Investigation of Al2O3 and Fe2O3 transmission and transformation during the decomposition of phosphogypsum☆
Jie Yang,Bin Zhu,Liping Ma*,Hongpan Liu
1 Faculty of Environmental Science and Engineering,Kunming University of Science and Technology,Kunming 650500,China
2 School of Environmental Science&Engineering,Huazhong University of Science&Technology,Wuhan 430074,China
3College of Materials and Chemical Engineering,Chongqing University of Arts and Sciences,Chongqing 402160,China
Keywords:Phosphogypsum Reductive decomposition Simulation calculations Aluminum oxide Iron oxide Ternary diagram
ABSTRACT Phosphogypsum(PG)is a solid waste produced in the phosphate fertilizer industry and is environmentally harmful.The decomposition of PG to recycle calcium and sulfur is a proper way to reutilize PG.Current work aims at enriching the basic theory of coal decomposition process of PG.The emphasis was laid on the exploration of impact of main impurities on the process.On the other hand,according to Reaction Module,Equilib Module,and Phase Diagram Module of FactSage,the simulation computation was done on the systems of pure gypsum mixed with coal,with or without impurities for avoiding other impurities interference.Later,possible reactions in the process were deduced.Additionally,experiments were conducted in a TG-DTA integrated thermal gravimetric analyzer and a tube furnace.The products from the experiments were characterized and analyzed to verify the accuracy of theoretical calculations.The results showed that these impurities can change the decomposition process of PG.For example,aluminum oxide was transformed to calcium sulfoaluminate,while iron oxide was transformed to dicalcium ferrite.Furthermore,the results help to further improve the basic theory of phosphogypsum decomposition.
1.Introduction
Phosphogypsum(PG)is composed primarily of calcium sulfate dehydrate,and is a sort of industrial slag which results as a by-product during the production of phosphoric acid. Its low utilization rate,large-tonnage output,and environmental harm hinders the development of the phosphate fertilizer industry.However,it is an important source of calcium and sulfur, and has been used in the production of construction materials, chemical agents and the amendment of soils[1-4].
There are many ways to recycle phosphogypsum,including direct and indirect utilization.The direct way mainly contains removing the impurities of phosphogypsum to prepare CasO4whisker,using as additive for dehydrating agent in sludge,applying for additive of cement and so on[5-7].And the indirect utilization is fully applied each element in of CaSO4in is phosphogypsum based on thermal decomposition.For example,Yang discovered that phosphogypsum can be used oxygen carrier in chemical looping gasification with lignite to prepare high added value material syngas[8-10].Zhao used the product of phosphogypsum as the material of Ca-looping for CO2capture[11-13].And,Zheng prepared CaO by changing the atmosphere of phosphogypsum thermal decomposition[14,15].Additionally,during the phosphogypsum thermal decomposition process, H2S and SO2probably be released, and they are the raw materials for preparing sulfur and sulfuric acid.Thus,according to the way of recycling phosphogypsum,thermal decomposition is a recommended method.
FactSage is one of the largest fully integrated database computing systems in the world,and has been widely used in various fields,including material science, metallurgy, electricity, glass industry, combustion,ceramics,and geology[16-19].Additionally,it can be used for predicting multiphase equilibrium,proportions of liquid and solid phases,phase transitions in a specified atmosphere and temperature for the heterogeneous slag systems[20,21].The group of Lawrence P.Belo tested pure salts to allow for the identification of species in ash which capture sulfur[22].The group also carried out thermodynamic modeling of the process using FactSage[22].Recently,FactSage Thermodynamics Model was used to understand the ash fusion behavior and to predict the phase transformations occurring during the process of coal combustion[23].
In order to explore the decomposition of PG,it has conducted significant research work on the effective decomposition of PG in different atmospheres with or without additives[24-31].Furthermore,PG's impurities,such as SiO2,P2O5,Al2O3,and Fe2O3can reduce the decomposition temperature to some extent [32]. In order to improve the basic theory,research work on many impurities in PG, such as SiO2,Al2O3and Fe2O3was needed to be included.In previous studies,it has confirmed that silicon dioxide can change the decomposition process of PG and is finally transformed to Ca3SiO5[33].Therefore,in the present work,the impact of Al2O3and Fe2O3on the reductive decomposition of PG has been discussed, and is combined with several modules of FactSage6.4 and laboratory experiments.
2.Experimental
2.1.Sample preparation
The PG sample was procured from Yunnan Natural Gas and Chemical Engineering Company, Yunnan, China. Coal was also produced in Yunnan,China(see Table 1).According to the study of Zheng Shaocong[34],the particle size of coal and PG were selected to be 109-120 μm(120-140 mesh).
