Methane hydrate formation and dissociation behaviors in montmorillonite☆
Kefeng Yan,Xiaosen Li*,Zhaoyang Chen,Yu Zhang,Chungang Xu,Zhiming Xia
Key Laboratory of Gas Hydrate,Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510640,China Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development,Guangzhou 510640,China
University of Chinese Academy of Sciences,Beijing 100049,China
Guangzhou Center for Gas Hydrate Research,Chinese Academy of Sciences,Guangzhou 510640,China
Keywords:Methane hydrate Porous media Formation/dissociation behavior Molecular dynamics simulation
ABSTRACT The methane hydrate formation and the methane hydrate dissociation behaviors in montmorillonite are experimentally studied.Through the analyses of the microstructure characteristic,the study obtains the porous characteristic of montmorillonite.It is indicated that methane hydrate in montmorillonite forms the structure I(sI)crystal.Meanwhile,molecular dynamics simulation is carried out to study the processes of the methane hydrate formation and the methane hydrate dissociation in montmorillonite.The microstructure and microscopic properties are analyzed.The methane hydrate formation and methane hydrate dissociation mechanisms in the montmorillonite nanopore and on the montmorillonite surface are expounded.Combining the experimental and simulating analyses,the results indicate the methane hydrate formation and methane hydrate dissociation processes have little influence upon the crystal structure of porous media from either micro-or macro-analysis.It is beneficial to the fundamental researches on the exploitation and security control technologies of natural gas hydrate in deep-sea sediments.
1.Introduction
Natural gas hydrates is a clean energy in the earth,which mainly exists in the marine sediment regions and in the permafrost regions.Recently,the investigation of gas hydrate focused on CO2capture and sequestration[1,2],gas storage and transportation[3],and water desalination[4].In particular,some promoters of the hydrate formation have been investigated to reduce the increase in the reaction rate of the hydrate formation process[5-7].In the research of the natural gas hydrate exploration,the hydrate equilibrium and hydrate kinetic experiments were used to investigate the behaviors of the hydrate formation and the hydrate dissociation in porous sediment.In previous researches,the silica sand,the glass bead and the activated carbon as the man-made porous sediment were used to investigate the formation and the dissociation of methane hydrates in porous sediments[8].Montmorillonite is one kind of porous sediment in marine,which is distributed in the reservoirs of natural gas hydrate in nature.Recently,the equilibrium and the kinetic behaviors of the hydrate formation in the montmorillonite were investigated by the hydrate formation experiments[9,10].The results demonstrated that the hydrate formation condition in the montmorillonite was milder than that in the pure bulk solution.However,the hydrate formation and hydrate dissociation mechanisms in montmorillonite were do not clearly understand by experiments.The molecular dynamics simulation has been successfully used to study the hydrate formation and hydrate dissociation mechanisms[11-13].The simulation results explained some behaviors of the hydrate formation in the pure bulk solution.As for the porous sediment,the mechanism of the hydrate formation in the silica was expounded by the molecular dynamics simulations[14-16].The results indicated the hydrate formation behaviors in silica were more complex than that in the pure bulk solution.Water molecules neighboring the silica surface constructed the ice-like structure.And the hydroxylated silica surface can promote the hydrate formation.The hydrate formation mechanism in the montmorillonite also was carried out by the molecular dynamics simulations [17-19]. The results indicated methane molecules were absorbed on the montmorillonite surface. The hydrate formation and the molecular diffusion presented the cooperativity in the pore of the montmorillonite.The gas water ratio was one key factor affecting the hydrate formation in the montmorillonite.However,there are few investigations done on the dissociation mechanism of the hydrate in the montmorillonite.Therefore,the aim of the work was to study the methane hydrate formation and methane hydrate dissociation behaviors in the montmorillonite by experiment and the molecular dynamics simulation.We focused on the hydrate formation and hydrate dissociation mechanisms in montmorillonite. We report the microstructure characteristic of the systems containing a montmorillonite layer with 3 water hydrate layers and a bulk solution layer.The structure effects of montmorillonite in the methane hydrate formation and the methane hydrate dissociation process are discussed.
