Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
Mohammad Reza Aghajanzadeh,Mohammad Sharifi*
Department of Petroleum Engineering,Amirkabir University of Technology,Tehran,Iran
Keywords:Formation damage Enhanced oil recovery Salinity Asphaltene Core flooding Nano particles
ABSTRACT In this study,the performance of stable nanofluid containing SiO2 nanoparticles dispersed and stabilized in high salinity brine for asphaltene inhibition in dynamic condition is evaluated.In the first stage of this work,the stability of silica nanoparticles in different range of water salinity(0-100000 mg·L-1)is investigated.Next,stable nanofluid containing highest salinity is selected as asphaltene inhibitor agent to inject into the damaged core sample.The estimated values of oil recovery for base case,after damage process and after inhibition of asphaltene precipitation using nanofluid are 51.6%,36.1%and 46.7%,respectively.The results showed the reduction in core damage after using nanofluid.In addition,the relative permeability curves are plotted for the base case,after damage process and also after inhibition of asphaltene precipitation using nanofluid.Comparison of relative permeability curves shows,relative permeability of oil phase decreased after damage process as compared with the base case.But after using nanofluid the oil relative permeability curve has shifted to the right and effective permeability of oil phase has been improved.
1.Introduction
Asphaltene deposition and precipitation is still one of the challenging issues in oil and gas industry.Asphaltene precipitation and aggregation in the near wellbore region of oil and gas reservoir has a strong negative impact on rock properties(porosity and permeability)and petroleum operations such as production and refining[1].Asphaltenes are defined as the wax-free fraction of crude oil,which is soluble in toluene,benzene and other light aromatic materials.Changing the thermodynamic conditions or injection of a gas such as carbon dioxide as an EOR may cause precipitation and adsorption of asphaltene onto the rock surface[2].Asphaltene adsorption onto the rock surface causes two main alterations in porous media,(1)permeability reduction(2)alteration of wettability from water wet toward oil wet[3-5].The adsorption of precipitated asphaltenes onto rock surface can lead to damage formation in oil reservoirs by reducing the oil effective permeability[6].
There are a number of techniques used to detect and measure the extent of asphaltene precipitation,including:absorption spectroscopy,light scattering, acoustic resonance, and the conventional gravimetric approach.Some researchers used sensors to detect and monitor precipitating asphaltenes in real time. These sensors demonstrated reliable performance in industrial,environmental,and biomedical applications[7-9].
It is also well known that salinity can affect the oil recovery efficiency in secondary and tertiary oil production scenarios especially in the presence of asphaltene[10].There are two methods to control this problem including prevention and removing[1,11].Recently,many researchers are looking for an efficient method for prevention of asphaltene deposition.They found that asphaltene molecules can be adsorbed onto metal surface such as gold and steel[12,13],mineral surfaces such as calcite and clay [14,15], and nanofluids contain metal oxide nanoparticles such as TiO2,SiO2and Fe2O3[16,17].
Nanofluids are a new category of fluids that are made by the dispersion of nanoparticles in a base fluid(typically water).One of the key factors in optimizing the properties of these fluids is their stability issue.Making stable suspension is still a technical challenge due to the strong Van der Waals attraction forces among the particles and their tendency to aggregation.The simplest method for assessing the nanofluid stability is the sedimentation method and the visual examination of sedimentation amount[18-21].The nanoparticle deposition due to the weight in the nanofluid indicates degree of their stability. Experiments showed that nanoparticle-stabilized emulsions can be effective in enhanced oil recovery[21].According to the definition of nanofluids stability,nanofluid can be considered stable when the concentration and size of particles in the suspension remain constant and no sediment is observed at the bottom of the test tube.In this way,in the method of photography and visual examination,nanofluid samples will be photographed at different times and a comparison will be made of the amount of deposition of each nanofluid[22].Matin et al.in 2010 divided the images of the nanofluid into three parts in terms of stability.First:an early time stage characterized with a single clear phase,second:a precipitation stage with a single turbid phase and third:a sedimentation stage with two separate phases[23].The particle-particle interaction plays a very important role in controlling aggregation and deposition of nanoparticles.This interaction is traditionally defined by the DLVO theory. However non-DLVO forces,such as spatial, magnetic and hydration forces can play more roles in the stability of nanofluid.In the classical theory of DLVO,nanofluid stability is described as the total energy of the nanoparticle interaction.Based on this theory,the nanofluid stability depends on the total force of the Van der Waals and the Electrical Double Layer force.
The addition of stable nanofluids to the injecting fluid can lead to enhance oil recovery by changing the wettability of rock surface and inhibition of asphaltene precipitation[24-27].Due to their small sizes,they are mobile in porous media and due to unique surface properties and high surface area per unit volume,they can be used for asphaltene inhibition in porous media and near wellbore region[11,27,28].There are many studies on the impact of metal oxide nanoparticles on asphaltene inhibition in static conditions.For instance,Mohammadi et al.(2011),investigated the effect of three different metal nanoparticles(TiO2,ZrO2,SiO2)to prevent or delay asphaltene precipitation.They found that,TiO2nanoparticles have higher impact on asphaltene stability in acidic conditions[29].Franco et al.(2013),studied the effect of twelve types of nanoparticles on asphaltene adsorption. They found that the adsorption of asphaltene is dependent on the type of nanoparticles[30].Tarboush and Husein showed that NiO nanoparticle has higher affinity toward asphaltene adsorption[31].
The aim of this study is to examine the effect of SiO2nanoparticles on inhibition of asphaltene precipitation and reduction of formation damage under dynamic condition.
2.Materials and Method
2.1.Materials
The carbonate rock used in this research was obtained from the outcrop of the Iranian reservoir formation.The mineralogical properties of the rock are presented in Table 1.Crude oil was obtained from one of the reservoirs in the southwest of Iran.The properties of used oil are in Table 2.N-heptane was supplied by Merck(Germany).It was used as the solvent and precipitant agent.Commercial hydrophilic mono-dispersed silica(SiO2)nanoparticles of 180-600 m2·g-1specific area were used in the experiments.The average particle size was 20-30 nm and purity of nanoparticle was >99% (Table 3). The SEM image for the used nanoparticles is shown in Fig. 1. Polyvinylpyrrolidone (PVP) was purchased from Samchun Pure Chemical Company.PVP was used as the stabilizer[32].Since PVP has hydrophilic as well as hydrophobic functional groups,therefore interactions with various solvents are possible[33].
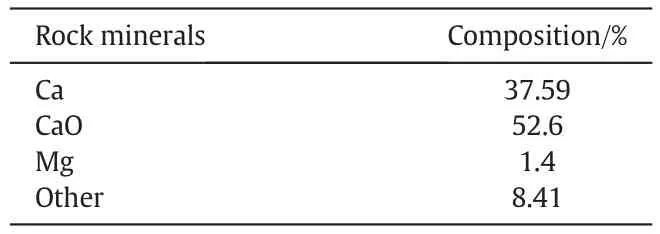
Table 1 Mineralogy of the carbonate rock

