.Flower-like morphologies of Li2CO3are also formed in this way.Li2CO3nanobranch appeared as the lithium ion concentration in organic gets higher.The crystallization behavior for the small particles is completely different. Li2CO3small particles are formed with significant growth space constraints which prevent continuous radial crystal growth,resulting in the nanobranch particles; nanosphere and nanorod MgCO3·3H2O is also formed in this way.
Many literatures have explained the crystal morphology and size control in microemulsion structure which has a significant growth space constraints[35],it can prevent continuous radial crystal growth.The formation mechanism of nanoparticle has been explained in the coupled reaction and solvent extraction[23].Thus,the reaction model for small particles should be similar to a water-in-oil structure(Fig.7).As the reaction is carried out, the crystal should be restricted in a constrained organic structure,which can permit the CO2and H+pass through.The nanoparticles were restricted in the organic phase.The thermodynamic analysis is supplied in the supporting information.
In order to validate the proposed model,the organic phase was determined by FT-IR spectroscopy(Fig.8).The samples are the organic phase composed of the alcohol phase extracted with n-butanol in different concentrations of LiCl and Alamine 336.The results showed that the absorption peak in 3000 cm-1and 1000 cm-1presents two kinds of absorption intensity;this means there exist two crystallization behaviors.It is consistent with the results from the SEM images and the particlesize distributions.Moreover,the red-shift was found in the hydroxyl bond in IR spectra from 3447 cm-1to 3357 cm-1with the increase of LiCl concentration; it is because the hydrogen bond is enhanced.The water-alcohol-organic structure was formed with the initial LiCl concentration more than 3 mol·L-1,the crystals are formed in nanometer,while the micro-size particles were obtained with the initial LiCl concentration less than 3 mol·L-1.

Table 2 Lithium ion concentration in organic phase
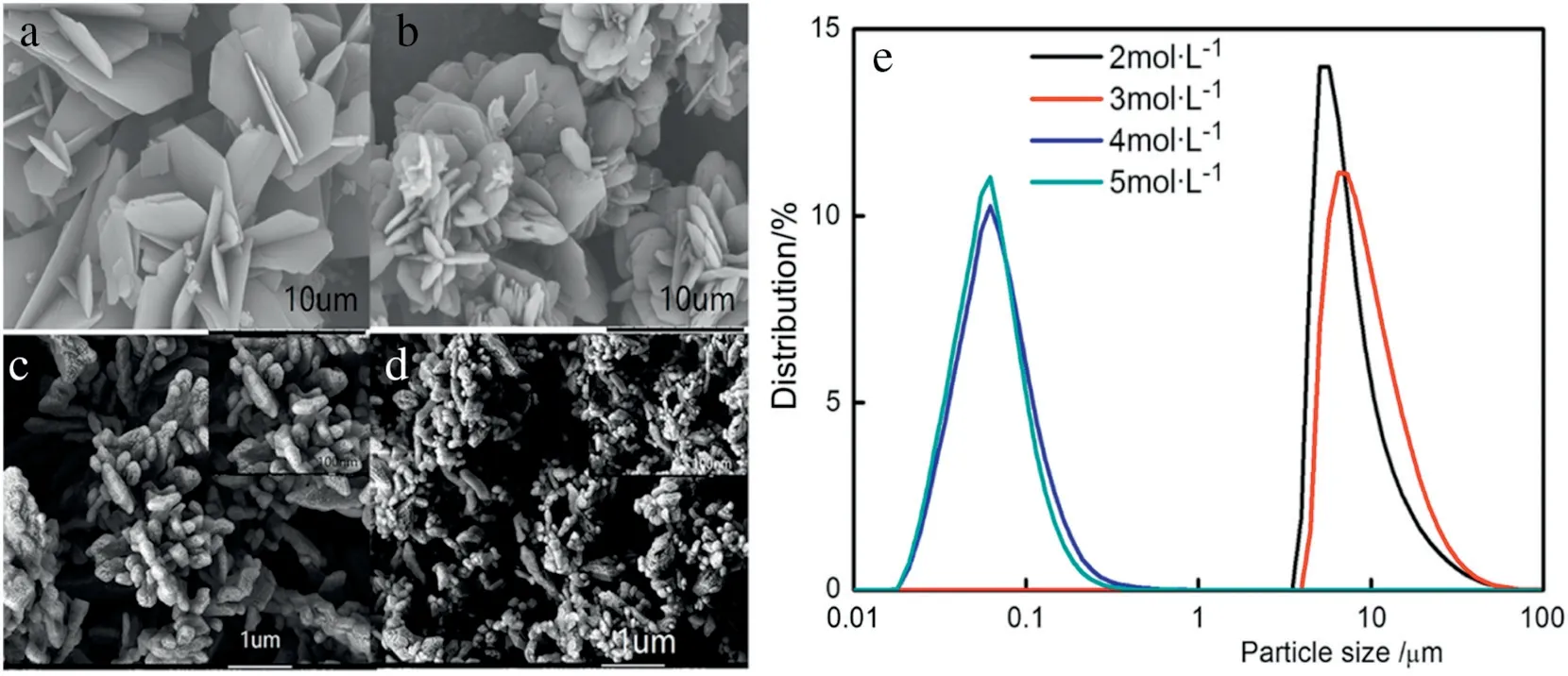
Fig.6.SEM images of Li2CO3.(a)Flaky,(b)flower,(c)and(d)nanobranch,(e)Particle size distribution of Li2CO3 crystals.

Fig.7.Schematic diagram of nanoparticles formation mechanism.