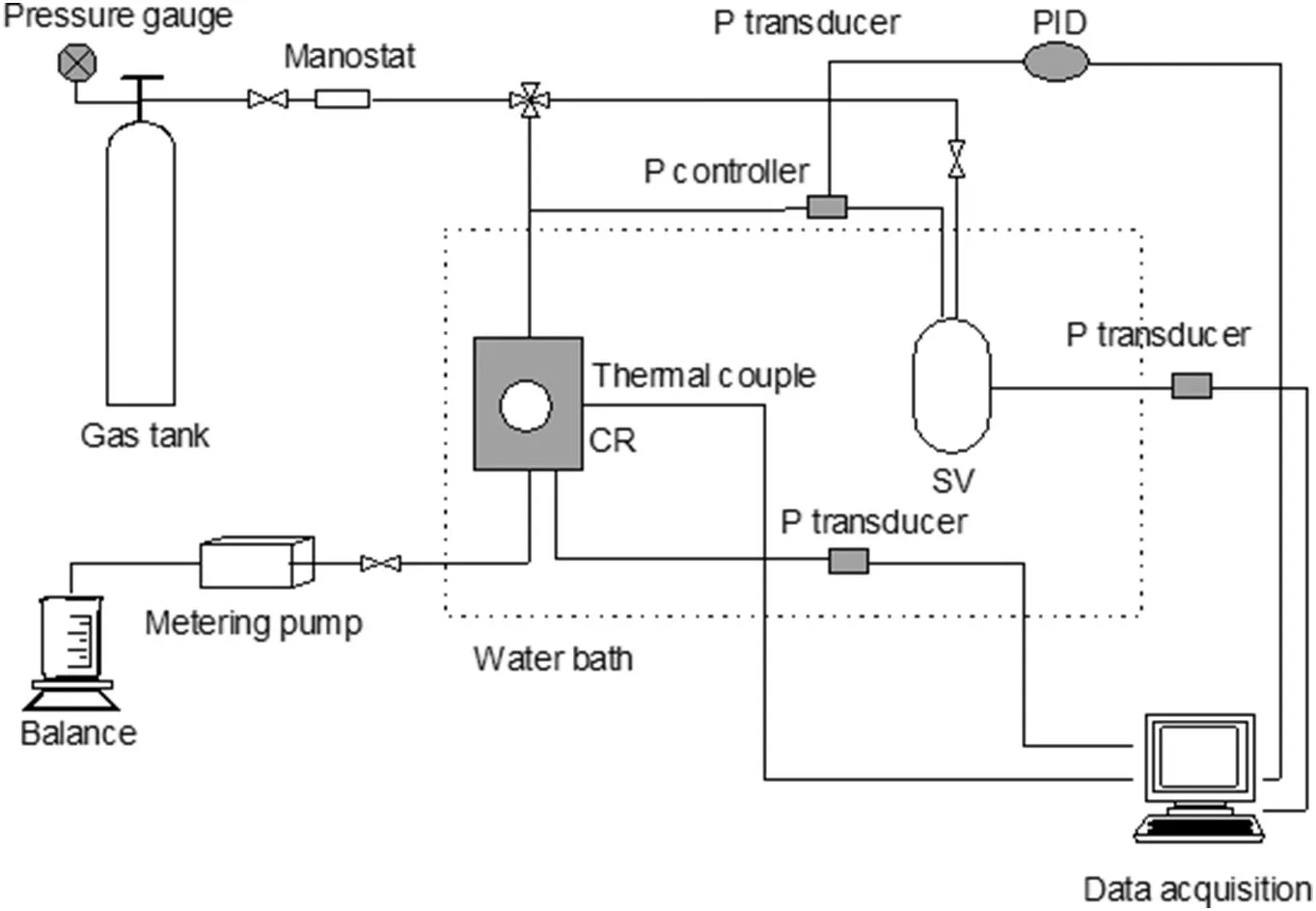
Fig.1.Schematic diagram of the hydrate formation and the hydrate dissociation system.
