Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
Zechen Jin,Lijie Yin*,Dezhen ChenYuanjie JiaJun YuanBoyang Yu
1 Thermal and Environmental Engineering Institute,Tongji University,Shanghai 200093,China
2 Shanghai Municipal Engineering Design Institute(Group)Co.,Ltd,Shanghai 200092,China
Keywords:Molten plastic Heat transfer Pyrolysis Vertical falling film reactor
ABSTRACT The heat transfer efficiency during the pyrolysis process is a key factor to be considered in the design of pyrolysis reactors.In this study,the average apparent heat transfer characteristics of molten plastic pyrolysis in a vertical falling film reactor were explored by experiments and numerical simulation and the apparent heat transfer coefficients were determined.In addition,the temperature distribution and the thickness of the liquid film in the reactor were predicted and the influences of pyrolysis temperatures on the average apparent heat transfer coefficients were discussed.
1.Introduction
In China,about 25 million tons of waste plastics were generated in 2015[1,2],but only 25%-30%of them were recycled in chemical,mechanical or energy ways[3].As one of the energy and chemical recycling ways of waste plastics,pyrolysis has been widely concerned.
Plastic pyrolysis is an endothermic process.The heat transfer characteristics of molten plastics largely affect the quality of pyrolysis products and the design of pyrolysis reactors,especially industrial reactors[4].Previous studies on plastic pyrolysis were mainly performed with thermogravimetric analysis(TGA)and focused on the reaction temperature range,the properties of products and kinetic parameters[5].Heat transfer characteristics of plastic pyrolysis were seldom reported.TGA experiments were generally carried out under static conditions without considering the effects of flows on heat and mass transfer.In the actual pyrolysis process,the interactions between flows,heat transfer,and reactions are complex.The fluid flow affects the temperature distribution,which affects the pyrolysis rate,products,and the viscosity and density of the fluid.The changes in the components and reaction heat generated in the pyrolysis process affect the velocity field and temperature distribution.Therefore,it is necessary to explore the heat transfer characteristics of the pyrolysis process.
Bockhorn et al.[6]modeled the non-stationary heat transfer inside a plastic particle.Navarro et al.[7]studied the pyrolysis of plastic,tyre,coal and biomass with the particle model and just gave the global heat transfer coefficient of wood and coal particles.In fact,the pyrolysis process of plastics is divided into three stages:melting,volatile evaporation and coke formation[8].That is to say,when pyrolysis starts a plastic has melted into a liquid and the volatile evaporation process is a liquid-to-gas process.
Molten plastic is characterized by high viscosity and low thermal conductivity.Its flow velocity is slow and it is difficult to heat molten plastic.Liquid film heat transfer is an effective method for heat transfer enhancement [9] and has been widely applied in various industrial fields[10,11].Liquid film heat transfer can be used to deal with the materials with high viscosity and low thermal conductivity[12,13].Jin et al.[14]experimentally studied the pyrolysis of molten waste plastics in a vertical falling film reactor and analyzed the influences of temperature on pyrolysis products.However,due to limited measurement conditions,local velocity or detailed temperature field inside the liquid film was not obtained and the thickness of the liquid film could not be measured.In order to explore the flow mechanism and heat transfer characteristics during the pyrolysis process of plastics, the gas-liquid twophase flow with heat and mass exchange should be deeply studied.
The VOF model has been successfully used to track the liquid surface of the large-scale gas-liquid two-phase flow[15,16].This model tracks the interface by solving the volume fraction equation[17,18].Guo et al.[19]established the falling film model of the gas-liquid two-phase flow in an evaporative condenser and analyzed the effect of wall heating flux on the temperature of the liquid film.Liu et al.[20]simulated the evaporation process of water in a filler-type saturator and discussed the influences of inlet velocity and feeding rate on the heating and mass transfer coefficients.VOF model had been used to simulate gas-liquid two-phase flow in falling film reactors mainly based on water or aqueous solution,but its application in the simulation of highly viscous molten fluids was seldom reported.
In this work,the heat transfer characteristics in the plastic pyrolysis process were experimentally explored in a vertical falling film reactor and simulated with VOF model.In addition,the effects of pyrolysis temperature on the heat transfer coefficients were discussed.
