Mineral phase and structure changes during roasting of fine-grained carbonaceous gold ores and their effects on gold leaching efficiency☆
Jianping Jin*,Yuexin Han,Hui Li,Yangyang Huai,Yongjun Peng,Xiaotian Gu,Wei Yang
1 School of Resources&Civil Engineering,Northeastern University,Shenyang 110819,China
2 Xi'an Northwest Geological Institute Company of Nonferrous Metals Co.,Ltd.,Xi'an 710054,China
3 College of Material and Resource,Xi'an University of Architecture and Technology,Xi'an 710055,China
4 School of Chemical Engineering,The University of Queensland,St.Lucia,Brisbane 4072,Australia
Keywords:Carbonaceous gold ore Roasting Leaching X-ray diffraction(XRD)Scanning electron microscopy(SEM)
ABSTRACT While roasting has been widely applied to reduce the negative effect of carbonaceous matters on gold extraction from fine-grained carbonaceous gold ores,the phase and structure changes of minerals during roasting and their influences on the leaching rate of gold have not been fully understood.This limits the extraction of carbonaceous gold deposits.The current work examines the oxidation process of a fine-grained carbonaceous gold ore during roasting using a range of techniques including X-ray diffraction(XRD),scanning electron microscopy(SEM),Energy Dispersive Spectrometer(EDS)analysis and pore structure analysis together with gold leaching tests.The results show that during the process of oxidative roasting,the carbonaceous matters(organic carbon and graphitic carbon)and pyrite were completely decomposed at 600°C with the carbonaceous components burned and pyrite oxidized into hematite.At 650°C,while dolomite was decomposed into calcia,magnesia,calcium sulfate etc.,the calcine structure became loose and porous,leading to a high gold leaching rate from the roasted product.Above 750°C,the porous calcite structure started to collapse along with the agglomeration,leading to the secondary encapsulation of gold particles,which contributed to the sharp drop in the gold leaching rate of the roasted product.This study suggests optimum phase and structure changes of minerals during roasting to achieve maximum gold extraction from fine-grained carbonaceous gold deposits.
1.Introduction
Carbonaceous gold ores often contain amorphous carbon,graphite,lignite or organic matters with a high hydrocarbon ratio [1]. Due to the natural adsorption of these carbonaceous components on gold,which is referred to as“preg-robbing”,the gold extraction from carbonaceous gold ores by cyanidation is severely inhibited,especially for those with more than 0.2%organic carbon constituents[2].Therefore,prior elimination of the carbonaceous components to gold cyanidation is of great significance to gold extraction.
Roasting is a well-developed technique to treat carbonaceous gold ores,in which not only are the carbonaceous components oxidized to carbon dioxide but also the gold-encapsulated minerals are destroyed so that the finely disseminated gold particles can be exposed to cyanide during leaching[3-7].Temperature is one of the most critical factors during roasting,determining the final chemical reactions and physical structures of gangue minerals.From the literature,the normally used temperature for the roasting of carbonaceous gold ores is 500 °C-700 °C. At a low temperature(<400 °C to 450°C),the reaction rates of calcines are slow causing the insufficient oxidation of carbonaceous components,while at a high temperature(>700°C to 750°C),the porous structure of calcines may be collapsed,resulting in a decreased leaching rate of gold,which is called“sintering”or the secondary encapsulation of the gold particles[8-11].
A number of studies have been conducted to improve the extraction of gold from fine-grained carbonaceous gold ores.Nanthakumar et al.[3]investigated the microwave roasting process of a double refractory gold ore,in which gold is locked in both pyrite and carbonaceous matters. They found that it was necessary to keep the temperature of roasting above 650°C to convert pyrite to hematite and to eliminate the organic carbon in the microwave roasting.In addition,dolomite in the ore was decomposed into magnesium oxide and calcium oxide at 690°C.However,the pore structure transformation of the major associated minerals,such as quartz,dolomite,pyrite and muscovite,during roasting was not studied by Nanthakumar et al.[3],but it determines the gold extraction rate.Afenya[12]and Wang et al.[13]found that carbon and sulfur dioxide were produced during roasting of carbonaceous gold ores. They suspected that the emission of gases might cause a structure change of the carbonaceous gold ores,but did not determine whether a structure change of roasted product occurred during roasting and how this structure change affected gold leaching. Liu et al. [14]found that during roasting,the matters with a low melting point such as Ba2SiO4and CaAl2O4were melted at an over-high temperature,probably leading to the secondary encapsulation of gold particles and hence a decrease in the leaching efficiency of gold. However, the phase changes of gangue minerals during the melting and encapsulation process were not confirmed in that study.