2.Methodology
2.1.Materials and experimental methods
The experimental apparatus is shown in Fig.1,which has been used in previous work[20].The high pressure crystallizer(CR),the supply vessel(SV),the gas/liquid supply system,the temperature controlled water bath and the data acquisition system are included in the experimental apparatus.The pressure(P)controller and the Proportion Integration Differentiation(PID)regulator are used to control the pressure of the CR. Two P transducers are used for pressure measurement(MBS3000, Danfoss, Copenhagen, Denmark), with a maximum certainty of 0.01%of the span(0-25 MPa).A Pt1000 thermoprobe is used to measure the temperature in the CV within a precision of±0.05 K(JM6081,JinMing,Tianjin,China).The CV and SV temperature is controlled by the temperature-controlled water bath with a precision of 0.1 K(263.15-303.15 K).The signals of the temperature and the pressure are monitored and recorded by the data acquisition system.
Methane is supplied by Foshan Hua Te Gas Co with the purity of 99.9%.The montmorillonite is a naturally occurring mineral from America,which is supplied by Alfa Aesar China(Tianjin)Co.,Ltd.Tables 1 and 2 list the element components and the physical properties of the montmorillonite sample,respectively.The element component of the montmorillonite samples is measured by the elemental analyzer(vario EL cube,Elementar Analysensysteme GmbH,Germany).The density of the dry samples is measured by the true density meter(VPY-30,Quantachrome,Boynton Beach,FL,USA).The particle diameter distribution of the dry samples is measured by the Mastersizer 2000 particle size analyzer(Malvern Instruments Ltd., Worcestershire, UK). Specific surface area and pore size analyzer(ASIQMO002-2,Quantachrome,Boynton Beach,FL,USA)are used to measure the pore diameter and the porosity of the samples.
The procedures for the methane hydrate formation and the methane hydrate dissociation are like the previous work [21]. Water is mixed with the dried montmorillonite sufficiently.The mass ratio of water to dry montmorillonite is 20%.Firstly,the montmorillonite saturated with water is loaded into the CR.Then,methane flushes the experimental system 5 times to flush the air out.In order to ensure the hydrate formation,the experimental conditions are set at 5.1 MPa and 275.15 K,which is higher than the equilibrium pressure. The temperature of the water bath is then adjusted to 275.15 K.Methane is pressurized in the CV to 5.1 MPa.In this work,the experiments are carried out using the Constant volume method.During the hydrate formation,the pressure continuously decreases.When the pressure remains constant for more than 3 h,it is indicated that the process of the hydrate formation is complete.In the hydrate dissociation process,the CV is opened.The pressure decreases to atmospheric pressure.Then the hydrate gradually decomposed.While the hydrate formation process or the hydrate dissociation is ended,to analyze the microstructure characteristic of the hydrate in montmorillonite,a precooled medicine spoon is used to sample the hydrate from the opened CR. And then the samples immediately immerse into liquid nitrogen.
2.2.Model and simulation method
As the model montmorillonite, we use the generic type Namontmorillonite,which is constructed from our previous work[22],for which the molecular formula is Na3(Si31Al)(Al14Mg2)O80(OH)16.The 3 water layer hydrate intercalates in the montmorillonite corresponding to 96 water molecules in a unit cell,which is defined from the previous researches[23].The simulation cell along x-direction corresponds to the basal space of the 3 water layer hydrate. The simulated system is amontmorillonite layer in contact with a bulk solution layer along the(0 1 0)face of the montmorillonite(Fig.2).The montmorillonite layer contains the supercell(1×1×2)of Na-montmorillonite model along the z-direction.The(0 1 0)face edges are obtained by cutting the montmorillonite layer.And the broken bonds are saturated with OH groups[24].

