Study on the emission characteristics of nitrogen oxides with coal combustion in pressurized fluidized bed☆
Zheng Gong,Yingjuan Shao,Lei Pang,Wenqi Zhong*,Chao Chen
Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education,School of Energy and Environment,Southeast University,Nanjing 210096,China
Keywords:Pressurized fluidized bed coal combustion Operating parameter NOx N2O
ABSTRACT Nitrogen oxides are one of the most significant pollution sources during coal combustion. This experimental study was conducted in a 15 kWth lab-scale pressurized fluidized bed (inner diameter = 81-100 mm, H =2100 mm) firing with bituminous coals. The effects of operating parameters, including bed temperature(800 °C-900 °C), operating pressure (0.1-0.4 MPa), excess air level (16%-30%) and flow pattern on NOx and N2O emissions were systematically studied during the tests. During each test the interaction effects of all the operating parameters were properly controlled. The results show that most operating parameters have an opposite effect on NOx and N2O emissions, and the N2O emissions mainly depend on the bed temperature. Increasing the operating pressure can significantly suppress the fuel-N conversion to NOx but enhance its conversion to N2O. With the rise of the excess air level and fluidization number, NOx emissions grow distinctly while N2O emissions remain almost unchanged. Total nitrogen oxide emissions increase with the bed temperature while decrease with the operating pressure.
1.Introduction
Pressurized fluidized bed combustion(PFBC)has been proved as a highly clean and efficient power generation technology[1-4].Pressurized fluidized bed is the core of the second generation pressurized fluidized bed combustion combined cycle(2R PFBC-CC)[5]and integrated gasification combined cycle (IGCC) [6], which is one of the most promising combustion technologies.Compared with atmospheric fluidized combustion(AFBC),PFBC has higher oxygen partial pressure,so the reaction rates are expected to increase if the reaction takes place under kinetically controlled conditions.Therefore,the combustor vessel has a small plan area per unit output [7]. What's more, due to the higher carbon load in the bed and lower fluidizing velocity,PFBC can achieve lower NOx reduction in the dense bed compared with that in AFBC[8,9].
Nitrogen oxide(NOx), which mainly consists of nitric oxide(NO)and nitric dioxide(NO2),is one of the most significant pollutants produced by thermal power plants.NO is the main component(generally over 90% [10])of the NOxin the tail flue gas,and the majority of NO will convert to NO2in the air[11,12],which is the main cause of nitric acid rain.Emission of Nitrous oxide(N2O)has attracted great attention because it enhances the greenhouse effect and the destruction of stratospheric ozone layer.Because of the much lower temperature in fluidized bed combustion, N2O emission in fluidized bed is relatively higher compared with conventional pulverized coal boiler,and the N2O concentration is even higher than the NOxconcentration in some fluidized beds[13,14].
The formation and control of NOxand N2O in the pressurized fluidized bed have been a focus of attention.The effects of some operation parameters(bed temperature,operating pressure,excess air level)on the NOxand N2O emissions in PFBC have been studied by researchers over the past two decades.The effects of the temperature on the N2O and NOxemissions in both AFBC [13-15] and PFBC [16-18] are negatively correlated. The N2O emissions decrease while the NOxemissions increase with the increase of temperature.Increasing operating pressure contributes to a reduction of NOxemissions in the pressurized fluidized bed and further NOxdecrease can be obtained by means of staged combustion and/or ammonia injection to comply with further NOxemissions. It has been shown that PFBC facility can achieve the NO reduction of 75%to 85%with NH3injection and/or air staging[18].The effect of operating pressure on N2O emissions was studied in a lab-scale PFBC by[19],and they found that the N2O emissions in PFBC exerted a maximum when the operating pressures are in the range of 0.2-0.5 MPa. Another study conducted in a 130 kW PFBC test rig showed that N2O emissions were slightly reduced from 0.6 to 0.9 MPa[17]. However, it has been found that compared with the operating pressure,oxygen partial pressure has opposite influence on the conversion of fuel-N to NOx,while both operating pressure and oxygen partial pressure cause little effects on the fuel-N conversion to N2O[20].In both AFBC and PFBC,the excess air level plays an important role in the NOxemissions,increasing the excess air level in fluidized bed combustion will lead to a significant rise of NOxemissions[21-23].
