Removal of chloride from simulated acidic wastewater in the zinc production☆
Weizao Liu,Li Lü,*,Yao Lu,Xiaowei Hu,Bin Liang
1 College of Chemical Engineering,Sichuan University,Chengdu 610065,China
2 China Tin Group Co.,Ltd.,Liuzhou 545000,China
Keywords:Ion-exchange Dechlorination Ozone Zinc production Oxidation
ABSTRACT The removal of chloride from the zinc electrolyte produced during hydrometallurgical zinc production is challenging.The ion-exchange method is a promising way to remove chloride if the resin washing wastewater can be recycled. This paper focuses on chloride removal from resin washing wastewater to enable its reuse.Various processing factors including the oxygen gas velocity,temperature,and reaction time were investigated systematically. The results show that the optimal conditions for dechlorination are an oxygen gas velocity of 0.5 L·min-1,a reaction temperature of 80°C,and a reaction time of 30 min.A dechlorination efficiency of 80%with a residual chloride ion concentration less than 200 mg·L-1 was achieved,which meets the requirements for the recycling of wastewater.The presence of manganese accelerates the dechlorination by forming a Mn2+-MnO2-MnO4--Mn2+redox cycle.In this process,about 15 kg of the MnO2 and all of the zinc can be recovered from 100 m3 wastewater,and the wastewater can be reused,which makes the ion-exchange method a promising technique for chloride removal.
1.Introduction
Currently, hydrometallurgical processes account for almost 80%-85%of the zinc production in the world[1]. In this process, chloride ions accumulate rapidly and sometimes reach concentrations of several thousand parts per million because of the use of chlorine-containing ores as raw materials, as well as the recycling of the electrolyte.The presence of chloride ions corrodes not only pipes and devices but also the electrodes of the whole electrowinning system.The corrosion of the Pb-Ag anode increases the Pb content in the electrolyte, which results in low-grade Zn products[2].In addition,the corrosion becomes more severe if the oxidation of Cl-to ClO3-proceeds at the anode.The mechanism of corrosion can be expressed as follows:
Because of the corrosion,the anode surface becomes uneven,which results in increasing electric power consumption.The corrosion to the cathode accelerates the dissolution of the deposited zinc,which reduces the current efficiency[3].It has been well established that,if the concentration of chloride ions in the electrolyte is less than 100 mg·L-1,the above adverse effects can be significantly reduced.
There are various methods for the removal of chloride ions from the zinc electrolyte,including chemical precipitation[4-9],flocculent precipitation[10],solvent extraction[11],membrane separation[12],and ion exchange[13-15].Among them,chemical precipitation with silver salts and Bi2O3achieves a high dechlorination efficiency.Unfortunately,the precipitates,AgCl and BiOCl,are expensive and difficult to recycle,resulting in poor cost-effectiveness and difficulties in scaling up the process.The cuprous chloride precipitation method uses the readily available copper slag by-product from zinc electrolyte purification as a raw material.Because of its high efficiency and relatively low cost,this method is widely used in zinc metallurgical plants. However, the amounts of zinc powder and CuSO4added are considerable, and the acidity and reaction temperature must be strictly controlled in this process. In the solvent extraction method, the loss of extractant is inevitable, which leads to high costs. Compared with the above methods,ion exchange has many advantages such as speed,simplicity,low investment,and low cost.Ion exchange has been used in industry and shows good prospects. Generally, dilute sulfuric acid is used to wash and recycle the chlorine-containing resin after dechlorination.Thus,the washing wastewater produced is a dilute sulfuric acid solution containing 20-40 g·L-1H2SO4and 0.8-1.0 g·L-1chloride ions.In addition,a fraction of Zn2+and Mn2+in the electrolyte will be entrained into the resin during adsorption and finally enter the wastewater after washing.The large quantity of chlorine-containing acidic wastewater cannot be discharged directly and must be further treated before disposal.Currently,neutralisation with lime is the most common way to deal with the acidic waste.However,the generated gypsum not only causes secondary pollution but also wastes sulfur,zinc,and manganese resources.Therefore, many zinc metallurgical plants avoid using the ion-exchange method because of the large amount of unmanageable wastewater generated in the regeneration process.However,if the chloride ions are oxidised to chlorine gas and separated from the solution,the wastewater can be reused to wash the resin for regeneration.In this way,the emission of wastewater is reduced,and the sulfuric acid is recycled,which makes the ion-exchange method more promising for widespread use.