Table 1 Elemental composition of the montmorillonite

Table 2 The properties of the montmorillonite
The molecular dynamics simulations are performed by using DLPOLY package [25]. The montmorillonite is represented by the CLAYFF force field [26], methane molecules are represented by the OPLS-AA Force Field[27]and the TIP4P/ice water model is employed for water molecules[28],which have been widely used in related studies[18,29,30].The hydrate formation simulation is maintained at 260 K with the timescale of 500 ns and a timestep of 1 fs.Then the confgiuration of hydrate formation simulation at 500 ns has become the initial confgiuration of the hydrate dissociation simulation.And the dissociation simulation is maintained at 350 K with the timescale of 10 ns.
3.Results and Discussion
3.1.Analysis of the montmorillonite sample

Fig.2.Molecular configuration at x-z plane in the simulation at 260 K with 0 ns,at 260 K with 500 ns and at 360 K with 10 ns.Methane molecules are shown as yellow sphere shows,water molecules are shown as red and white stick,montmorillonite molecule is shown as yellow and purple stick,Na+ions are shown as purple blue sphere,and the H-bond is shown the blue dashed line indicates.
The corresponding elemental analysis data of the montmorillonite sample is presented in Table 1.From Table 1,we can see the purity of the montmorillonite sample, and corresponding elements have the same compositions as those of the montmorillonite in previous studies[31,32].The major appearance of silicon(Si)and Oxygen(O)elements in montmorillonite indicates that the Si--O ring is the major part of the montmorillonite.And the components of the elements in Table 1 are similar to those of the montmorillonite structure in the simulation work.Table 2 lists the physical parameters of the montmorillonite sample.It can be seen from Table 2 that the main distribution of the pore diameter is on the nanometer scale.It reflects the porosity characteristic in the montmorillonite sample.Fig.3 gives micrographs of the montmorillonite sample by a scanning electron microscope(SEM).From Fig.3,we can see some pores present in a part of the montmorillonite sample.As the above analysis of the montmorillonite sample,the montmorillonite sample is pure and has a porous characteristic.Thus the result of the methane hydrate formation and the methane hydrate dissociation experiment is reliable in this montmorillonite sample.

Fig.3.The SEM image of the montmorillonite.
3.2.Microstructure characterization of the methane hydrate formation and the methane hydrate dissociation in the montmorillonite
Under a high pressure condition,methane is injected in the montmorillonite to form hydrate at the temperature of 275.15 K and the initial pressure of 5.1 MPa.When the process of the hydrate formation is complete,the montmorillonite with the hydrate saturation of 23%is sampled from the opened CR.It can be seen that the crystal of hydrate is white and like ice in the montmorillonite surface,as shown in Fig.4(a).The XRD measures the montmorillonite with the methane hydrate formation at low temperature,and XRD patterns are shown in Fig.5.From Fig.5 we can see a(d 001)diffraction peak at 2θ=5.4°.It means the interlayer spacing(d-spacing)is 1.62 nm.The interlayer d-spacing is represented by this(001)basal plane peak from the montmorillonite-interlayer surface,which agrees with the result of methane hydrates in the 25 wt%montmorillonite (d-spacing (001) is 16.06 Å) [32]. This interlayer dspacing corresponds to the basal spacing of the montmorillonite structure with a 3 water layer hydrate[33].Therefore,we do the simulation work of the methane hydrate formation and the methane hydrate dissociation in the montmorillonite with the 3 water layer hydrate at the next section.In Fig.5,some main peaks reflect the cubic structure I(sI)hydrate.It suggests that the sI hydrate forms in the montmorillonite pore.Additionally,some peaks reflect the ice.It indicates that water does not completely form methane hydrate in the montmorillonite pore.Some ice structures are present in the montmorillonite pore. Regrettably, the amount of hydrate formed in the montmorillonite pore in this work is difficult to obtain by the analysis of XRD.