Table 3 Silicon oxide nanoparticles(SiO2)certificate of analysis
2.2.Nanofluid preparation
The nanofluid suspension is made by adding a certain amount of nanoparticle powder and polyvinylpyrrolidone(PVP)stabilizer to distilled water.PVP was selected as an agent for stabilizing the nanofluid and preventing the nanoparticle aggregation[34].In order to increase the stability of the suspension and make the fluid uniform,first it was mixed by the magnetic stirrer and then it is placed inside the ultrasonic device.If only a magnetic stirrer is used,nanoparticles will precipitate over a short time.The reason is that nanoparticles are not separated from each other and the suspension is not uniform.The ultrasonic device helps to increase nanofluid stability by breaking nanoparticle clusters.In this way,to prepare a nanofluid,first 1 g of silica nanoparticles and 1 g of PVP stabilizer were added to 1000 cm3distilled water and it was mixed for 1 h using a magnetic stirrer.Then,in order to increase the nanofluid stability,the solution was placed inside the ultrasonic vibration apparatus at 100 W for 10 min.The nanofluid is used as a base nanofluid and adding different electrolyte concentrations,the desired nanofluid are produced.
2.3.Nanofluid stability evaluation
In this section,the stability of SiO2nanofluid in the presence of different sodium chloride concentrations(0-100000 mg·L-1)is evaluated by three different methods including visual observation,Spectral Analysis, measuring size of the hydrodynamic diameter of the particles(DLS method).The stability evaluation procedure is demonstrated in Fig.2.In this part,the effect of salinity brine and PVP stabilizer on stability of nanofluid will be investigated.
2.4.Core flood experiment
Based on the results of nanofluid stability test, the optimum nanofluid was selected to evaluate its performance by core flooding run.The physical properties of prepared core samples are reported in Table 4.
Fig.3 shows a schematic of the core flood setup.The setup consists of three stainless steel transfer vessels,a dual high pressure syringe pump(VINCI Technology Co.,France),a hydraulic pump for applying confining pressure,fraction collectors,and a hydrostatic core holder.
The purpose of doing the core flooding test was to investigate the effects of the nanofluid on the reduction of asphaltene damage in the core sample in dynamic condition.