Ozone is an oxidiser and can react with chloride ions in an acidic environment as follows:
Tsarevitch Ivan crept nearer, and as it was about to pluck a golden apple in its beak9 he sprang toward it and seized its tail. The bird, however, beating with its golden wings, tore itself loose and flew away, leaving in his hand a single long feather. He wrapped this in a handkerchief, lay down on the ground and went to sleep.

The reaction between chloride ions and ozone in H2SO4and HCl solutions has been widely investigated [16-27]. The strengthening measures,reaction kinetics,and reaction mechanism between O3and Cl-have been discussed in depth.It has been shown that both hydrogen ions and some transition metal ions, such as Fe3+, Cu2+, and Co2+,accelerate the reaction remarkably. The transition metal elements in the process catalyse the oxidation of chloride ions.However,most studies concern the kinetics and mechanism of this process, and,thus,the chloride ion concentrations and acidity employed are quite high(Cl->1 mol·L-1,H+up to 8 mol·L-1).Thus,it is meaningful to investigate whether this reaction can be used to deal with chlorinecontaining industrial solutions.Our previous studies have focused on the removal of chloride ions from concentrated phosphoric acid[28]using ozone.Our results show that hydrogen ions improve the dechlorination significantly.The Cl-concentration can be effectively reduced from 1000 mg·L-1to 5 mg·L-1in an 80 wt%concentrated phosphoric acid at 70°C.However,concerning the resin washing wastewater with low acidity and chloride ion concentration,it is unknown if the reaction is feasible.If the chloride ion concentration is less than 200 mg·L-1,the wastewater can be recycled for washing the saturated resin, which could make the ion-exchange method more popular.
The interference of H2SO4on the potentiometry can be eliminated by the standard addition method.Based on the compositions of wastewater,50 g·L-1H2SO4,0.1 g·L-1Mn2+,and 4 g·L-1Zn2+were simultaneously added into the standard chloride solutions. The standard addition curve of potential(E)versus lg[Cl-]is also given in Fig.3,and it also shows good linearity,even in the presence of impurities.Thus,the chloride ion concentration was determined by the standard addition method.In each test,50 ml of ozone-treated filtrate was heated to 60°C,and, then, a certain amount of oxalic acid was added to reduce the MnO4-to Mn2+.After the solution had been cooled to room temperature, the potential of the filtrate was compared with the standard curve to determine the chloride concentration.
The concentration of ozone in the mixed gas (O2+ O3) mainly depends on the type of gas, gas velocity, and the efficiency of the ozone generator.Air and oxygen can be used as the gas sources,and the latter produces a higher concentration of ozone. Thus, in this study,oxygen gas was selected as the gas source for ozone generation.The concentrations of ozone in the mixed gas at different initial O2velocities were measured,and the results are shown in Fig.4.As shown,the ozone concentration was inversely proportional to the oxygen gas velocity.When the oxygen gas velocity was 0.1 L·min-1,the ozone concentration reached 80 mg·L-1,whereas it decreased to 57 mg·L-1at a gas velocity of 0.8 L·min-1.
2.Experimental
2.1.Experimental materials and procedure
Simulated acidic wastewater was prepared according to the actual compositions of wastewater from China Tin Group Co.,Ltd.,in which zinc sulfate electrolyte was treated with strong basic phenyl ethyl anion exchange resin(type 201×7)[29].The composition of the simulated wastewater,unless otherwise noted,H2SO4was 40 g·L-1,Cl-was 1.0 g·L-1,Zn2+was 4 g·L-1,and Mn2+was 0.1 g·L-1.All chemical regents used including ZnSO4·7H2O,MnSO4·H2O,NaCl and H2SO4were of analytical grade and high-purity deionized water with a resistivity large than 18.25 MΩ·cm was used in all experiments and tests.