Fig.4.The montmorillonite(a)with the formation methane hydrate,(b)with the dissociation methane hydrate.
Fig.4 shows the montmorillonite with the methane hydrate formation(Fig.4a)and with the methane hydrate dissociation(Fig.4b).In the montmorillonite surface,it can be seen from Fig.4(a)that the montmorillonite particles are agglomerated and methane hydrate covers the montmorillonite. It suggests that the hydrate forms not only in the nanopore of montmorillonite but also on the montmorillonite surface.The result agrees with the experimental result on the formation of hydrate in the marine sediments from South China Sea [34]. As can be seen from Fig. 4(b), the white ice-like crystal disappears after the hydrate dissociation.And the montmorillonite is a honeycomb structure.There are some pores present on the montmorillonite surface.It is because methane hydrate forms in the montmorillonite and swells the pores of montmorillonite.Some channels form in the montmorillonite after the hydrate dissociation.The stacking structure of montmorillonite particles is changed.However,it's worth noting that the cementing montmorillonite does not collapse after the hydrate dissociation.The XRD patterns of the initialized and ultimate montmorillonite are shown in Fig.6.From Fig.6,we can see that there has few changes to the crystal structure of the initialized and ultimate montmorillonite.It means that the molecular arrangement of the montmorillonite is not changed.As the results of Figs.4 and 6,it concludes that the influence of the methane hydrate formation and the methane hydrate dissociation upon the crystal structure of the montmorillonite is small.However,the above analyses of the experiments do not sufficiently prove that the methane hydrate forms in the pore of the montmorillonite.Therefore,some simulation works of the methane hydrate formation and the methane hydrate dissociation are done in the montmorillonite system.
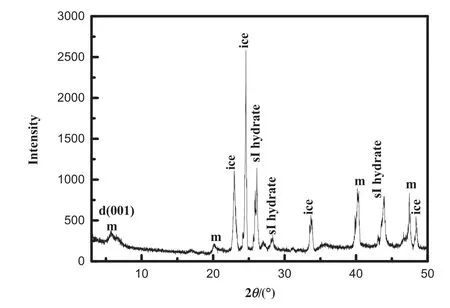
Fig.5.The XRD patterns in the montmorillonite with methane hydrate.
3.3.Simulation of the methane hydrate formation and the methane hydrate dissociation in the montmorillonite
As the above experimental results,the montmorillonite configure with the 3 water layer hydrate is constructed in the molecular dynamics simulation work.Therefore,the systems of the methane hydrate formation and the methane dissociation have been performed in a montmorillonite layer with 3 water hydrate layers and a bulk solution layer.Fig.2 shows the molecular configuration at the x-z plane in the simulation at 260 K with 0 ns,at 260 K with 500 ns and at 360 K with 10 ns.At the simulations of 0 ns at 260 K,it can be seen from Fig.2 that a bubble is formed by some gathering methane molecules in the bulk solution region.Subsequently,the methane bubbles disappear with the dissolution of methane molecules in the solution.Water molecules with these dissolved methane molecules form an amorphous cluster and continually form irregular cages.These irregular cages have been rearranged,until the regular clathrate hydrate structure is formed.The molecular configuration of 500 ns at 260 K on the x-z plane in Fig.2(b)and the molecular configuration of 500 ns at 260 K on the y-z plane in Fig.7(a)show the arrangement of methane molecules in the bulk solution layer and the arrangement of water molecules is quite regular. It means that the clathrate methane hydrate appears in the bulk solution layer.