At present, according to the author's knowledge, the relationship between nitrogen oxide emissions and fluidization number in PFBC has rarely been reported.The flow pattern and superficial gas velocity change dramatically with the fluidization number,which may have an impact on NOxand N2O emissions.An experiment conducted in an atmospheric fluidized bed showed a positive correlation between the N2O emissions and superficial velocity[14].It still needs further study on the influence of the fluidization number under pressurized conditions.
In this study,a kind of typical Chinese bituminous coal was used to study the effects of operating conditions on the emissions of NOxand N2O systematically.Operating parameters,including bed temperature,operating pressure,excess air level and fluidization number,are taken into consideration with a 15 kWthlab-scale pressurized fluidized bed combustor. The goal of the study is to find out the rule about the emission characteristics of NOxand N2O during the combustion process in a pressurized fluidized bed.
2.Experimental
2.1.Experimental setup
The investigation was performed on a 15 kWth lab-scale fluidized bed combustor, as is shown in Fig. 1. The system consists of a gas distribution system, a coal feeding system, a bubbling fluidized bed reactor, a dust removal system, a water-cooled refrigeration system and an automatic control system. The combustor is made of a stainless-steel tube with high temperature and pressure resistance.The combustor is 2600 mm in height with three sections:dense zone of 81 mm i.d.and 1000 mm height,transition zone of 600 mm height and dilute zone of 100 mm i.d.and 1000 mm height.A water-cooled heat extraction pipe in the dense zone of combustor is used to control the bed temperature.What's more,the flow rate of the cooling water inside the pipe can be adjusted to control the heat transfer and hence the bed temperature.
The air flow rate is adjusted by a mass flow meter. During all the tests,little flows of compressed air were fed into the coal hopper and the feeding pipe to avoid backfire and prevented the sand particles from entering the feeding pipe. An electric air preheater and four external electric half-cylindrical heating elements are installed to preheat the combustion air as well as the combustor during the start-up of each test.A star feeder is set up with an inverter to control the feeder motor frequency for controllable and stable coal feed rate under elevated pressures.After de-dusted by an efficient cyclone and cooled by the condenser tube,the flue gas is exhausted to the vent-pipe and some of which is extracted to be analyzed by the online gas analyzer continuously.O2,CO2,CO,NO,NO2and N2O concentrations are measured by a Madur online continuous gas analyzer(Madur,GA-21plus).Pressure differentials and temperatures across the combustor are measured by pressure gauges and K-type thermocouples respectively.The combustor is equipped with a computer system to display and record all the measured process data, including pressure differentials,temperatures and gas composition.
2.2.Experimental conditions
Before the experiment,silica sand was introduced into the combustor and then heated to 600-700°C by an air preheater and electrical heating elements. After that, a certain amount of coal and air (as shown in Table 1)was fed into the combustion zone continuously and then the electrical elements were turned off.After the introduction of coal, the fluidized bed temperature was controlled in the ranges of 800-900°C.The total pressure inside the combustor was maintained from 0.1 MPa to 0.4 MPa.The exhaust gas from the combustion process was cooled,depressed and then introduced into the gas analyzer.In this study,parameters including temperature,pressure,excess air level and fluidization number were adjusted to different values for investigating their effects on NOxand N2O emissions.All of the data,including pressure, temperature and gaseous emissions were collected online and used in this paper.
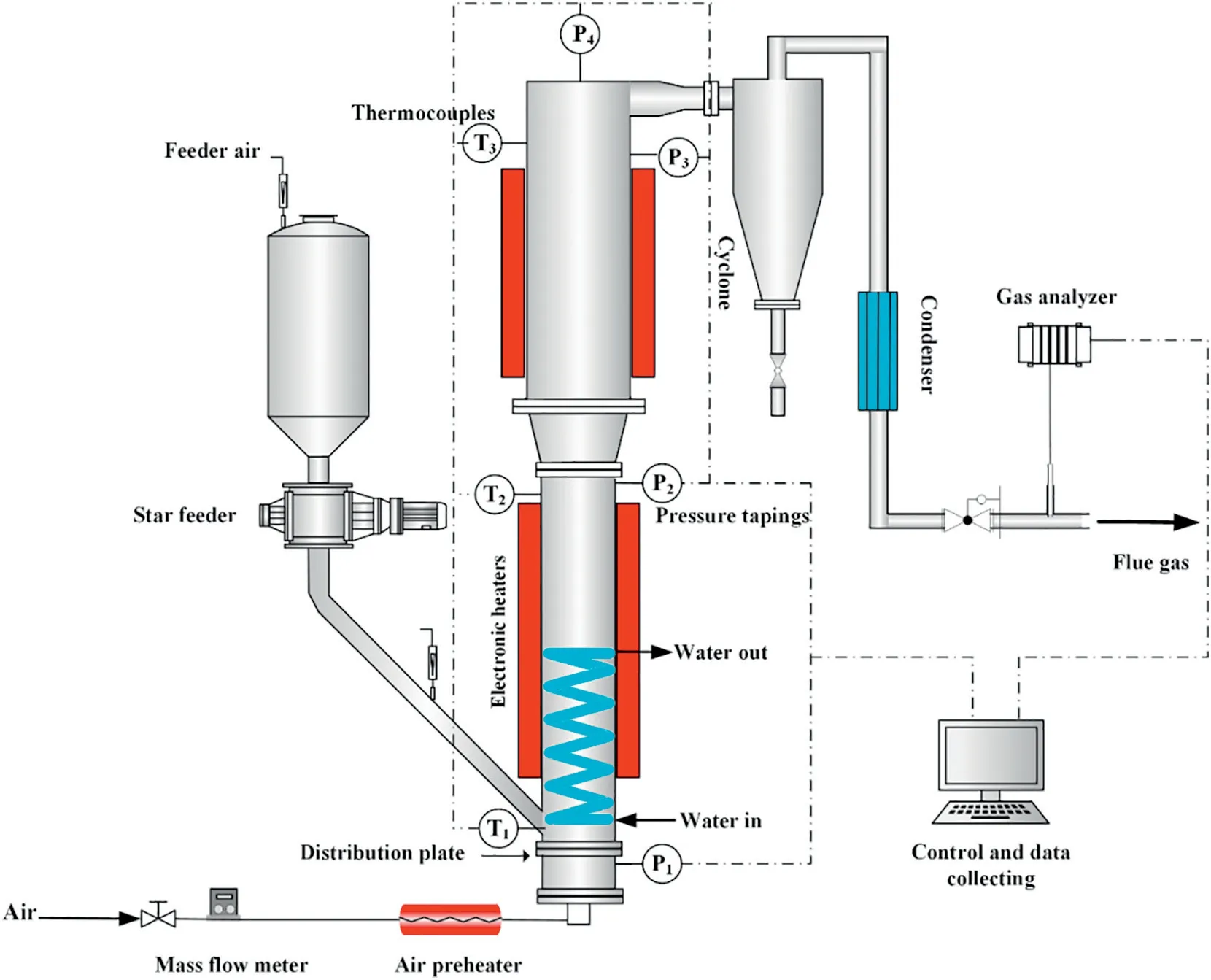
Fig.1.Schematic representation of the experimental unit for PFBC.