So he walked, and he walked, and he walked, until he met the giant, and he asked, Have two young men, making for yonder mountain, passed this way? And the giant answered, Yes, they have passed by, but they never came back, and by this I know that the spell has fallen upon them
2.2.Analysis and characterisation

Fig.1.Flowchart of the dechlorination procedure for zinc electrolyte by ion exchange combined with ozone oxidation.

Fig.2.Schematic of the experimental apparatus.
The chloride ion concentration in the simulated wastewater was determined by potentiometry with a PHS-3C potentiometer(Fangzhou Technology Co.Ltd.,Chengdu).A chloride-ion selective electrode(Type 7107, Ruosull Technology Co. Ltd., Shanghai) was used, and its test range was 10-1to 10-5mol·L-1(1.775-3550 mg·L-1). The Clcontaining standard solutions were prepared using a reference chemical, NaCl. The standard potential (E) versus lg[Cl-] curve is given in Fig.3,and it shows good linearity.To investigate the influence of impurities on the potentiometry,0.1 g·L-1Mn2+,4 g·L-1Zn2+,50 g·L-1H2SO4,0.1 g·L-1oxalic acid and 0.1 g·L-1MnO4-were added into the standard chloride solutions,respectively.The variations of E with the different impurities are shown in Table 1. As shown, the effects of Mn2+and Zn2+on the potential can be neglected,although the effect was significant in the cases of H2SO4and MnO4-.The effects of dissolved ozone and oxygen on the variations of standard potential were also investigated by bubbling the gases into a Cl-depleted solution(50 g·L-1H2SO4,0.1 g·L-1Mn2+,and 4 g·L-1Zn2+).After that,oxalic acid and Cl-(the amount was equal to standard chloride solution)were added into the solution successively. The results showed that the standard potentials were equal to the standard chloride solution,which indicated that the effects of the dissolved ozone and oxygen on the variations of standard potential can be excluded.
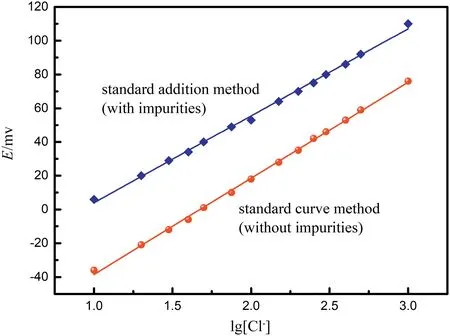
Fig.3.The working curves of standard solutions with and without impurities.

Table 1 Variation of the potential in the presence of different impurities
To obtain a better understanding of the reaction,we measured the concentrations of manganese ions(total concentrations of Mn2+and MnO4-)at different reaction times,and the results are shown in Fig.9.In addition,the average concentration of ozone in the exhaust was measured,and these data are given in Table 2.At the beginning of the reaction,the ozone concentration at the entrance was 67.14 mg·L-1,but that at the exit was very low.When the reaction time was 5-10 min,the average of the exit ozone concentrations was only 10.12 mg·L-1,a reduction of about 85%.During the reaction period,the residual chloride concentrations ranged from 839 to 765 mg·L-1,corresponding to a dechlorination efficiency of 16.1%to 23.5%.In contrast,the manganese ion concentration decreased rapidly from 100 to 1.84 mg·L-1,corresponding to a conversion to MnO2of 98.16%.This indicates that the oxidation of Mn2+to MnO2was dominant in the initial stages and restrained the reaction between ozone and chloride, which agrees with the results shown in Fig.8.When the reaction time was extended to 15-20 min,the solution became red-brown with a slight increase in the manganese ion concentration, whereas the chloride concentration declined to 280 mg·L-1.It can be inferred that MnO2was oxidised to MnO4-.However,the interaction of O3-MnO2-MnO4-was a gas-liquid-solid phase reaction, which is restricted by kinetics. Therefore, the degree of oxidation was limited.On the other hand,the generated MnO4-easily reacted with Cl-in the acidic conditions,as follows:
“What strange conduct,” said the emperor, when her flight had been discovered; and all the courtiers blamed her, and said she was a very ungrateful creature.