2.Experimental
The pyrolysis plant[14]is shown in Fig.1.The vertical falling film plate is 0.05 m×1 m.A feed inlet and a nitrogen inlet are located at the top of the melting tank.Three rows of type-K thermocouples with three in each row are mounted from top to bottom at equal distances to monitor the falling film plate temperatures Tw,the liquid film temperatures Tfand the volatile temperatures Tg.
The experimental materials were the mixture of polyethylene(PE),polypropylene(PP)and polystyrene(PS)(PetroChina Daqing Refining&Chemical Co.,Ltd.).According to the proportion of fresh waste plastics in the municipal solid waste [21,22], the blending ratio of PE:PP:PS is 24:9:8. The proximate and ultimate analyses of the materials are shown in Table 1.
In the pyrolysis process,the heat transfer equation is:

where Er is heat absorption of the mixed plastic, J·g-1; Φ is the mass flow of molten plastics, g·s-1; a is the conversion degree; hais an apparent heat transfer coefficient,W·m-2·K-1,indicating the influence of chemical reaction,fluid flow and volatile evaporation on the pyrolysis process;A is the area of the molten plastics flowing through the heating wall, m2; Twis the temperature of heating wall, K; Tfis the average temperature of liquid film,K.Tinis the inlet temperature of liquid film. Er is measured by differential scanning calorimetry(DSC).During the thermogravimetric experiment,the amount of feed was 15 mg each time,the heating rate was set as 100°C·min-1and the pyrolysis temperature ranged from 50°C to 650°C,the results are shown in Fig.2 and Table 2.
3.Mathematical Methods
Since there is little solid residue[14],the pyrolysis products include non-condensable gas and oil.The oil is also gaseous at high temperatures.The chemical reaction equation is:
Molten plastics →pyrolysis oil+non-condensable gas (2)

where Rris the pyrolysis reaction rate,kg·m-3·s-1;ρlis the density of liquid phase,kg·m-3;αlis the volume fraction of liquid phase.
The conservation equations are provided in Table 3.
The structure scheme and grid of the falling film plate of the reactor are shown in Fig. 3. The time step size is 10-4s. The physical parameters used in the simulation were obtained in falling film pyrolysis experiments. The heating wall temperature, inlet velocity and inlet temperature were set as experimental parameters. In the experiment, molten plastic entered the falling film plate through a U-shaped tube under the pressure. The inlet velocity of the liquid film was controlled by adjusting the flow rate of nitrogen on the top of the melting box (Fig. 1). The density, specific heat, viscosity and thermal conductivity of pyrolysis oil and non-condensable gas were calculated with the measured components and contents(Table 4).

Table 1 Proximate and ultimate analyses of the materials

Fig.2.TG(solid line)and DSC(dotted line)curves at 10 K·min-1 for plastics:(a)PE,(b)PP,(c)PS,(d)PE/PP/PS.
4.Results and Discussion
4.1.Velocity distributions
Fig. 4(a) shows the velocity field at different heights at 823 K.The pyrolysis products flow downward with the liquid film due to the action of gravity, and the velocity of the flow gradually increases.Fig.4(b)shows the velocity magnitudes of the fluid along the height direction at different distances from the falling film plate,in which the positions (x = 0.001 m and x = 0.0025 m) are located in the liquid film.In the liquid film,the velocity increases firstly and then decreases along the flow direction.The changes may be interpreted as follows.As the liquid film flows downward,the temperature increases and the pyrolysis reaction gradually increases. The upward flow of volatiles generates more rapidly as the pyrolysis reaction increase,resulting in the decrease of the liquid film flow rate.
4.2.Temperature distributions
Fig.5(a)and(b)shows the temperature distributions of fluid at different heights at 823 K.The temperature of the fluid near the falling film plate is the highest and the temperature gradient inside the liquid film is large.As the liquid film flows downward, the temperature increases gradually.Fig.5(b) also gives the average temperatures of Tw,TfandTgat z=0.59 m measured in the experiment.It can be seen that the simulated result is in accordance with the temperature distribution measured in the experiment.