Table 1 List of the main experimental conditions
Fig.2 presents the temperature profiles and the gas product(O2,NOxand N2O)distribution at the outlet of the combustor from a typical experiment.All the tests were started with the atmospheric air combustion.After stabilization, the combustion environment was converted from atmospheric to pressurized combustions with continuous coal feeding.
2.3.Fuel and bed material
Table 2 shows the ultimate and proximate analysis of the typical Chinese bituminous coal used in the experiment.The particle size of bituminous coal ranges from 0.55 to 1.4 mm (true density: 1200 kg·m-3).Silica sand (particle size range: 0.38-1.7 mm, mean particle size:1.1 mm, true density: 2400 kg·m-3) was used as the bed material.Table 3 shows the chemical composition from the bituminous coal ash.
3.Results and Discussion
3.1.Effect of temperature on NOx and N2O emissions
Fig. 3 illustrates the impact of bed temperature on NOxand N2O emissions under 0.2 MPa and 0.4 MPa respectively. The bed temperature is controlled by adjusting the star feeder and the watercooling device.The overall oxygen concentration at the outlet remained 3.5%-4%in this test.The results are expressed by the amount of emissions per energy unit (mg·MJ-1).This kind of emission unit is used for better comparing the conditions of different volumetric flow rates and fuel input. The NOxemissions increase steadily with increasing bed temperature.As the bed temperature increases,firstly,the combustion efficiency increases,more char-N is released from the N site in the coal matrix to form NOxprecursors.Secondly,more active O and OH radicals are produced from molecular O2,and the precursors are easier to be oxidized to NOx. Thirdly, CO and char concentrations in the combustor decrease with the increment of bed temperature,and both the homogeneous and heterogeneous reduction reactions of NO are suppressed[25]:

The N2O emissions are strongly affected by the bed temperature in PFBC. N2O emissions decrease fast as the bed temperature rises up. Similar results were also observed by [26]. Because of the relatively low combustion temperature (800 °C-900 °C) in PFBC, the oxidation of N2in the air can be neglected, so the nitrogen in N2O is mainly from the fuel-N. HCN and NH3are the precursors of both volatile-N and char-N conversion into the nitrogen oxide.The homogeneous oxidation of HCN plays the most important role in the mechanism of homogeneous formation of N2O [27],the main elementary reactions of the conversion are as follows[28]:

Fig.2.Emissions of NOx and N2O at flexible change of the operating pressures.

Table 2 Proximate and ultimate analysis of the bituminous coal samples

As the temperature increases, more O and OH radicals were produced, the key intermediate product NCO is easier to react with these radicals,which reduces the effect of homogeneous formation of N2O[29].Meanwhile,the concentration of H radical increases as the temperature increases,the N2O is reduced by the following reaction,which has been proved to be the fastest homogeneous N2O reduction reaction[28,30,31]:

Despite the decline of N2O emissions,total nitrogen oxide emissions increase with bed temperature under pressurized conditions.
3.2.Effect of pressure on NOx and N2O emissions
Because of the relatively low combustion temperature in the fluidized bed, both prompt-NOxand thermal-NOxcan be neglected, the NOxemissions are mainly originated from the fuel-N conversion[32].In order to better investigate the source of nitrogen of NOxand N2O,two paths of conversion from fuel-N to NOxand N2O are calculated as follows:
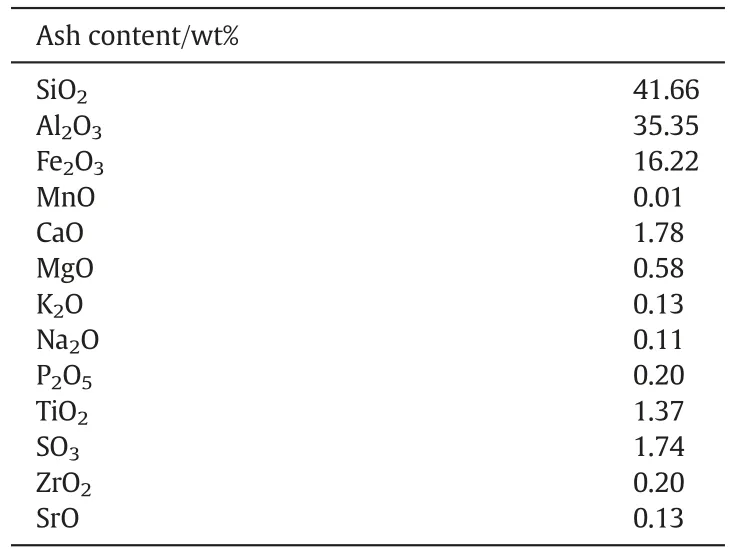
Table 3 Chemical composition of ash①

Fig.3.Effect of temperature on NOx and N2O emissions under 0.2 MPa and 0.4 MPa.

where MNis the automatic mass of nitrogen in g·mol-1,and Q is the gas flow rate.[NOx]and[N2O]are the NOxand N2O concentrations in μl·L-1.Vmis the mole volume under normal conditions(22.4 L·mol-1),mcis the mass of fuel input in g,and xNis the mass fraction of nitrogen in the fuel in wt%.
The effect of operating pressure on NOxand N2O emissions in PFBC is shown in Fig. 4 where the bed temperature is in the range of 840°C-860°C.For further investigations,we compared the conversion rate of fuel-N to NOxand N2O of our experimental results with the calculated values modeled by AHO [33] of pressurized pulverized coal combustion in an entrained flow reactor, which is shown in Figs. 5 and 6.