Therefore,in this study,the reaction between ozone and chloride ions in the resin washing wastewater was investigated,and a flowchart of this process is shown in Fig.1.The effects of various process factors including reaction time,temperature,and gas velocity were systematically investigated.Notably,the behaviour and promotion mechanism of Mn2+in the solution are discussed.This work provides theoretical and experimental guidance for the industrial application of the ionexchange method for chloride ion removal from zinc electrolyte.
An iodometric method was used to measure the ozone concentrations in the oxygen stream.The ozone in the O2stream was sufficiently absorbed with two-stage 2 wt%KI solutions in series to form I2.The I2concentration was then titrated with 0.3 mol·L-1Na2S2O3.The ozone concentration was estimated based on the O2flow and bubbling time.
Then the white dove came flying and settled down on the pile of wood, and cooed and said, Shall I help you? Yes, said the prince, many thanks for your help yesterday, and for what you offer to-day
A schematic of the experimental apparatus is presented in Fig.2.Compressed oxygen gas was depressurised,metered,and fed to an ozone generator (HY-005, Yifenghong Environmental Protection Technology Co., Ltd., Chengdu). The generated ozone was mixed with oxygen and reacted with the wastewater in a 350 ml selfmade bubble reactor.The reactor includes three parts:a cylinder at the top (diameter 50 mm, height 350 mm), a circular truncated cone at the bottom (bottom diameter 70 mm, height 30 mm), and a sand core gas distributor between them(thickness 5 mm,average hole sizes 3-4 μm). With this reactor, the gas introduced from the bottom can be distributed uniformly, making adequate contact with the solution. In each test, 100 ml simulated wastewater was added to the reactor and preheated in a super thermostatic water bath with a temperature tolerance of±1°C.When the desired temperature had been reached,oxygen with a purity of >99.2%was introduced, and the reaction was timed. The exhaust gas was discharged after absorption with a saturated sodium hydroxide solution.After the reaction,the solution was filtered,and the concentrations of residual chlorine and manganese were determined.The filter slag was analysed by X-ray diffraction.
I didn t know as his attendant7 carried him back to the car that it would be the last time I saw him. He missed several weeks, then I went back to college. I found out months later that he died not too long after that.But instead of mourning, I thought of him in heaven, running out to his favorite horse, not having to wait until Thursday or for his attendants8 to help him. He and his horse would gallop9 across clouds, with him laughing and the horse s tail streaming freely behind as the wind sang through their hair.
The manganese ion concentration in the simulated wastewater was determined by atomic absorption spectrophotometry(4510F,INESA Analytical Instrument Co.,Ltd.,Shanghai).
XRD analyses of the filter slags were performed using an X-ray diffraction spectrometer (DX-2007, Haoyuan Instrument Co. LTD.,Dandong)with a CuKαradiation source filtered with a graphite monochromator at a wavelength (λ) of 1.54 nm. The generator voltage and anode current were 40 kV and 30 mA,respectively.A continuous scanning mode with a 0.03 s interval and 0.05 s set time was used to collect the XRD patterns.
UV-Vis spectra of the solutions were recorded on an ultraviolet spectrophotometer(L6S,INESA Analytical Instrument Co.LTD.,Shanghai)with a scanning range from 1100 nm to 190 nm and scanning interval of 1 nm.
3.Results and Discussion
3.1.Effects of the oxygen gas velocity
The big old monster greedily accepted my dime, and I heard the bottles shift. On tiptoes I reached up and opened the heavy door. There they were: one neat row of thick green bottles, necks staring directly at me, and ice cold from the refrigeration. I held the door open with my shoulder and grabbed one. With a quick yank() , I pulled it free from its bondage13. Another one immediately took its place. The bottle was cold in my sweaty hands. I will never forget the feeling of the cool glass on my skin. With two hands, I positioned the bottleneck14 under the heavy brass15() opener that was bolted to the wall. The cap dropped into an old wooden box, and I reached in to retrieve16 it. I was cold and bent17 in the middle, but I knew I needed to have this souvenir(,) . Coke in hand, I proudly marched back out into the early evening dusk. Grampy was waiting patiently. He smiled.