X ray diffraction(XRD),pore structure analysis,scanning electron microscopy(SEM)and energy spectrometer(EDS)analysis are modern analytical methods, which are widely used in material preparation,nanomaterials and detection of bacterial pathogens [15-17]. In this study,in order to fully understand how the phase and structure changes of gangue minerals in carbonaceous gold ores during roasting affect the subsequent gold leaching,the oxidation of a fine-grained carbonaceous gold ore at various roasting temperatures was investigated using XRD,pore structure analysis, SEM and EDS analysis in conjunction with gold leaching tests.The mechanism of roasting which is favorable for leaching was analyzed.This study offered fine-grained carbonaceous gold ores;the fundamental reason for dressing difficultly.And the research will further provide theoretical and technical guidance to advance gold recovery from carbonaceous fine-grained gold ores.
2.Materials and Methods
2.1.Samples
The carbonaceous fine-grained gold ore was obtained from a gold mine in Qinling Mountains,Shaanxi Province,China.The main metal minerals in the ore are pyrite and limonite.The valuable minerals are essentially native gold and argentite. The non-metallic minerals are mainly quartz,calcite-dolomite,vanadium sericite,kaolinite,graphite,organic carbon,barite,etc.The gold in the ore exists as a native gold with 83.03% gold grains being the range of 0.005 mm-0.01 mm and 16.97%gold grains being the range of-0.005 mm.The carbon content in the ore is relatively high with 1.33%graphite and 1.50%organic carbon.Carbonaceous matters and quartz are closely associated and embedded evenly with the particle size of 0.01 mm. Gold particles are partially encapsulated in carbonaceous siliceous slates and their fragments.Table 1 shows the elemental composition of the ore.Tables 2 and 3 show the gold carriers and carbon components, respectively.The gold grade was determined by foamed plastics-enriched atomic absorption spectrophotometry.As can be seen,the main component in the ore is SiO2.The gold content in the ore is 5.46 ppm.The total carbon and sulfur content are 6.84%and 0.81%,respectively.The gold content in silicates and sulfides is 27.50%and 20.38%,respectively,while 38.27%gold is free gold.The content of organic and graphitic carbon in the ore was over 1%,which may adsorb solubilized gold cyanide as activated carbon[18-22].

Table 1 Elemental compositions of the ore
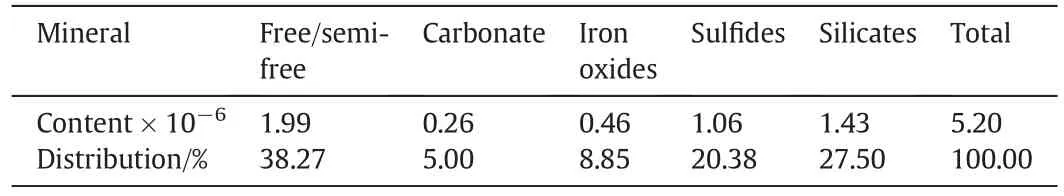
Table 2 Gold contents in its carriers in the ore

Table 3 Carbon minerals,their contents and distributions in the ore
2.2.Methodology
2.2.1.Roasting
The roasting tests were carried out in a rotary resistance-heated furnace (Model: HB-J-30, Xi'an, China) with a drum rotation speed of 4 RPM.500 g ore sample(-2 mm)was roasted in the furnace at a certain temperature with an air flow rate of 0.8 m3·h-1for 2 h.Then the calcine was cooled to room temperature,weighed and analyzed.Finally,the loss of the calcine and the grade of the calcine were calculated.