Fig.1.SEM image of nanoparticle.

Fig.2.Procedure of nanofluid stability evaluation.
The core flood test was carried out by three scenarios,the first scenario is obtaining the oil recovery and relative permeability curve during primary brine (contains 100000 mg·L-1NaCl) flooding. In this scenario,the clean and dry core sample was saturated with brine then it was placed in a hydrostatic core holder and crude oil was flooded into the core sample to achieve the irreducible water saturation and measure the effective oil permeability.Then same brine was flooded at the rate of 0.2 cm3·min-1into the core sample under a confining pressure of 1000 psi and ambient temperature. During flooding, the pressure drop across the core sample was monitored and plotted versus pore volume injection.Brine injection was continued until no oil was observed at the outlet of core holder.In each time step by use of a fraction collector the volume of the produced oil and water was collected and oil recovery curve versus pore volume injection was plotted.Finally,according to differential pressure and oil production,the relative permeability curve was constructed. The second scenario is conducting the oil recovery and relative permeability curve during secondary brine(contains 100000 mg·L-1NaCl)flooding after asphaltene damage that was generated into the core sample.In this scenario,after finishing the first scenario and completing primary brine flooding,crude oil was re-injected into the core sample until the initial water saturation and then about 1 PV of n-heptane was injected simultaneously with crudeoil into the core sample to induce damage in the porous medium.The n-heptane was soaked for 24 h into the core sample then the secondary brine was injected and the oil recovery,pressure drop across the core sample and finally relative permeability curve after damage were measured.In the third scenario,after secondary brine flooding,the inhibition process was performed by injection of two pore volumes of nanofluid.The nanofluid was soaked for 4 days into the core sample then crude oil was injected into the core sample until the initial water saturation and then oil saturated core was flooded by the same brine of primary flooding(tertiary brine)to construct the oil recovery and relative permeability curves after treatment by the nanofluid.Fig.4 shows the schematic for all scenarios.In each scenario,the oil effective permeability at initial water saturation(ko@Swi)and water effective permeability at residual oil saturation(kw@Sor)were measured.