Fig. 4. The residual chloride ion concentration and O3 concentration vs. O2 velocity.Reaction conditions:T=80°C,time=30 min.
Fig.4 also shows the effects of oxygen gas velocity(0.1-0.8 L·min-1)on the residual chloride concentration in wastewater using a reaction temperature of 80°C and a bubble time of 30 min.As shown,the residual chloride in the simulated wastewater decreased first and then increased with increasing O2gas velocity. The best dechlorination, having a residual Cl-concentration of less than 200 mg·L-1,was achieved at an O2gas velocity of 0.5 L·min-1,which corresponds to an O3concentration of 67 mg·L-1. The dechlorination efficiency is related to the ozone concentration and the level of gas-liquid contact.At lower oxygen gas velocities, the wastewater could not be effectively dispersed by the mixed gas,leading to poor gas-liquid contact.Hence,the reaction was insufficient despite the higher ozone concentration in the gas.In contrast,at higher oxygen gas velocities, although the gas-liquid phases were in sufficient contact, the ozone concentration was too low at short reaction periods. In this case, the dissolution of ozone was impeded,which led to a poor dechlorination efficiency.In other words,the gas velocity should be controlled within a certain range depending on the reactor. In this study, the optimum oxygen gas velocity was selected as 0.5 L·min-1and applied in the subsequent experiments.
3.2.Effects of reaction time
Fig.5 shows photographs of the reaction at different contact times using a reaction temperature of 80 °C and an oxygen gas velocity of 0.5 L·min-1. As shown, the mixed gas was dispersed into a large number of tiny bubbles and in full contact with the solution.In addition,the colour of the solution gradually changed from colourless to amaranth with time,accompanied by the formation of black particles.This was because Mn2+in the solution was gradually oxidised to MnO2and MnO4-. XRD analysis of the black particles obtained after 10 min was conducted,and the XRD pattern is shown in Fig.6,which confirms that the black particles were MnO2and its formation occurred before the formation of MnO4-.Fig.7 compares the UV-Vis spectra of the wastewater subjected to ozone oxidation for 30 min in the presence and absence of Mn2+and that of a KMnO4solution.The Mn2+containing wastewater had almost the same absorption peaks as the KMnO4solution while the Mn2+-free wastewater almost had no peaks. The typical absorption peaks at 525 and 545 nm and the amaranth colour confirmed the oxidation of manganese to MnO4-.
The variation in the residual chloride concentration with the reaction time in the Mn2+-containing and Mn2+-free wastewater are shown in Fig.8.Compared with the Mn2+-free solution,the dechlorination of the Mn2+-containing solution was faster.The residual chloride concentration was less than 300 mg·L-1after 20 min in the Mn2+-containing solution,and the dechlorination efficiency was calculated to be more than 70%.However,the dechlorination efficiency was less than 50%in the Mn2+-free solution.Apparently,the presence of Mn2+accelerated the dechlorination. The dechlorination efficiency of the Mn2+-free solution was higher than that of the Mn2+-containing solution reacted for 10 min.The residual chloride concentrations were 770 and 670 mg·L-1,respectively.This is because the oxidation of Mn2+to MnO2is dominant in the initial stages,which restrained the reaction between ozone and chloride.

Fig.5.Reaction phenomena at different times at 80°C and an O2 velocity of 0.5 L·min-1.