Clearly showing the clathrate hydrate structure,we delete some molecules in the system of 500 ns at 260 K to exhibit the cage configurations,as shown in Fig.7(b).From Fig.7(b),we can see the regular 512cages(12 pentagonal faces)and 51262cages(12 pentagonal and 2 hexagonal faces)present in the bulk solution layer in the system,which also present in a typical sI hydrate.It means that the sI hydrate presents in the montmorillonite system,and this result agrees with the above experiment result.This structure type of the formed hydrate is probably mostly due to the gas content(methane).In the interface between the bulk solution layer and the montmorillonite layer, clathrate hydrate connects the montmorillonite surface with the ice-like structural water molecules,which is the same as the ice-like hydrate structure near the silica surface[14].This result reflects the ice-like structure present in the montmorillonite system,which agrees with the above XRD result from the hydrate formation experiment.
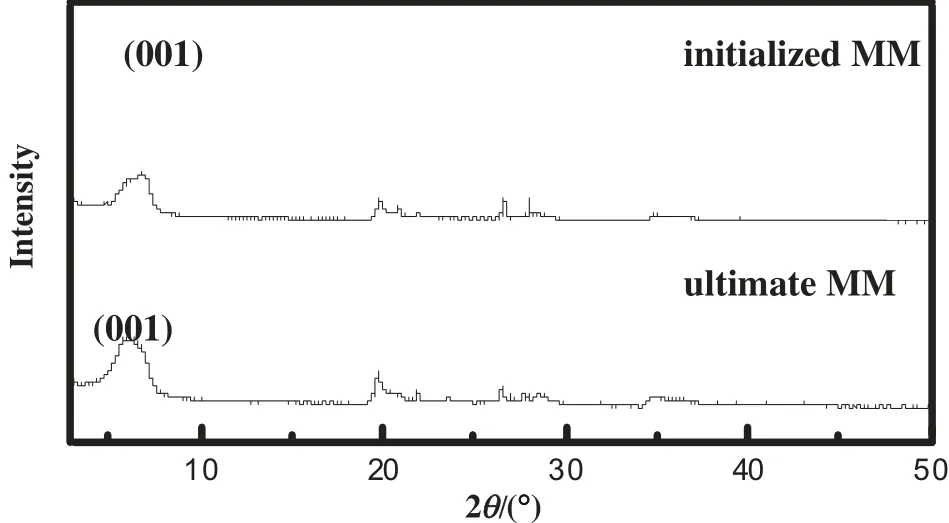
Fig.6.The XRD patterns of the initialized and ultimate montmorillonite.
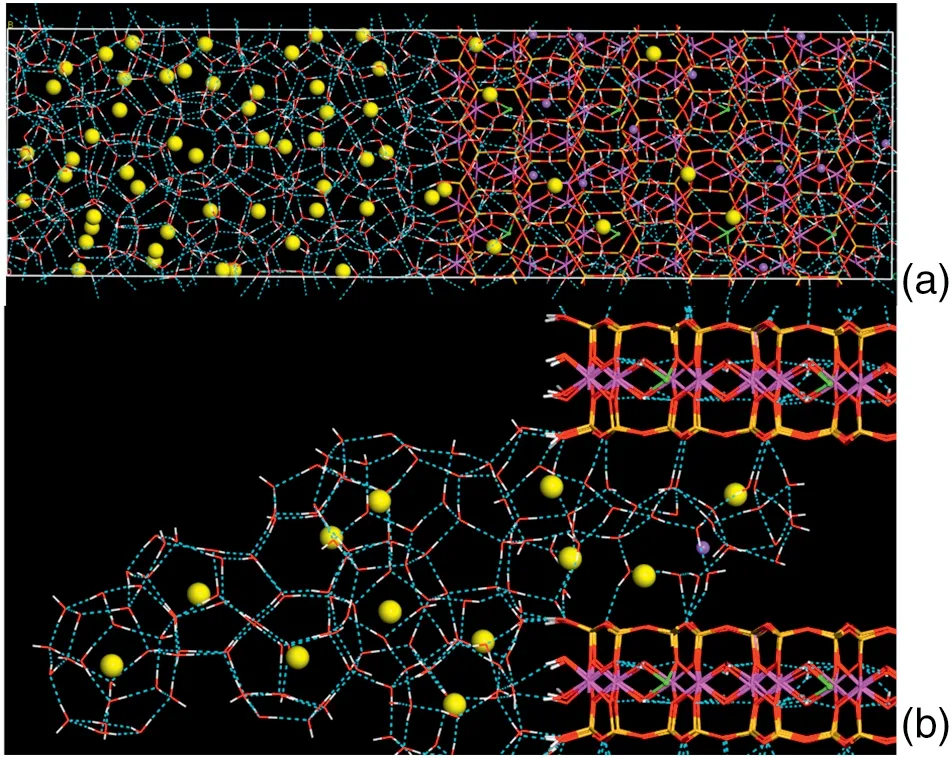
Fig.7.Molecular configuration of the hydrate formation at 260 K with 500 ns(1)at y-z plane,(b)with integrated cages.