Fig.4.Effect of pressure on NOx emissions,T ≈850°C.

Fig.5.Comparison of calculated values(by AHO)with experimental results for fuel-N to NOx of different pressures.
As is mentioned in the Introduction, an increase in pressure can significantly reduce the NO emission.Fig.5 shows that the reduction is more remarkable when the pressure increases from 0.1 MPa to 0.2 MPa,then it declines steadily as the pressure continues to increase.Similar tendency is shown about the conversion rate of fuel-N to NO from 0.1 MPa to 0.4 MPa in Fig.6,and the experimental results match well with the calculated values.The NO partial pressure and CO partial pressure grow with the increase of total pressure,which strengthens CO reducing action for NO. Moreover, the rise of pressure increases the residence time for the diffusion of NO throughout the char particles and further reduces the amount of NO in the char particles consequently.In summary,the lower NOxemission can be explained by the creation of preferential conditions for the homogeneous and heterogeneous reduction reactions shown in Eqs.(1)and(2).
As it can be observed from Fig.5,the increase of bed pressure causes more N2O emission (from 17 mg·MJ-1to 32 mg·MJ-1).The similar dependence of conversion rate of fuel-N to N2O on operating pressure is shown in Fig.7.However,the calculated conversion rate from fuel-N to N2O(from 4%to 13%)grows significantly than that of our experimental results(from 3.5%to 6%).It can be explained by the different conditions between the pressurized entrained flow reactor and the fluidized bed when considering the particle size and particle temperature,which have been proved to play a significant role in the combustion kinetics and nitrogen chemistry[34,35].The resistance of volatiles diffusing in the coal particles accelerates with the increase of bed pressure, which results in a decrease in the volatile release [36].Therefore,more fuel-N are converted to char-N,promoting the heterogeneous generation of N2O[37,38]:
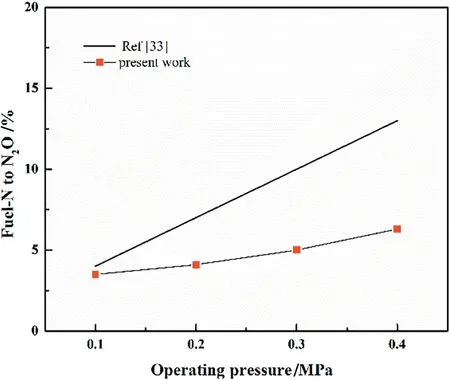
Fig.6.Comparison of calculated values(by AHO)with experimental results for fuel-N to N2O of different pressures.

Fig.7.Effect of excess air level on NOx emission,T ≈850°C.

All of these mentioned above lead to higher values for total N2O emissions.
Due to the dominant role of NOxemissions, total nitrogen oxide emissions show a decline with the operating pressure.
3.3.Effect of excess air level on NOx and N2O emissions
In this work,the excess air level can be changed by adjusting the total air flow,which is shown in Table 1.The effect of excess air level on the NOxemissions is shown in Fig. 7.The following expression is used for more detailed and direct display of the excess air level:

where[O2]is the value of the outlet oxygen concentration in vol%.As the excess air level increases from 16%to 32%,the NOxemissions for all the pressures have an obvious increase, while the dependence of NOxemissions is weaker under higher operating pressure.The reason is that as the excess air level increases,the NO formation also increases due to the oxidation of nitrogen species,such as HCN and NH3.What's more,the surface temperature of the burning coal particles is relatively lower in less O2concentration atmosphere[35,39].At the same time,both the volume and the surface area of char available for NO reduction reactions decrease,and we observed a lower CO concentration in the flue gas with the increase of O2concentration.Consequently,both the homogeneous and heterogeneous reductions of NO are suppressed,which leads to an increase of NOxemissions.
As is shown in Fig.8,the N2O emissions remain almost constant or increase slightly with the excess air level at different pressures.Redick[40]investigated the pollutant formation during coal combustion in a fluidized bed test rig and found that the formation of the H radicals of the H2/O2mechanism is significantly suppressed by the absence of oxygen. Therefore, the concentration of H radicals declines with the excess air level,which weakens the homogeneous destruction of N2O.The relatively low excess air level leads to low concentrations of O and OH radicals,which decreases the NCO formation reactions(3),(4)and(5). As a result, the drop of NCO concentrations moderates the formation reactions of N2O. This explains a slight increase of N2O emissions with the excess air level.
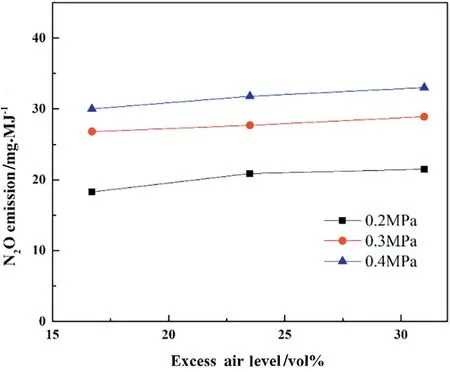
Fig.8.Effect of excess air level on N2O emission,T ≈850°C.
3.4.Effect of flow pattern on NOx and N2O emissions
In this work,the flow pattern was mainly changed by controlling the fluidization number.Excess air level was kept constant during all the tests. The critical fluidization velocity is calculated by simultaneous equations of Ergun[41],and Wen and Yu[42]:

Fig. 9 shows that both NOxand N2O emissions increase with the increasing fluidization number in PFBC,and the growth of NO emissions(from 216 mg·MJ-1to 264 mg·MJ-1)far exceeds that of N2O emissions(from 33.4 mg·MJ-1to 38.9 mg·MJ-1).The result of N2O emissions is similar with the experiment conducted in the atmospheric fluidized bed combustion by[14].Adding the fluidization number equals to an increase in the superficial gas velocity in the bed.As is shown in Table 4,the gas residence time is corresponding to the superficial gas velocity.When the gas residence time decreases,there is less time for the NO and N2O to react with the char particles,which weakens the heterogeneous reaction of NOx(as shown in Eq. (2)) and N2O on the char surfaces[37]:

Fig.9.Effect of fluidization number on N2O emission,T ≈850°C,P=0.4 MPa.

Table 4 Superficial gas velocity corresponding to the residence time in different fluidization numbers

Furthermore,due to the same oxygen concentration in the air,more oxygen is introduced to the bed at higher superficial gas velocity.As a result,the char combustion is enhanced,accelerating the heterogeneous formation of NOxand N2O from char-N.As is mentioned above,more fuel-N will be converted to char-N under elevated pressure,and NOxis mostly formed by heterogeneous oxidation of char-N during char combustion, while the N2O is mainly formed by the homogeneous reaction of HCN[43,44].This explains why NOxemissions have a larger increment with higher fluidization number.
4.Conclusions
This paper presents the results of a typical Chinese bituminous coal tested in a 15 kW·h-1lab-scale pressurized fluidized bed combustor.The effects of bed temperature, operating pressure, excess air level and fluidization number on NOxand N2O emissions were investigated systematically.Based on the cited literature and obtained experimental results,the following conclusions can be drawn:
(1) The bed temperature is quite an important operating variable in NOxemissions and the N2O emissions mainly depend on the bed temperature.An increase of NOxemissions was observed when the bed temperature rises from 800°C to 900°C,while N2O emissions decrease rapidly when the bed temperature is over 800°C and reaches a relatively low level around 900°C.(2) A drastic decrease of NOxemissions can be achieved for bituminous coal under pressurized conditions when the operating pressure increases from 0.1 MPa to 0.4 MPa. On the contrary,elevating operating pressure from 0.1 MPa to 0.4 MPa enhances the fuel-N conversion to N2O.(3) Excess air level is a dominant operating variable on NOxemissions because of its influence on CO concentration and surface temperature of coal particles.However,excess air level has little effect on the N2O emissions,only a slight increase of N2O yield was observed with the outlet O2concentration.(4) Influence of fluidization number on NOxemissions under elevated pressures cannot be neglected.The increase of fluidization number reduces the gas residence time,which weakens the heterogeneous reduction of NO and hence enhances the NOxemissions,while the N2O emissions only increase slightly with the rise of fluidization number.
Nomenclature
dpparticle diameter,m
H height,m
i.d. inner diameter,m
t time,s
ugsuperficial gas velocity,m·s-1
umfcritical fluidization velocity,m·s-1
μ gas viscosity,Pa·s
ρpparticle density,kg·m-3
ρggas density,kg·m-3
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