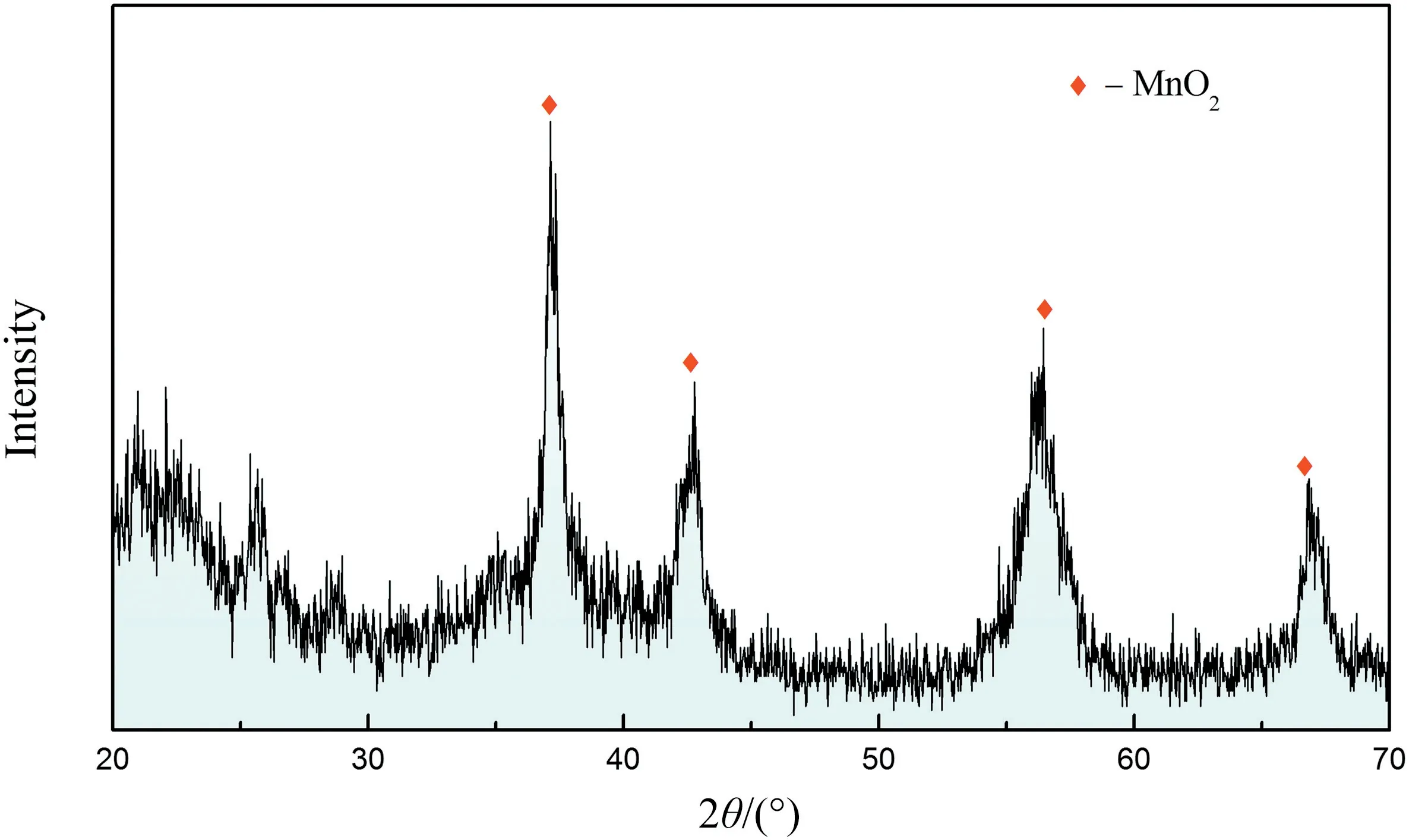
Fig.6.XRD pattern of solid precipitation obtained from the simulated wastewater after bubbling for 10 min at 80°C and an O2 velocity of 0.5 L·min-1.

Fig.7.UV-Vis spectra of the wastewater after bubbling for 30 min at 80 °C and an O2 velocity of 0.5 L·min-1 in the absence(a)and presence(b)of Mn2+and spectrum of a KMnO4 solution(c).

Fig.8.The effect of reaction time on the residual chloride concentration at 80°C and an O2 velocity of 0.5 L·min-1.

Fig.9.The effect of reaction time on the manganese concentration at 80 °C and an O2 velocity of 0.5 L·min-1.