The formation mechanism of Li2CO3with flower morphology is discussed,the relationship of reaction time and morphologies size was analyzed(Fig.9).The results showed that the formation mechanism is of typical radial growth; the flower begins to reveal the embryonic form which is gathered by particles of flaky in nanometer after 15 min of reaction.As the reaction carries on,the crystals aggregate and grow up; the aggregation and growth process ended after 45 min, a blossoming full flowers were formed with the petals and the flower heart consisting of horizontal and longitudinal flakes respectively.The conversion of lithium ions and reaction time was investigated at the same time(see image in Supporting Information Fig.S3),after 45 min of reaction,the concentration of lithium ions no longer changes,and the growth of crystal had ceased,only the crystal aggregation occurred.In the whole process,the reaction was conducted in the organic-like phase.Thus the lithium ion conversion rate was not restricted.All the lithium ion entering the organic can be reacted;commonly,lithium carbonate can dissolve a few in the aqueous solution,but in this organic solution,it will crystallize and precipitate. The curves showed that the lithium ion conversion rate is higher than 95%by controlling the reaction time in 45 min.

Fig.8.FT-IR spectra of the organic phase with the initial concentration of LiCl(a),(b),(c),(d)and(e)1,2,3,4,5 mol·L-1 respectively.
Temperature has a great influence on the model structure. High temperature can damage the model structure,thus affect the crystal morphologies(Fig.10a,b,c,d and e).Experiments under different reaction temperatures were conducted to investigate the effects on the model structure. The reaction temperatures at 10, 20, 30, 40, 50 °C were analyzed.The results in Fig.10f showed that at low temperature,the crystal morphologies were in uniform micron;however,the nanometer particles were coupled with the micron particles at a higher temperature,there exist two crystal distributions at a high temperature.It is mainly because with the increase of temperature,the movement between water molecules and alcohol molecules intensified;the organic structure was then constructed.The nanometer particles were formed under the organic structure,while the water molecule and the alcohol molecules were separated at a low temperature;the organic structure was then destroyed.The formation mechanism once again changes to radial growth mode.The effects of temperature on crystal morphology are mainly owed to the influence on the reaction model structure which is consistent to the organic structure.