In the montmorillonite region,it can be seen from Fig.7(b)that the methane molecules transfer from the bulk solution region into the montmorillonite region.Water molecules connect with the Si--O ring of the montmorillonite surface and the nearest-neighboring water molecules to form the network by the hydrogen bonds.Methane molecules near the montmorillonite surface are surrounded by the network to form cages in the montmorillonite surface.The Na+ions,as the cations which balance the charge of the montmorillonite,disperse in the montmorillonite interlayer and form cages with water molecules. These cages distort under the steric effect of cations.This steric effect comes from a fact that the cations occupy the space which should be occupied by water molecules to construct the network of cage in the montmorillonite.Therefore,the steric effect influences the structure of cage.While methane molecules diffuse,some cages form in the bulk solution region.Thus,the low frequency diffusion for methane molecules and the slow hydrate growth in the montmorillonite layer are caused by the obstruction of the hydrate crystals in the entrance of the montmorillonite.It can be seen from Figs.2(b)and 7(b)that the cage is difficult to form in the montmorillonite layer.It is mainly due to the larger effect of ions on the hydrate formation in the montmorillonite with the smaller pore size.When the pore size increases,this effect of ions reduces.Therefore,it is easier to form hydrate in the montmorillonite with the larger pore size[22].While the hydrate dissociates,the hydrogen bonds between water molecules are broken and the cages are collapsed,as shown in Fig.2(c).As can be seen from Fig.2(c),water molecules and methane hydrate are dispersed in the montmorillonite layer and the bulk solution layer.Comparing with Fig.2(a)and(c),it is found that the crystal structure of montmorillonite is unchanged.The result indicates that the hydrate formation process and the hydrate dissociation process have no influence upon the crystal structure of montmorillonite.
The order parameter of water molecules can identify hydrate formation events,which has the high resolution traces to the hydrate formation. F4is one of the order parameters of water molecules, which is defined in previous research[35].The 0.4 value of F4is the site of the interface between the liquid and the solid hydrate[36].Fig.8 shows the F4at the z-profile for the systems.As shown in Fig.8,the values of the F4in the bulk solution layer of the simulation systems of 500 ns at 260 K approximately approach that value in the hydrate region(0.7),which implies water molecules of the simulation systems present the solid structure in this region.In the montmorillonite layer,the F4falls suddenly under the basic line of the solid water(4.0)in the simulation systems of 500 ns at 260 K.It indicates that it is difficult to form an integral cage in the montmorillonite layer.At the same time,in the simulation systems of 0 ns at 260 K and the simulation systems of 10 ns at 360 K,the F4is under the basic line of the solid water(4.0)both in the bulk solution layer and in the montmorillonite layer,shown in Fig.8.It means the arrangements of water molecules in the two systems present a liquid state.There is no hydrate formation in the systems.