Table 2 The average concentration of ozone at different periods of time
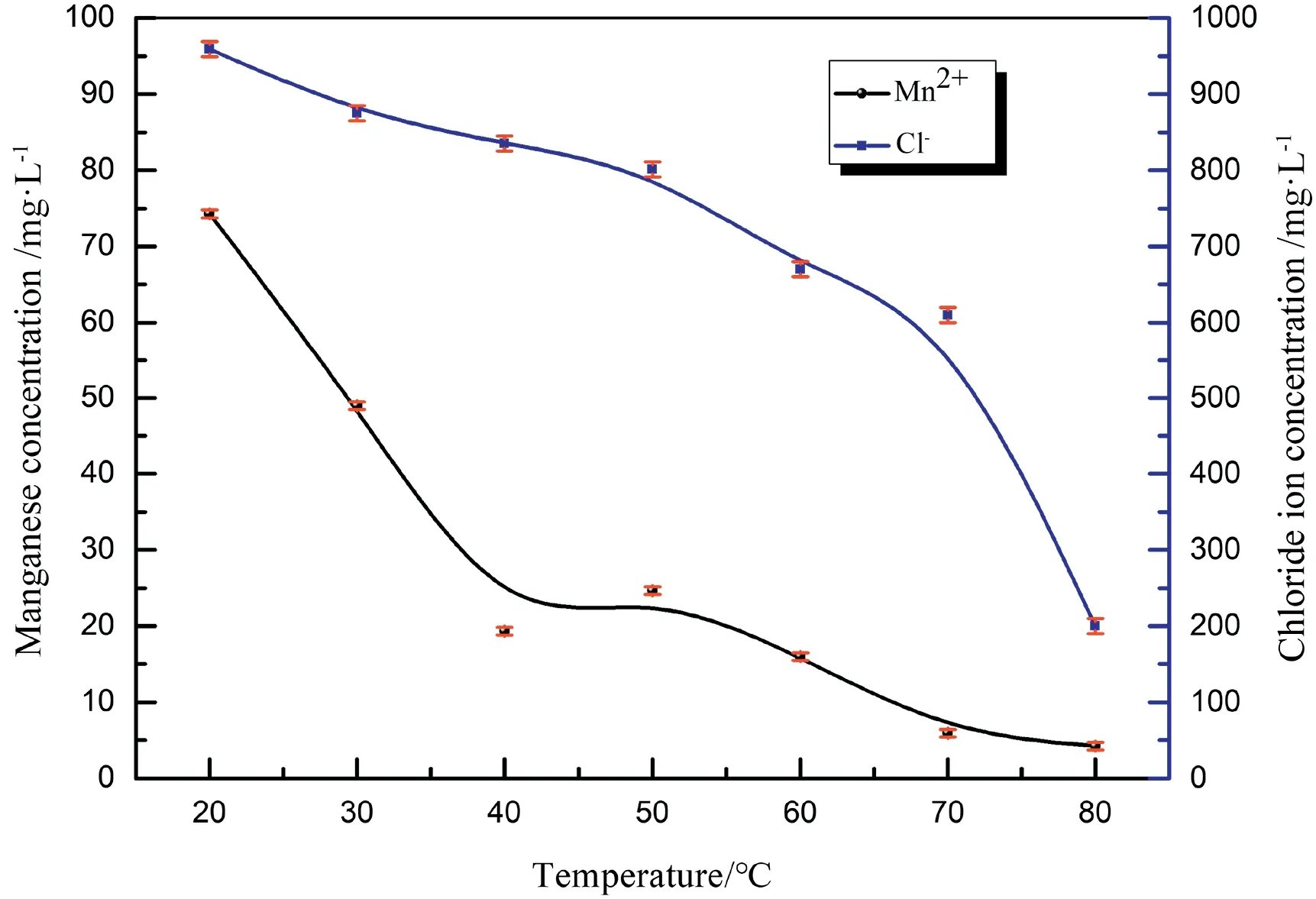
Fig.10.The effect of reaction temperature on the chloride and manganese concentrations at an O2 velocity of 0.5 L·min-1 bubbling for 30 min.