The grade of calcine(β)was calculated as follows:

where α is the gold grade of the raw ore,β is the gold grade of the calcine,m0is the mass of the raw ore and m1is the mass of the roasted product.
2.2.2.Leaching
Leaching experiments were conducted for the roasting-treated with different temperature and raw ore.A Sample(500 g)was first ground in an φ160 mm×240 mm wet rod mill.After grinding,the slurry was put in agitation leaching trough(Model:XJTII,Changchun,China),followed by 3 min conditioning of water to be 2.5:1 of liquid-solid ratio of leaching.Then,CaO was added and conditioned for 30 min to adjust the pH at about 12. NaCN of industry grade were used as leaching agent with its concentration 0.3%in the slurry.The duration for gold leaching was 24 h.The leaching residue was filtered and dried before measuring the gold grade.The leaching rate of gold(η)was calculated as follows:

where η is the leaching rate of gold,β is the gold grade of calcine and θ is the gold grade of leaching residue.
2.2.3.Pore structure and surface area measurements
N2-adsorption/desorption isotherms were acquired at liquid nitrogen temperature(-196°C),after a pre-treatment performed for 2 h at 200°C under vacuum.The pore structure and specific surface area of the ore and calcine were measured by the surface area and porosity analyzer(ASAP2020,Micromeritics,America).The surface area and porosity were determined using respectively the BET equation and the Barret Joyner and Halenda (BJH) method applied to the desorption branch of the isotherms that was based on capillary de-condensation and theoretically the volume of the internal pores of the calcine was calculated through the pressure and the liquid mercury consumption volume as follows:

where D is the diameter of the inner hole of calcine,γ is the surface tension of mercury,ϑ is the contact angle between the liquid mercury and the hole in the calcine,and P is the applied mercury pressure.
45. Finger: The forces of the unconscious that can emerge without warning and hinder efforts of the conscious are represented by the finger (Olderr 1986).Return to place in story.
2.2.4.X-ray diffraction analysis
The raw ore and calcine were analyzed by the X-ray diffractometer(D8,Bruker,German).Each powdered sample was evenly placed onto a circular stainless steel holder,flattened with a glass slide and covered by a Kapton film.X-ray powder diffraction patterns were obtained on a Bruker D8 Endeavor X-ray diffractometer fitted with a copper tube(copper Kα radiation),an incident beam monochromator and a scintillation detector.The diffractometer was operated at 40 kV and 35 mA.Diffraction patterns were collected over the range of 5°-70°2θ with a scan step of 0.033°and a dwell time of 2s per step.The total pattern collection time was 20 min.Mineral phase identification was operated at a high vacuum(<133×10-5Pa)with an accelerating voltage of 15 kV and a beam current of 0.3 nA.
2.2.5.Scanning electron microscopy
The SEM-EDS measurements were conducted using a Quanta 650 Scanning Electron Microscope(FEI,Hillsboro,USA)coupled with double X-ray spectrometer(AMETEK,Inc.,Berwyn,USA)for electron image acquisition and elemental analysis,respectively.SEM images were obtained at a high vacuum employing an acceleration voltage of 30 kV and a working distance of 10 μm to 400 μm.Different magnifications(up to×5000)were used for secondary electron images and an integration time of 50 s was employed to improve the signal-to-noise ratio.Furthermore,imaging studies were also conducted in some specific microscopic areas of the samples investigated,allowing the evaluation of distribution of the elements present throughout the sample.
3.Results
3.1.The effect of roasting temperature on gold leaching rate
Fig.1 shows the effect of roasting temperature on the gold leaching rate from the roasted product. As can be seen, with the increase of roasting temperature from 400°C to 650°C,the leaching rate of gold increased from 82.33%to 91.28%with the maxima at 650°C.After that,the leaching rate experienced a sharp decrease from 91.28% to 78.36%.Clearly,the leaching rate of gold was closely related to the temperature used to roast the ore and the most suitable temperature here was 650 °C. It is expected that the temperature during roasting determines the phase and structure changes of gangue minerals and the exposure of gold in calcine which govern the leaching rate of gold.