The radial distribution functions(RDFs)in the montmorillonite layer are analyzed to describe the status of hydrate in the montmorillonite layer. In Fig. 9, the gO--O(r) presents the RDF between O--O atoms of water molecules in the montmorillonite layer,and the gC--C(r)presents the RDF between C--C atoms of methane molecules in the montmorillonite layer.It can see from Fig.9(a)that the initial three peaks for the gO--O(r)s of the system of 500 ns at 260 K in the montmorillonite layer agree with the peaks of gO--O(r)for the sI hydrate[37,38].The result indicates the clathrate-like arrangement of water molecules in the montmorillonite layer of the system of 500 ns at 260 K.In the montmorillonite layer of the system of 10 ns at 360 K,the second peak of gO--O(r)is almost horizontal,and it is indicated that water molecules exist in liquid phase[39].It suggests that the hydrate in the montmorillonite layer is completely dissociated at 360 K with the simulation at 10 ns.
In Fig.9(b),the clear different profile for gC--C(r)in the montmorillonite layer of the system of 500 ns at 260 K and the system of 10 ns at 360 K can be found. The first peak value in the montmorillonite layer of the system of 10 ns at 360 K approaches the peak value of gC--C(r) in liquid [40]. In the montmorillonite layer of the system of 500 ns at 260 K,the first peak value of gC--C(r)which approaches the first peak value of gC--C(r)in liquid[40]is lower than the second peak values of gC--C(r)which approaches the first peak value of gC--C(r)in methane hydrate[37,38].The results indicate that the arrangement of molecules in the montmorillonite layer of the system of 500 ns at 260 K is close to the arrangement of methane molecules in methane hydrate.In the montmorillonite layer of the system of 10 ns at 360 K,the methane molecules distribute in liquid.
In the above simulation analysis,at the low temperature(260 K),methane molecules dissolve in the bulk solution layer and diffuse into the montmorillonite layer.Water molecules arrange to form hydrate around methane molecules in the bulk solution layer,and water molecules connect with the Si--O ring of the montmorillonite surface to form the semi-hydrate with the diffused methane molecules.The structure of methane hydrate most presents the structure of the sI hydrate with some ice structure in the montmorillonite.While the formed hydrate in the montmorillonite at high temperature(360 K),the hydrogen bonds are broken.And then the hydrate is dissociated.Water molecules and methane molecules present the liquid distribution. The crystal structure of montmorillonite is unchanged within the hydrate formation process and the hydrate dissociation process.

Fig.8.F4 at z-profile for the systems.

Fig.9.RDFs in the montmorillonite layer of the systems(a)gO--O(r),(b)gC--C(r).
4.Conclusions
The methane hydrate formation and methane hydrate dissociation behaviors in montmorillonite are studied by experiment and the molecular dynamics simulation.From the experiment,the porous characteristic of montmorillonite is analyzed by the SEM and the XRD.The results indicate that methane hydrate in montmorillonite forms the sI crystal and the structure of some water in the montmorillonite forms the icelike structure.From the simulation,the microstructure and microscopic properties of the hydrate formation and the hydrate dissociation in the systems containing the montmorillonite layer with 3 water hydrate layers and the bulk solution layer are analyzed. The results indicate that methane hydrate not only forms in the bulk solution layer but also forms in the montmorillonite layer,which presents in the typical sI hydrate.Water molecules form the ice-like structure in the montmorillonite surface.Combining the experimental and simulating analyses,it concludes that there is little influence upon the crystal structure of the montmorillonite in the methane hydrate formation and in the methane hydrate dissociation. It is beneficial to the fundamental researches on the exploitation and security control technologies of natural gas hydrate in deep-sea sediments.
Acknowledgments
The authors gratefully acknowledge support from the Guangzhou Branch of the Supercomputing Center of Chinese Academy of Sciences References and the Supercomputer Center of the Computer Network Information Center,Chinese Academy of Sciences.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