Table 3 Conversions of Mn2+to MnO2 at different temperatures
The interference of MnO4-on the potentiometry can be eliminated by adding a small amount of oxalic acid until the purple colour of the solution disappears.The reaction between MnO4-and oxalic acid can be expressed as follows:
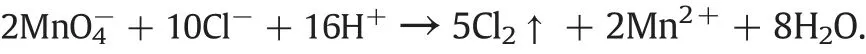
Therefore,about 95%of the manganese was in the form of MnO2,while the remainder was in the Mn2+-MnO2-MnO4--Mn2+cycle over the whole reaction period.This cycle could be responsible for the increased dechlorination. When the reaction time was extended to 30 min,the residual chloride concentration was less than 200 mg·L-1,which meets the requirements for the recycling of wastewater. The optimal reaction time was,thus,selected as 30 min.The consumption of ozone decreased accordingly with an exit ozone concentration of up to 33.34 mg·L-1.Given the high exit ozone concentration,multilevel processing could be designed to increase the utilisation of ozone.
3.3.Effects of reaction temperature
The variation in the residual chloride and manganese concentrations with temperature is shown in Fig.10.The chloride concentration decreased with increasing reaction temperature.There was little dechlorination at 20°C,whereas the residual chloride was less than 200 mg·L-1at 80°C.At the same time,the manganese concentration also decreased with increasing reaction temperature.Manganese mainly existed in the form of Mn2+at low temperatures,as determined by the weak reddish colour of the solution.Thus,MnO4-had little effect on the dechlorination process.Table 3 shows the conversion of manganese to MnO2at different temperatures.About 95%of MnO2was precipitated at 80°C,indicating that the oxidation of Mn2+to MnO2was susceptible to temperature.
In summary, the optimal conditions for dechlorination were an oxygen gas velocity of 0.5 L·min-1,a reaction temperature of 80°C,and a reaction period of 30 min. A dechlorination efficiency of 80%with a residual chloride concentration of less than 200 mg·L-1was achieved,which meets the requirements for the recycling of wastewater.At the same time,about 95%of the manganese was recovered in the form of MnO2,which can be used as the oxidising agent for the removal of iron from the zinc electrolyte.Furthermore,about 15 kg of the MnO2could be recovered from 100 m3wastewater.With the circulation of wastewater,the zinc will gradually accumulate.Thus,when the zinc accumulates to 100 g·L-1, the wastewater can be added to the zinc electrolyte for electrolysis.In this way,zinc and manganese can be recovered,and the sulfuric acid can be recycled.
Then a young man better dressed and better looking than any of us presented himself at our table. “Good evening, my name is Paul, and I’ll be your waiter this evening. Would you like a few minutes before I take your order?”“No,” I said, “I’m just a meat-and-potatoes guy, so I’ll have the filet3 mignon and baked potato.”
3.4.Dechlorination mechanism

Fig.11.Schematic diagram of the dechlorination mechanism.
Based on our results, a dechlorination mechanism for Mn2+-containing wastewater is proposed,as shown in Fig.11.First,chloride ions are oxidised to chlorine gas at high temperature.The oxidation of Mn2+to MnO2occurs before that of Cl-,and the generated MnO2is slowly oxidised to MnO4-. Because MnO4-is a strong oxidant, it will also oxidise Cl-into Cl2, resulting in a Mn2+-MnO2-MnO4--Mn2+redox cycle.The cycle is synergistic with the ozone,promoting dechlorination.The main reactions in this process are listed in Table 4.

Table 4 Main reactions involved in dechlorination
4.Conclusions
The removal of chloride from simulated resin washing wastewater(Cl-11 g·L-1,H2SO450 g·L-1,Zn2+4 g·L-1,and Mn2+0.1 g·L-1)from zinc production by ozone oxidation was investigated with a focus on the effects of various process factors including the oxygen gas velocity,temperature,and reaction time.The results showed that the optimal conditions for dechlorination are an oxygen gas velocity of 0.5 L·min-1,a reaction temperature of 80 °C, and a reaction time of 30 min. A dechlorination efficiency of 80% with a residual chloride concentration of less than 200 mg·L-1can be achieved,which meets the requirements for the recycling of wastewater. The presence of manganese accelerates the dechlorination by forming the Mn2+-MnO2-MnO4--Mn2+cycle. In this process, considerable amounts of MnO2and zinc can be recovered,and the wastewater can be reused,making the ion-exchange method promising for chloride removal.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