Table 2 Volatile evaporation temperatures and heats of single-component polymers and mixtures determined from DSC measurements
The heating temperature affects the heating rate of liquid film and the stability of the fluid film during the pyrolysis process. Fig. 6shows the average fluid temperature in the longitudinal direction under different heating temperatures.The average temperature of the fluid increases with the increase of the pyrolysis temperature.At the bottom of the reactor,the temperature shows a wavy distribution,indicating that the pyrolysis reaction rate is large and consumes a lot of heat.The fluctuation may be caused by the shear stress generated by the relative velocity between the gas phase and the liquid phase.Pyrolysis is an endothermic reaction.As the liquid film flows downward,the temperature rises gradually,the liquid film begins to pyrolyze and the reaction intensifies gradually, and the amount of gas generated increases.At the same time,these gases move upward and the relative velocity between the gas and the surface of the liquid film increases, thus causing the fluctuation of the temperature of the liquid film.The higher the heating temperature,the more intense the pyrolysis reaction is,the greater the temperature fluctuation.
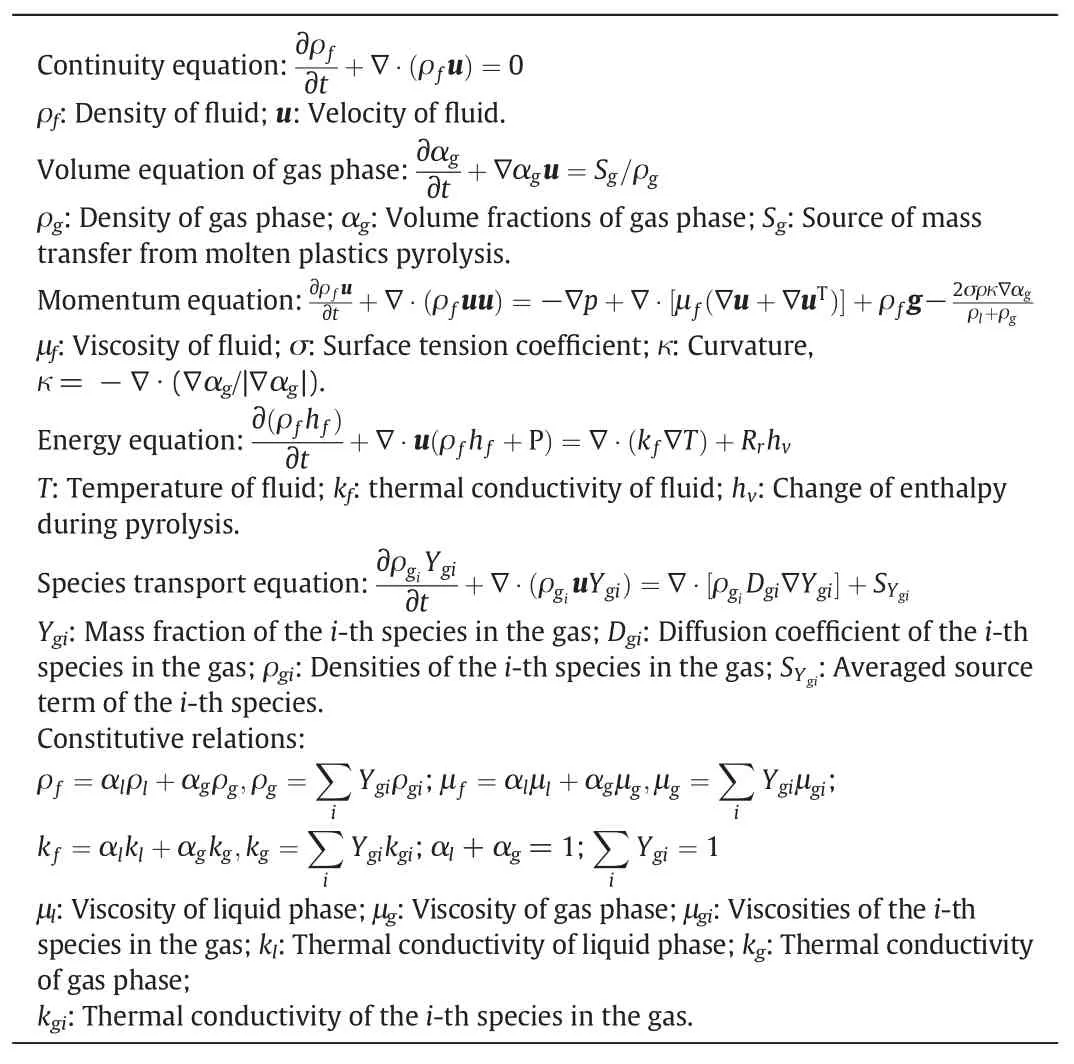
Table 3 Conservation equations of molten plastic pyrolysis in the falling film reactor
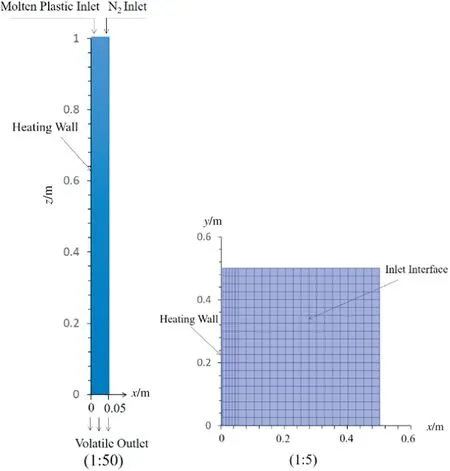
Fig.3.Structure scheme and grid.