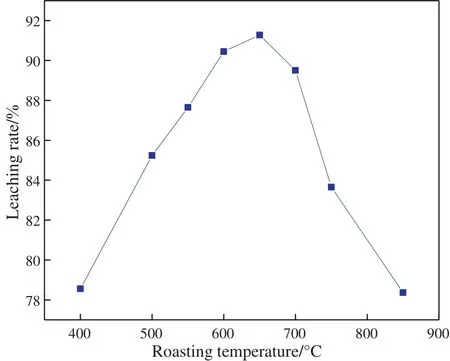
Fig.1.The effect of roasting temperature on the leaching rate of gold.
3.2.The effect of roasting temperature on phase compositions
The calcine compositions after roasting at different temperatures(400 °C, 500 °C, 550 °C, 600 °C, 650 °C, 700 °C, 750 °C, 800 °C and 850 °C) were determined by XRD. The phase changes from 400 °C to 650 °C were shown in Fig. 2(a) and from 700 °C to 850 °C were shown in Fig. 2(b). Partial enlarged XRD patterns were shown in Fig. 2(c). It can be seen that the main components of mineral phases in the ore were quartz, dolomite, calcite, vanadium containing sericite, pyrite, carbonaceous matter and graphite.

Fig.2.XRD of the ore after roasting for 2 h at different temperatures:(a)unroasted and 400°C to 650°C;(b)unroasted and 700°C to 850°C;(c)partial enlarged XRD patterns of selected sample.
Fig.2(a)and(c)shows that the diffraction peak of carbonaceous component (d=0.3311 nm)was weak but still noticeable after the ore was roasted at 400 °C. However, as the roasting temperature was up to 600 °C, the diffraction peak of carbonaceous component(reference code:01-074-1602)disappeared completely.The oxidation and combustion of carbonaceous matters and graphite are represented as Eq.(1).
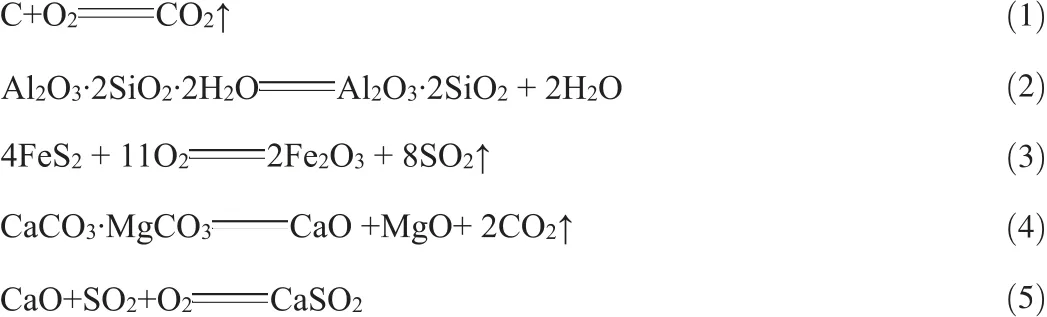
As shown in Fig.2(b),as the roasting temperature reached 850°C,the characteristic diffraction peak of calcite(d=0.3859 nm,reference code:01-085-1108)disappeared,resulting in a compound containing Si,Ca,Fe and other elements.This indicates that calcite was decomposed intensively at 850°C and can be expressed as Eqs.(6)-(8).