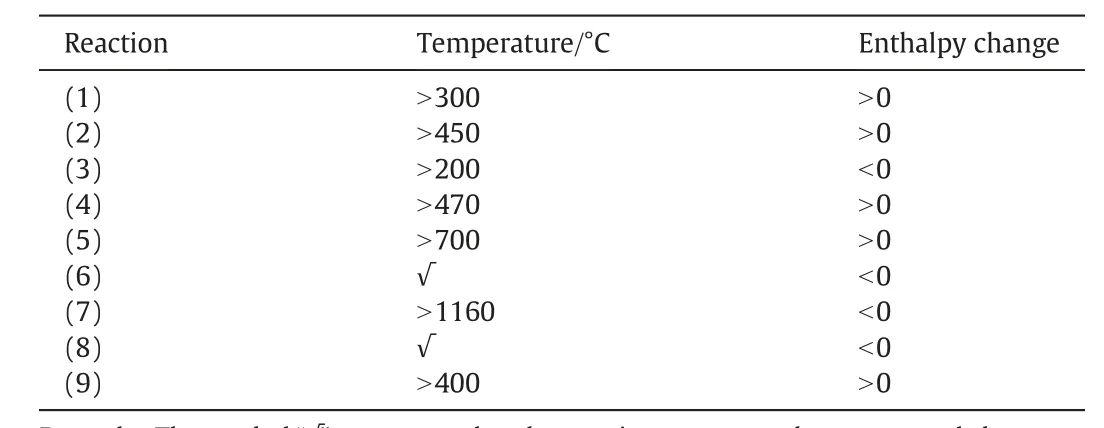
Table 1 The thermodynamic parameters of reactions involved in the decomposition process of PG
2.2.Experimental setup and procedure
2.2.1.Theoretical experiments
The Reaction Module,Equilib Module and Phase Diagram Module of FactSage were used to deduce the possible reactions as well as the characteristics in one system under a pressure of 1×105Pa.2.2.2.Laboratory experiments
Pure gypsum has been used as the raw material instead of PG for avoiding the unnecessary interference by other impurities. Both PG and coal had a mass ratio of 10:1,respectively[34].Experiments were conducted in a TG-DTA integrated thermal gravimetric analyzer with nitrogen flowing at 25 mg·min-1from ambient temperature to 1400 °C at 10 °C·min-1. The experiments were also carried out in a tube furnace with a flow of nitrogen at 100 ml·min-1from room temperature to 1400°C at 10°C·min-1.In addition,during the course of experiments,gas samples were analyzed continuously using a KM9106 gas analyzers.Furthermore,the slag was characterized using a D/Max 2200 X-ray diffractometer.
3.Results and Discussion
3.1.Reductive decomposition of phosphogypsum
3.1.1.Theoretical analysis
The Reaction Module of FactSage was used to figure out some of the key parameters of the possible reactions of pure phosphogypsum(without impurities)during the decomposition process.The reactions are represented by Reactions(1)-(9).
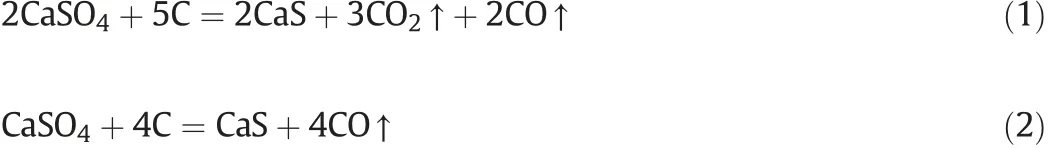

The results obtained about the reaction of PG with coal conformed to the ones already obtained in a previous study[35].And the initial temperature for the reactions taking place is listed in Table 1. CO, S2and S8are the products of the reaction process.FactSage was used to calculate the thermodynamic properties, which showed that theoretically, all reactions were spontaneous. In addition, Reaction (6)and Reaction (9) will happen, no matter what the temperature is.(See Table 2).

Table 2 Analysis of the coal used in the current work
CaS, CaO, and CaCO3are produced through reaction of PG with carbon(C).Besides,CaS can be obtained by Reactions(6),(8)and(9).Additionally,CaO can be obtained via Reaction(7)only when the temperature is higher than 1160°C.During these reactions,CaSO4is obtained through Reaction (9). The analysis showed that during the decomposition of PG,coal acts as a reductant,while the decomposition process mainly takes place via solid-solid and gas-solid reactions.
3.1.2.Laboratory experiments
Figs.1 and 2 show results when the temperature was changed from 70°C to 145°C.Due to the removal of crystallization water of phosphogypsum and the outer moisture of coal, there was a mass loss in the system.Additionally,the process showed an endothermic effect.