Table 4 Properties of the prepared core samples
2.5.Equilibrium adsorption isotherms

Fig.3.Diagram of the displacement test:(1)high pressure syringe pump,(2)valve,(3)distilled water,(4)brine cylinder,(5)nanofluid cylinder/n-heptane,(6)crude oil cylinder,(7)hydrostatic core holder,(8)pressure gauge,(9)the core sample,(10)fraction collector,(11)sleeve pressure(overburden pressure).
In this study, batch adsorption experiments were carried out in order to evaluate the effect of nanoparticles on the prevention of asphaltene precipitation.First,the ASTM-IP143 method was used to extract asphaltene from crude oil.Then,for preparation of synthetic oil samples, a certain amount of asphaltene was added to 10 ml of toluene/n-heptane with a ratio of 60/40 vol%to achieve the desired concentration (100 to 2000 mg·L-1). Next, silica nanoparticles were added to each synthetic oil solution with a ratio of 10:1 g·L-1. After that, the contents in the tubes were shaken at 200 r·min-1using an incubator at ambient conditions for 24 h.After reaching an equilibrium, the supernatant was examined by the DB-20S UV/Visible Double Beam Spectrophotometer at a wavelength of 700 nm in order to obtain its asphaltene content. The asphaltene adsorption by silica nanoparticles was measured using the following equation:where Q is the adsorbed asphaltene (mg·g-1), C0is the initial asphaltene content (mg·L-1), C is the asphaltene content after adding silica nanoparticles to the synthetic oil (mg·L-1), m is the mass of silica nanoparticles(g),and V is the volume of solution(L).

3.Results and Discussion
3.1.Stability study
3.1.1.Visual observation

Fig.4.Schematic summery of core flood experiments for all scenarios.

Fig.5.Photographs of sealed tubes that show the stability of silica nanoparticle dispersions without PVP stabilizer at various NaCl concentrations after 7 days, a) SiO2 nanofluid without salt,b)low saline(10000 mg·L-1)SiO2 nanofluid,c)high saline(50000 mg·L-1)SiO2 nanofluid.
Nanofluid stability was investigated using three methods.At first,the visual observation was performed for the SiO2nanofluid without PVP stabilizer and compared with the stability of nanofluid containing PVP stabilizer at similar conditions. As it is obvious from Fig. 5, the high and low saline (50000 and 10000 mg·L-1, respectively) SiO2nanofluid without PVP stabilizer began to precipitate after 7 days at room temperature.But,SiO2nanofluid without PVP stabilizer in distilled water remains visually stable for more than 7 days.The stability behavior of nanofluid in the presence of electrolyte can be explained by the DLVO theory. According to this theory, electrostatic force is strongly dependent on the electrolyte concentration in the nanofluid.The thickness of the diffuse electrical layer(DEL) is decreased by increasing the electrolyte concentration and reducing the repulsion force between the particles.The thickness of the electrical diffuse layer is one of the most important parameters affecting the stability of the nanoparticle.If the layer is thicker,it means that the electrostatic repulsion force between the particles is greater,which cause increase the stability of the nanofluid.
Fig. 6 shows the different stages of 1000 mg·L-1SiO2nanofluid containing PVP stabilizer in different electrolyte(NaCl)concentrations.As can be seen no precipitation is observed at the bottom of the sealed tubes with electrolyte concentration up to 75000 mg·L-1after 7 days.However,nanofluids with high content of electrolyte(100000 mg·L-1)began to precipitate after a week.The stability behavior of nanofluid in the presence of PVP stabilizer can be explained by steric stabilization in addition to the DLVO theory[35-37].In this mechanism,as the PVP stabilizer is introduced into nanofluid,it is adsorbed onto the silica nanoparticles through a head segment,while the other tail segment extended into the bulk medium.Under such circumstance,the vital factor to achieve steric stabilization is strong enthalpic interaction between dispersion medium and the stabilizing segment of the stabilizer[36,38].Since water is a good solvent for the PVP stabilizer, the interpenetration of adsorbed layers on the nanoparticles is thermodynamically disfavored.Thus,silica nanoparticles are more stable in the presence of PVP stabilizer[38].
At the end of the visual observation stage,because the visual observation is not enough to discuss the stability of nanoparticles,the solutions without sedimentation were selected for Spectral Analysis and DLS Analysis and the solutions with precipitation were considered unstable.
3.1.2.Spectral Analysis
In this section,the stability of silica nanofluid solutions containing PVP stabilizer with different electrolyte concentrations is investigated by ultraviolet-visible spectroscopy. Fig. 7 shows the absorbance for 1000 mg·L-1silica nanofluid in the presence of electrolyte during 7 days. It is recommended that the absorbance intensity be always read at maximum wavelength[39].The maximum wavelength is fixed in all specimens and indicated 225 nm.Thus all read absorbance numbers are in this wavelength.The results show that after 7 days,compared to the initial state, no appreciable change in the maximum absorbance intensity of nanofluids which contain 1000,5000,20000,50000 mg·L-1of electrolyte has occurred.In this way,it can be said that in the seven-day period there has been no change in the clarity of these nanofluids, and the nanoparticles have been well dispersed in the base fluid,and yet not too large aggregation of nanoparticles was formed.But for nanofluid containing 75000 mg·L-1of NaCl as electrolyte,the amount of absorbance intensity has been increased.According to the statement of Metin,the reason for this can be attributed to the aggregation of nanoparticle and the turbidity of nanofluid[40].The formation of large nanoparticle cluster by the addition of electrolyte can be demonstrated by interaction energy between particles.As mentioned before,by adding electrolyte to the nanofluid,the thickness of the diffuse electrical layer around each particle will be reduced.In this way,the repulsive force between particles is reduced and the particles'tendency to attract each other will be more.
3.1.3.Analysis of particle size in electrolyte
The average diameter of nanoparticles in the various concentrations of NaCl is shown in Fig.8.As can be seen,in this experiment,the increase in electrolyte concentration to 50000 mg·L-1,was achieved by increasing the average size of nanoparticles.Increasing in the size of nanoparticles means the absorption of nanoparticles to each other and the formation of nanoscale cluster.However, it is observed that the diameter of the nanoparticles is not much that is visible to the eye.