3.3.SEM and EDS analysis
The morphology of the ore before and after pretreatment by roasting was studied using SEM.Fig.3 shows the SEM images of the ore before roasting (a) and after roasting at 3 selected temperatures (b—500 °C,c—650 °C and d—850 °C). Consistent with the results from XRD,Fig. 3(a) shows that the surface of the ore sample was massive dense,fully covered with carbonaceous matters and closely associated with quartz.After the ore was roasted at 500°C,the coverage of carbonaceous matters gradually diminished and the mineral surface was getting loose with the appearance of some micro-pores as shown in Fig.3(b),though the surface structure change was not observed.After the ore was roasted at 650 °C, the microscale structure of minerals was clearly demonstrated and the porous surface structure of minerals started to appear as shown in Fig.3(c).The carbonaceous matter was depleted while massive fractures occurred.When the ore was roasted at 850 °C,although the typical micro-squama structure disappeared,some large pore cavity structures of mineral appeared as shown in Fig.3(d).The surface became compact and smooth with most of particles existing as“vitreous bodies”consistent with Zhou's investigation[25]. In addition, the samples were severely sintered and obviously the liquid phase was produced during roasting.
Table 4 shows the element contents determined by energy dispersive X-ray Spectroscopy of a micro-region on the SEM of the calcine after the ore was roasted at 850°C.It indicates that carbon was not detected,demonstrating that there was no carbonates and carbonaceous matters. In addition to Al and Si, there were K, Mg, Ca, Fe, V, Ti and other elements detected.This suggests that pyrite was gradually oxidized into hematite and the carbonates were decomposed into oxides during roasting at 850°C.The oxidation of carbonaceous matters during roasting can release a large amount of heat which can melt the calcineconsisting of Ca2SiO4,CaAlO4,etc.at a high temperature,hence leading to material sintering[26].This observation is consistent with the results from XRD analysis.
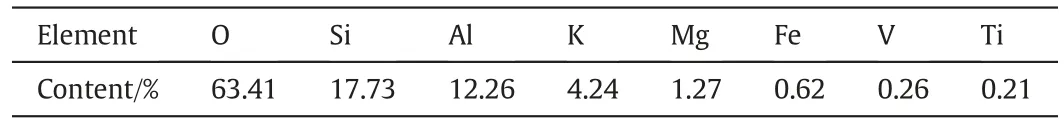
Table 4 Element compositions of the calcine after roasting at 850°C
3.4.Pore structure analysis
In addition to the phase change of the major minerals during roasting, the fine-grained carbonaceous gold ore also experienced changes of physical properties such as porosity and looseness.Although SEM can provide direct information of the porous structure of calcines,the quantitative analysis of the pore size distribution was not available.Therefore,the adsorption-desorption isotherms,pore size distribution and pore structure of calcines after roasting at various temperatures were analyzed by BJH.The results are shown in Fig.4.
The specific shape of sorption isotherms depends on the interaction between the adsorbate and adsorbent.Depending on the shape of the isotherms and the conditions used,IUPAC classified the sorption isotherms into six types.In the study,after the ore was roasted at 500°C,650°C and 850°C,the weak adsorption capacity of the calcines in the low pressure area indicated the weak interaction force between the samples and N2at a low pressure.The large adsorption occurred at a high pressure due to the filling process. Over the entire pressure range,the curve had no inflection point at low P/P0and the isotherms were convex to the P/P0axis over its entire range,which is consistent with typical characteristic of type III isotherms.Type III isotherms typically represent a weak interaction between adsorbent and adsorbate.Due to capillary condensation,if the adsorption-desorption of the adsorbate to the adsorbent is not completely reversible,the adsorptiondesorption does not match,leading to a hysteresis loop.According to the IUPAC classification of hysteresis loops[27,28],the current adsorption-desorption isotherms can be categorized into type H3.This indicates that the pores in the calcine after the ore was roasted at 500°C,650°C and 850°C were slit channels and the shape and size of these channels were not uniform.

Fig.4.N2 adsorption-desorption isotherm and pore diameter distribution of calcine after roasting at 500°C(a),650°C(b)and 850°C(c).
Fig.4 also shows the pore size distribution,in which the pore volume in the calcine first increased and then decreased with roasting temperature.Comparing the pore size distribution of the calcine after the ore was roasted at 500°C and 650°C,the main pore diameter decreased from 10 to 25 nm to 2-5 nm, indicating that a greater number of pores formed at 650 °C. This can help increase the diffusion of the leaching agent and hence benefits the gold leaching.In addition,the number of pores of 2-5 nm decreased significantly after roasting from 650°C to 850°C,indicating that some pores were closed and blocked during the roasting at 850 °C, which does not favor the diffusion of leaching agents,resulting in a slow leaching rate of gold.