4.3.Volatile distributions
Fig.7 shows the molar concentrations of non-condensable gas and pyrolysis oil along the height of the falling film plate at 823 K.The concentrations of the pyrolysis products increase continuously as the liquid film flows downward along the falling film plate.When the liquid film flows downward, the temperature of liquid film increases and the pyrolysis rate is gradually increased.Additionally, the products generating at the upper part of the reactor move downward and accumulate gradually. The concentration difference between the non-condensable gas and the pyrolysis oil increases gradually because that the noncondensable gas has a smaller molecular weight, a slower flow rate,and longer residence time in the reactor.
Fig.8 shows the effects of inlet velocities on the molar concentrations of non-condensable gas and oil vapor in the reactor.The molar concentrations of the two volatiles increase with the increase in the inlet velocity.Fig.9 shows the molar concentrations of non-condensable gas and pyrolysis oil at different pyrolysis temperatures.The molar concentrations of the two volatile components increase with the increase in heating temperature because the temperature rise accelerates the chemical reaction rate of molten plastics and leads to the higher volatile yield at the same inlet velocity.Combined with Figs.8 and 9,it can be seen that temperature and inlet velocity are the key factorsaffecting the pyrolysis of falling film.Increasing the pyrolysis temperature can increase the yield of pyrolysis products.When the pyrolysis temperature is constant, the conversion rate is not always increased with the increase of inlet velocity,which is related to the heat absorbed by the liquid film on the unit area of the heating wall.
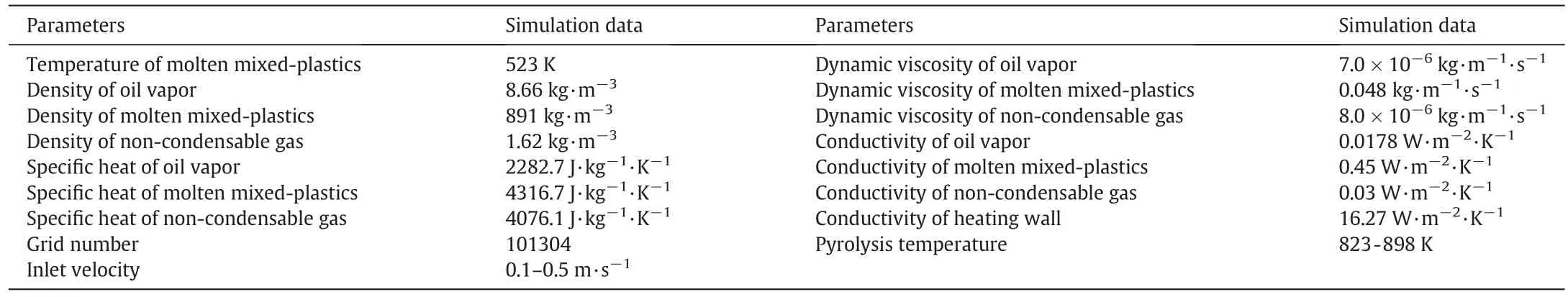
Table 4 Physical parameters used in the simulation
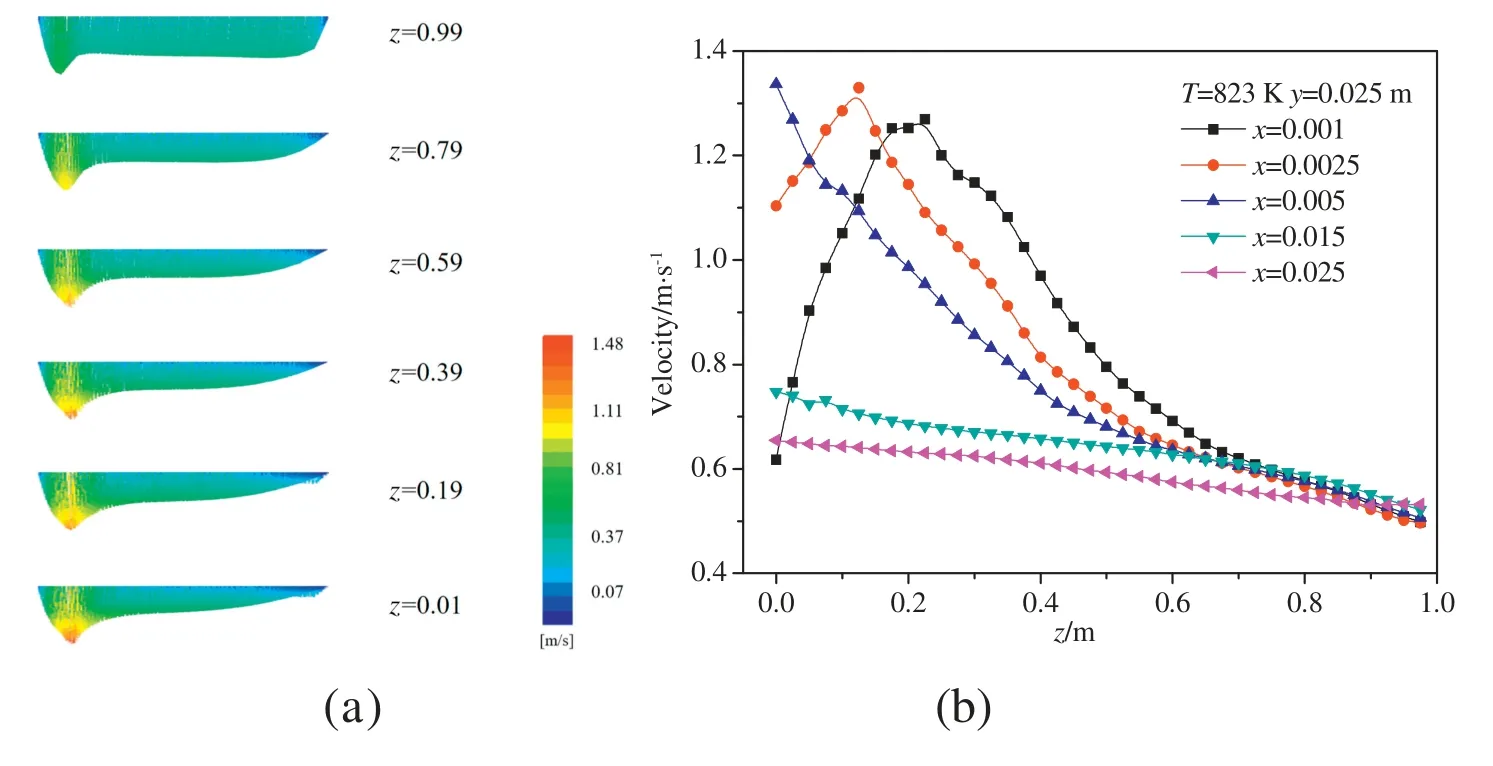
Fig.4.Contours of velocity distribution in the reactor(a)and velocity distribution in the longitudinal direction(b).

Fig.5.Contours of temperature distribution in the reactor(a)and temperature distribution in horizontal direction(b).