Fig. 6. Photographs of sealed tubes that show the stability of silica nanoparticle dispersions at various NaCl concentrations after 7 days. NaCl concentration is: a) 1000 mg·L-1,b)5000 mg·L-1,c)20000 mg·L-1,d)50000 mg·L-1,e)75000 mg·L-1,f)100000 mg·L-1.

Fig.7.UV-vis absorbance of a nanofluid at various NaCl concentrations a 225-nm wave length during 7 days.
The average diameter of nanoparticles in distilled water is about 69 nm and as the NaCl concentration increases,the average particle diameter somewhat increases,but as shown in Fig.8,this change is not appreciable.For example,after 7 days the average particle diameter is approximately 80 nm for 50000 mg·L-1of NaCl,which is not much different from the average particle size at the first hour, which was 76.5 nm.In fact,after 7 days,it can be said that the nanofluid with concentrations of less than 50000 mg·L-1of NaCl is completely stable and the nanoparticles are uniformly dispersed in the system and have not yet formed the cluster. But in higher concentrations than 50000 mg·L-1,the average particle diameter increases sharply after 7 days.So that for 750000 mg·L-1of NaCl,the average particle diameter will be increased from 78 nm to 420 nm after 7 days.This shows that in this nanofluid,the repulsion force between particles is sharply reduced,and particles are attracting each other and tend to aggregate.
3.2.Core flood experiments
Core flood tests were performed to see the ability of silica nanofluid at high salinity condition to inhibit the formation damage.As mentioned in Section 2.4,core flood tests were carried out by three scenarios.The value of oil recovery for the first scenario is about 51.6%. This value for the second scenario is 36.1%.A clear reduction in oil recovery in the second scenario compared with the first scenario is because of damage induced by n-heptane and precipitation of asphaltene into the core sample.In terms of permeability,the asphaltene precipitation causes a decrease in the water effective permeability(Kw)at the residual oil saturation from 0.13 mD in the first scenario to 0.08 mD in the second scenario.Comparison of the water effective permeability in the first and second scenarios clearly shows that the core sample has been damaged by precipitation of asphaltene. After completing the second scenario, about 2 PV of nanofluid was injected into the core sample and after that the system was shut-in for 4 days.Then,the oil was injected into the core sample and then the fully oil saturated core sample was flooded by water to measure the oil recovery after treatment of the core sample by nanofluid.The results show,the amount of oil production reaches 46.7%.This amount has increased considerably compared to the second scenario,and it is only slightly lower than the amount which obtained in the first scenario.The effective permeability of water in this scenario is as high as 0.091 mD.These results indicate that the nanofluid has been able to reduce the damage caused to the core sample by the asphaltene precipitation.A clear increase in oil recovery after using nanofluid shows the tendency of asphaltenes to adsorb on the nanoparticle surface rather than being in the bulk phase[11].