Table 5 shows the pore structure parameters of calcines including BET specific surface area,total pore volume and average pore diameter calculated from the pore size distributions of calcines at selected roasting temperatures.The change of the pore structure during roasting was mainly due to the release of CO2and SO2from the oxidation and decomposition reactions of carbonaceous minerals,pyrite,dolomite and calcite,resulting in the formation of micro-pores[29].Clearly,with the increase of roasting temperature, the BET specific surface area, total pore volume and average pore diameter first increased and then decreased with the highest peak at 650°C,which is consistent with the gold leaching rate as shown in Fig.1.This can be attributed to the fact that,during the roasting at 650°C,carbonaceous and carbonate minerals were strongly reacted,resulting in the greatest number of micropores. Those micro-pores are conducive to the diffusion of leaching agents during leaching and hence improve the leaching rate of gold.However,when the roasting temperature increased to 850°C,the specific surface area,total pore volume and average pore size began to decrease significantly,indicating that the roasting temperature was too high for the particles to melt and the micro pores inside the particles were again liquid-filled and closed so that the number of micro-pores was reduced and the internal structure became dense.This structure is not conducive to the proliferation of leaching agents, resulting in the decreased gold leaching rate.

Table 5 Micro pore structure parameters of calcines after roasting at different temperatures and corresponding leaching rates of gold
4.Discussion
This study shows that the fine-grained carbonaceous gold ore experienced phase and structure transformation with roasting temperature which was closely related to gold leaching rate of the roasted products.The illustration of this transformation is presented in Fig.5.
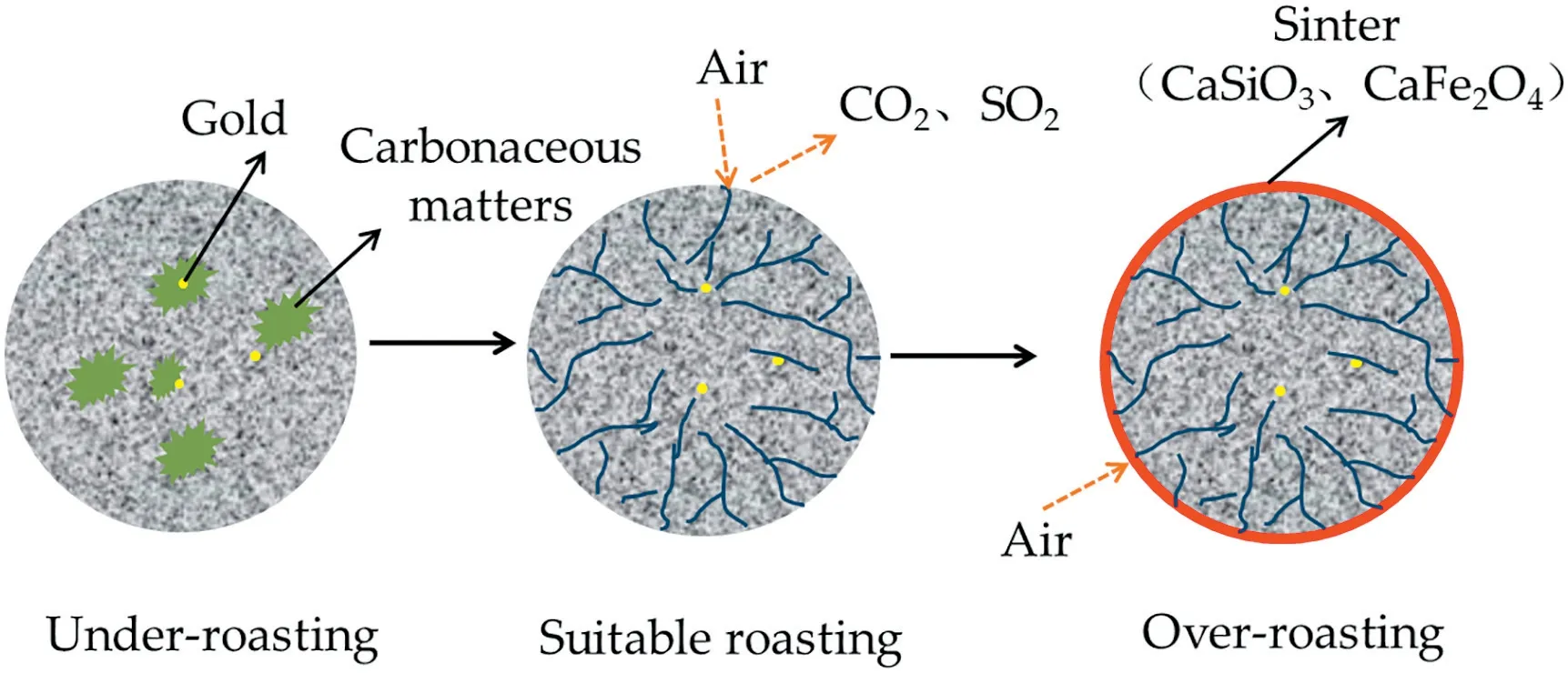
Fig.5.Schematic diagram of phase and structure transformation during roasting.
Under suitable roasting conditions,carbonaceous matters,pyrite,dolomite and clay minerals underwent oxidation and decomposition,so the effect of“gold preg-robbing”was mitigated.At the same time,the surface of particles became more porous along with the release of the CO2and SO2.These voids provided additional channels for the diffusion of CN-during leaching and would benefit the complexing of the CN-with Au,resulting in a significant increase of leaching rate.The structural changes can be further explained by the slight shift of the characteristic