4.4.Thickness of the liquid film
Fig.10 shows the change in the thickness of the liquid film along the direction of the height.The thickness of the liquid film decreases gradually along the flow direction.The surface of the liquid film is not smooth and presents a wavy structure.In the upper region,with the increase in the flow rate of the liquid film,the thickness of the liquid film decreases rapidly and the heat absorbed by the liquid film in this area is mainly used for heating.In the height range of 0.2-0.8 m,the thickness of the liquid film decreases slightly and the liquid film shows the wavy structure.In the height range of 0.0-0.2 m,the thickness of the liquid film decreases rapidly due to the violent pyrolysis reaction. At the bottom of the reactor,there are still some residues of molten plastics.

Fig.6.Average fluid temperature in the longitudinal direction.
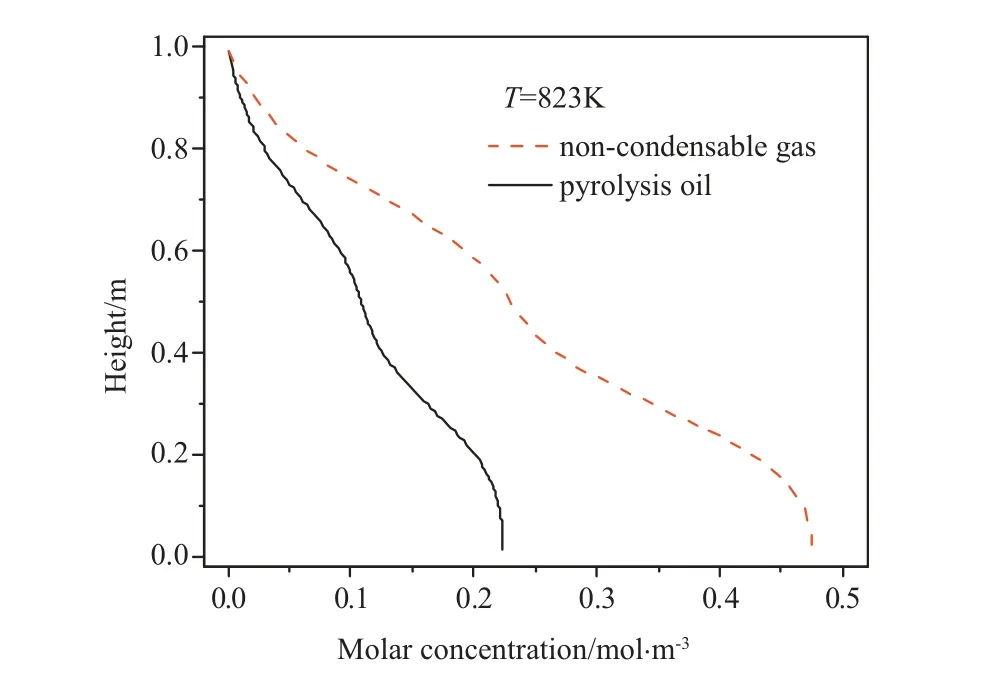
Fig.7.Molar concentrations of pyrolysis products.
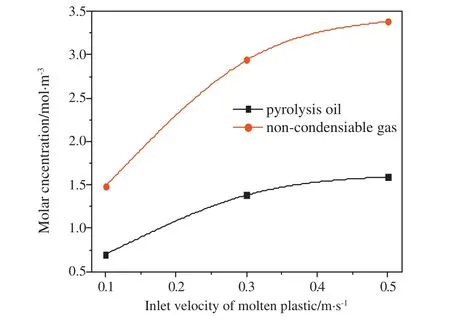
Fig.8.Molar concentrations of products under different inlet velocities.