Fig.8.Effective particle diameters of a nanofluid at various NaCl concentrations during 7 days.
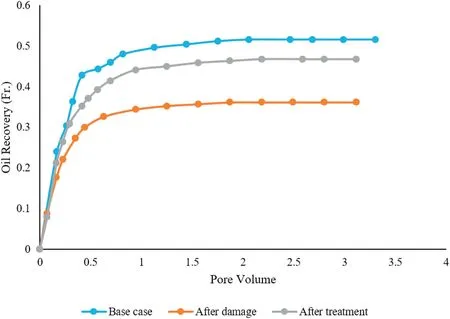
Fig.9.Oil recovery curve for each scenario.
Figs.9 and 10 show the oil recovery and pressure drop across the core sample for each scenario,respectively.According to these figures,the second scenario(after damage process)has the highest pressure drop and the lowest amount of oil production.By contacting the plug with the nanofluid,the pressure difference between ends of the plug will be decreased slightly and the production of oil will be increased significantly. The results obtained in these experiments show that nanofluid has been effective in reducing the damage caused by the asphaltene precipitation.
In order to get a better comparison of the damage caused to the plug,a non-dimensional parameter called damage factor is defined as follows:

In the above equations DF1and DF2are the damage factor after asphaltene precipitation and the damage factor after contact with the nanofluid,respectively.Kewis also the effective permeability of water at irreducible oil saturation for each scenario.Subscripts 1,2,and 3 correspond respectively to the first,second,and third scenarios(base case,after damage and after treatment respectively).According to the results obtained in the previous section,the value of DF1will be 0.58 and the value of DF2will be 0.65.The closer number to one is indicating that the damage to the plug is less.Thus,according to the obtained results,it can be claimed that the presence of nanofluid causes the reduction of damage to the plug that happened due to precipitation of asphaltene.
To show the effect of inhibition of asphaltene precipitation process on mobility of fluids in porous media,the relative permeability curve was constructed with respect to oil recovery curve and pressure drop across the core sample. In Fig. 11, the relative permeability curve of water and oil is plotted for all three scenarios.By comparing the relative permeability curve of the first and second scenarios,it can be seen that with increasing water saturation,the relative permeability of oil in the second scenario decreases sharply as compared to the first scenario and also it seems necessary to mentioned that the intersection of oil and water relative permeability curves was at water saturation of 0.59 in the first scenario while the intersection has happened at a water saturation of 0.50 in the second scenario.It means that in the second scenario,the asphaltene precipitation has intensified the oil wetness state and the ability of rock to pass the oil has been decreased.
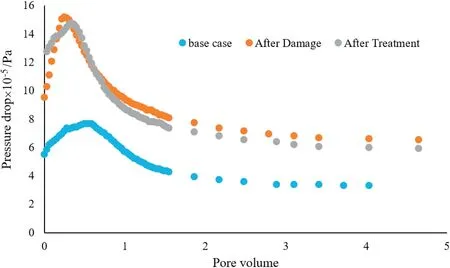
Fig.10.Pressure drop across the inlet and outlet faces of core sample for each scenario.