diffraction peaks of siliceous materials with the increase of roasting temperature as shown in Fig.6,where the crystal structural changes of quartz,the inter-planar spacing of quartz(100)and(101)planes were analyzed.The d100 and d101 values of quartz obtained at different roasting temperatures increased from 400 °C to 650 °C,reaching the maximum of 3.34606 nm and 4.25948 nm at 650°C,respectively.This increase indicates the crystal structure gradually became loose between 450 °C and 650 °C due to the oxidation of carbonaceous matter and pyrite and the decomposition of dolomite and calcite.Therefore,when the carbonaceous gold ore was roasted at 650°C,gold leaching rate was the highest.

Fig.6.The effect of roasting temperature on d100 and d101 of quartz.
Under over roasting conditions,CaSiO3and CaFe2O4formed and the samples would sinter.The higher the temperature was,the stronger the sintering would be.Along with the sintering,the specific surface area,total pore volume and average pore diameter of calcine became smaller and the“vitreous bodies”occurred,so the gold in the calcine would experience a secondary encapsulation,hence resulting in a decrease in gold leaching rate.
5.Conclusions
In this work,the process of extracting gold from fine-grained carbonaceous gold ore based on various roasting temperature was studied systematically,and calcine is characterized by X-ray diffraction analysis,pore structure analysis,scanning electron microscopy(SEM)and Energy Dispersive Spectrometer(EDS)analysis.The temperature used to roast the fine-grained carbonaceous gold ore had a direct impact on the subsequent gold leaching efficiency through the phase and structure change of associated minerals during roasting.Under suitable roasting temperature(650°C),the typical micro-squama structure disappeared with the appearance of some large pore cavity structures due to the additional emission of CO2.This loose and porous structure contributes to the fastest gold leaching rate.Under over roasting temperature,calcite decomposed and the material sintered,leading to a decrease in surface area of calcine,total pore volume and the average pore size,and contributing to a sharp decline in gold leaching rate.
Overall,the gold leaching rate of roasted fine-grained carbonaceous gold ores will be the highest when the oxidation reaction of carbonaceous matters is complete during roasting,during which the gas produced from the oxidation of carbon, pyrite and carbonate minerals makes the ore porous,while neither sintering nor secondary encapsulation of gold happens.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