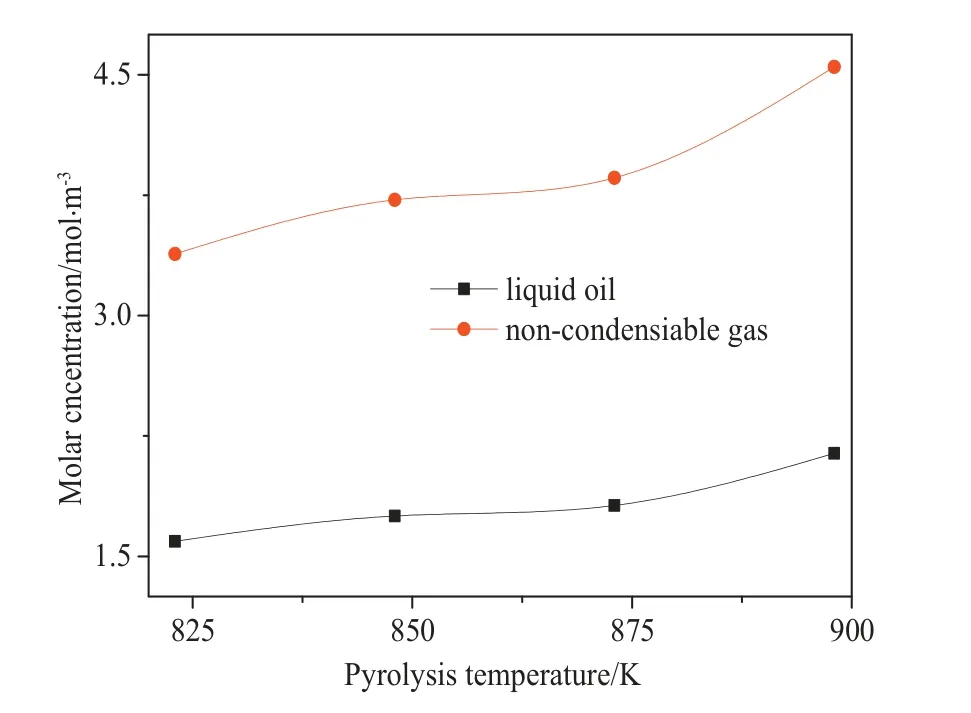
Fig.9.Molar concentrations of products at different pyrolysis temperatures.

Fig.10.Thickness of the liquid film in the longitudinal direction.
The corrugated structure of the liquid film surface observed by the simulation is consistent with the phenomenon of liquid film bifurcation and dripping during the experiment.In the actual operation,it is recommended to slightly tilt the falling film plate to ensure the uniformity of the film.
4.5.Apparent heat transfer coefficient
The apparent heat transfer coefficients haof PE, PP, PS and their mixture determined by Eq. (1) are shown in Fig. 11. The heat transfer coefficients of all materials decrease with the increase in pyrolysis temperature. Wang et al. [23] calculated haof mixed plastics pyrolysis in a rotary kiln reactor and also found that haof molten plastics decreased with the increase in temperature. As mentioned above, the thermal conductivity of the molten plastic is low [24] and due to the thermal delay, the temperature rise of the plastics is lower than the temperature rise of the falling film plate, thus causing an increase in the temperature difference. ha,ppis larger than ha,PS; ha,PEis the smallest; ha,mis between ha,PEand ha,PS. Fig. 11 also shows the average apparent heat transfer coefficient ha,sobtained by simulations, which has a similar trend to ha,mmeasured by experiments.The difference between ha,sand ha,mmay be interpreted as follows. The solid residue generated in the interface of the heating wall affects the flow of liquid film in the later experimental stage. However, the heating wall in the simulation is considered to be smooth.Besides,in the experiment,heat transfer dissipation also affects the heat transfer coefficient.
5.Conclusions
The heat transfer efficiency during the pyrolysis process is one of the key factors to be considered in the design of pyrolysis reactors.The molten plastics is characterized by high viscosity and low thermal conductivity,so the heat transfer characteristics of molten plastics in a vertical falling film reactor are explored by numerical simulation with the VOF model.The main conclusions as follows:

Fig.11.Apparent heat transfer coefficients obtained by experiments and simulations.
As the molten plastic flows downward,the temperature,flow rate and the molar concentration of the product increase,whereas the thickness of the liquid film decreases gradually.The surface of the liquid film is not smooth and presents a corrugated structure.
The concentration of pyrolysis products increased with the increase of pyrolysis temperature.When the pyrolysis temperature is constant,the concentration of pyrolysis products at the outlet of the reactor increases first and then remains almost unchanged with the increase of inlet velocity. The apparent heat transfer coefficients decrease with the increase in the heating temperature.
The apparent heat transfer coefficients of molten plastics pyrolysis in the falling film reactor is about 4000 W·m-2·K-1,which is higher than that in the rotary kiln reactor.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
- Removal of chloride from simulated acidic wastewater in the zinc production☆