Fig.11.Oil-water relative permeability curves for each scenario.
In the third scenario, the relative permeability curve of the oil decreases faster than the first scenario,while compared with the second scenario,the ability of rock to pass the oil has been increased.It means after treatment with nanofluid,the relative permeability curve of oil in the third scenario, compared to the second scenario decreases with less slope by increasing water saturation.In this scenario,the intersection of oil and water relative permeability curves was about 0.58 and the relative permeability curve shifted to the right compared with the second scenario.This can also confirm that the effectiveness of the nanofluid in reducing the damage to the plug happened due to the asphaltene precipitation.
3.3.Langmuir Isotherm
Due to their large surface area,silica nanoparticles have the ability to adsorb heavy components in crude oil such as asphaltene[41].Batch adsorption tests were performed in order to prove the hypothesis of asphaltene adsorption by SiO2nanoparticles. Fig. 12 illustrates the asphaltene adsorption onto silica nanoparticle surface at ambient conditions. In this figure, Q is the mass of the adsorbed asphaltenes onto nanoparticle surface per mass of the nanoparticles.As can be seen in this figure,silica nanoparticles adsorb noticeable amount of asphaltene.This equilibrium data was analyzed using the Langmuir Isotherm.The Langmuir Isotherm has been used to correlate experimental data on equilibrium adsorption[30,42,43].This isotherm shows a monolayer adsorption as follows:

Fig.12.Adsorption isotherms of asphaltenes onto silica nanoparticles at ambient conditions.

where Q is the amount of adsorbed asphaltene onto the nanoparticle surface(mg·g-1)and Ceis the concentration of asphaltene in the solution(mg·L-1)at equilibrium condition.Qmaxis indicative of adsorption capacity and KLis the Langmuir adsorption constant and it is indicative of adsorption affinity(L·mg-1).The linear form of the Langmuir Isotherm can be expressed by the following equation:

The plot of Ce/Q versus Ceprovides a straight line to determine Langmuir parameters(Qmax,KL)from the intercept and slope of this line as follows:

Fig.13 illustrates the linear plot of the Langmuir Isotherm model fitted to adsorption experimental data.As mention before,the Langmuir parameters can be obtained using Eq.(6)(Table 5).
As can be seen from Figs.12 and 13,the adsorption data fitted very well to the Langmuir Isotherm with R-squared close to unity,which indicates a Type I isotherm behavior(a monolayer adsorption),according to the IUPAC classifications.The results confirm that,silica nanoparticle is a good candidate for inhibiting the precipitation of asphaltenes on rock surfaces.
4.Conclusions

Fig.13.Linear plot of Langmuir Isotherm model fitted to adsorption experimental data.

Table 5 Adsorption isotherm parameters
In this paper,the effect of salinity on the stability of silica nanofluid was investigated.The results showed that increasing salinity decreases the stability of nanofluid. However, for concentration of less than 50000 mg·L-1no change was observed in nanofluid properties,such as nanoparticle size and concentration.Under dynamic condition,the stable nanofluid with the highest salinity(50000 mg·L-1)was selected for the core flooding experiments.This salinity was selected to be more close to sea water salinity which was widely used as a base fluid for chemical EOR.The results show that,asphaltene precipitation on rock surface decreases the oil recovery and increases the residual oil saturation compared with the base case(before asphaltene damage).In addition,after the treatment of the core sample using nanofluid the residual oil saturation of core sample has been decreased and the mobility of oil phase in porous media has been increased.It means that the nanofluid could be able to inhibit or decrease the asphaltene damage of the core sample.In summary,it can be concluded that the silica nanofluid can be very efficient in terms of inhibition of asphaltene damage.
Acknowledgements
The authors would like to acknowledge the support of the Department of Petroleum Engineering, Amirkabir University of Technology during this study.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
- Removal of chloride from simulated acidic wastewater in the zinc production☆