Fig.9.SEM images of Li2CO3 crystals at different reaction times.
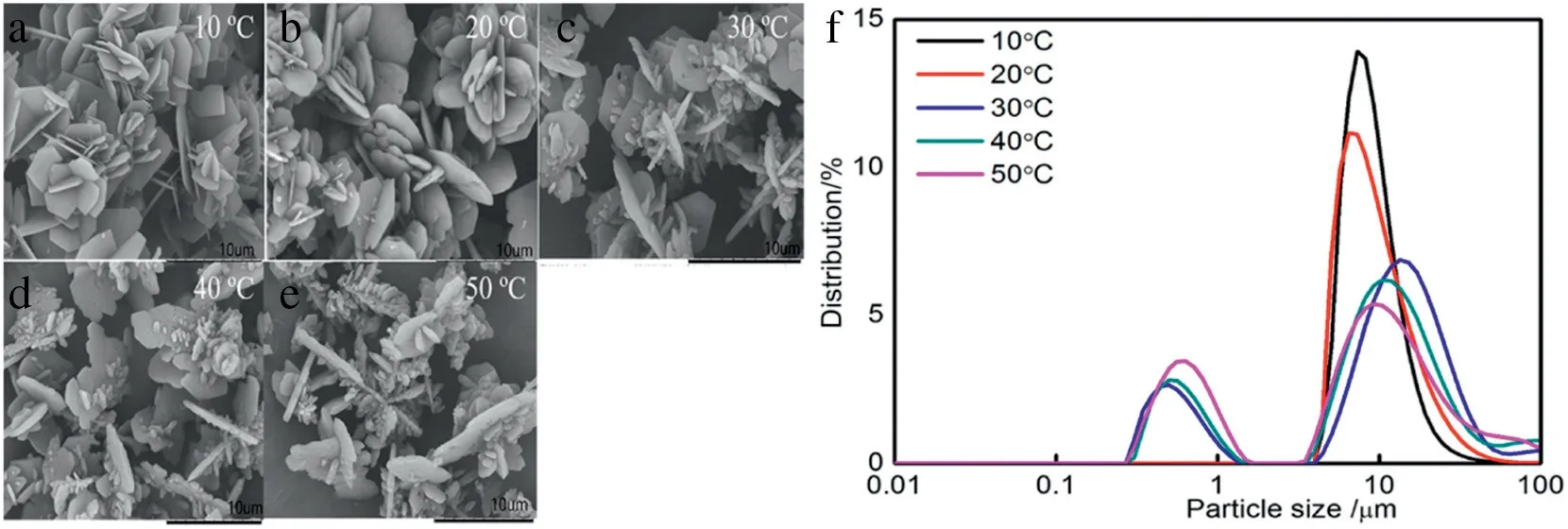
Fig.10.SEM images of prepared Li2CO3 under(a)10°C,(b)20°C,(c)30°C,(d)40°C and(e)50°C,(f)Particle size distribution of Li2CO3 at different reaction temperatures.
4.Conclusions
In summary,a new route for two-kind morphologies of MgCO3·3H2O and three-kind morphologies of Li2CO3in a homogeneous-like organic phase is proposed.The morphology transition of MgCO3·H2O from nanosphere to nanorod,Li2CO3from flaky to flower,and then to nanobranch was successfully achieved by tuning the initial concentration of MgCl2(LiCl).Compared with previous coupled reaction and solvent extraction process,the proposed method realizes the crystallization process in a single organic phase, which can control the crystal morphology and size.Moreover,the crystallization mechanism was discussed.The results showed that there exist two crystallization mechanisms.The model for the nanoparticle crystallization is proposed;variable experiments were carried out to validate the nanoparticles reaction model such as FT-IR,temperature and reaction time.The route of liquid-crystallization in a homogeneous-like organic phase was firstly proposed,which has been demonstrated to be a good method in the regulation of the morphology and size.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2019.02.009.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