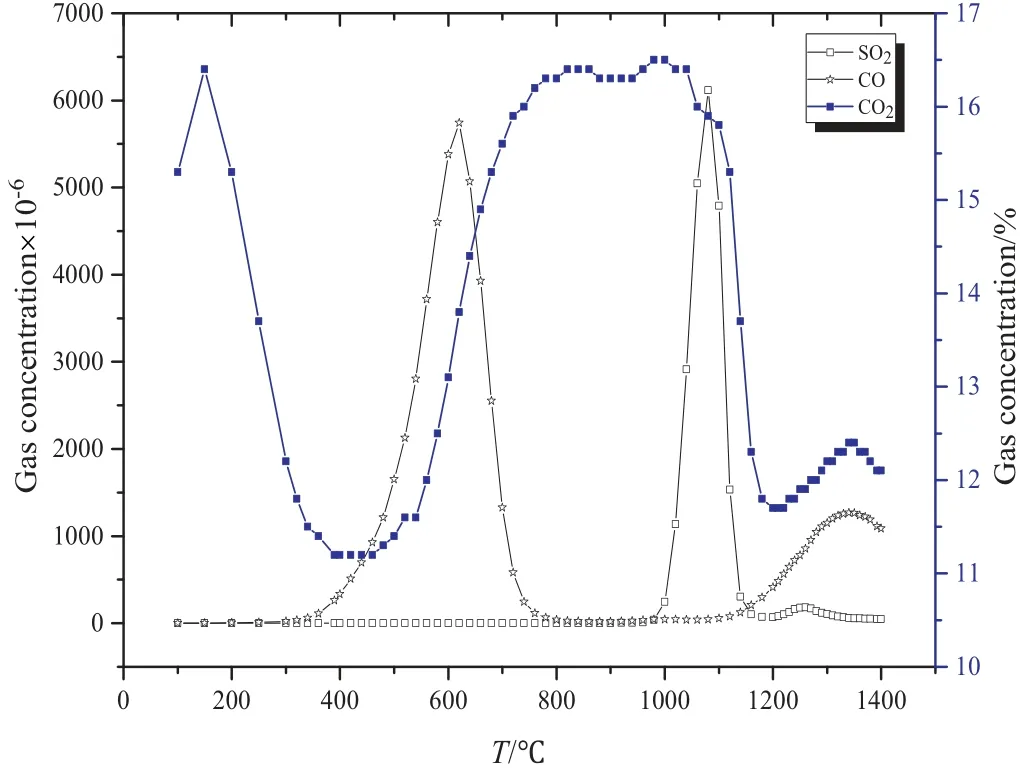
Fig.1.TG-DTA curves of PG mixed with coal under nitrogen atmosphere.
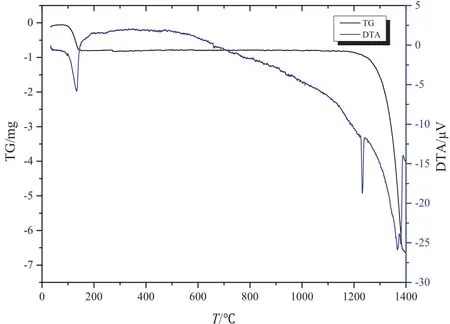
Fig.2.Gas components of PG mixed with coal under nitrogen atmosphere.
Furthermore,when the temperature was increased from 450°C to 660°C,a mass loss and an exothermic process took place.The concentrations of both CO and CO2increased with the increase in temperature,while some SO2was also produced during the process.It is worth mentioning that CO was generated due to the pyrolysis of coal,while CO2was yielded by Reactions (1),(2),and(4). Since the temperature of Reaction(5)is higher than 700°C,therefore Reaction(5)did not take place.When the temperature was increased from 660 °C to 1000°C,no obvious mass loss was observed.For the gas products,CO decreased sharply while CO2maintained a high level of concentration.It is to be noticed that Reaction(6)may happen under these conditions,while the decrease in CO was due to the cessation of pyrolysis of coal.As the temperature was increased to 1000°C,the peak of SO2was observed at 1080°C,which decreased to 0 at 1200°C.SO2was generated above 1080°C.In addition,there was no obvious mass loss or sharp decrease in CO2concentration. These results show that Reactions (7) and (8)were the most likely reactions which may take place at this stage.These results are supported by the solid products,which are confirmed through characteristic peaks of CaS and CaO(as shown in Fig.3).When the temperature was higher than 1170°C,there was a significant mass loss and an endothermic effect was also observed. At 1217 °C, the endothermic effect can be seen, which was due to the polymorphism transformation of calcium sulfate. This suggested that some calcium sulfate still existed under these conditions. When the temperature was increased up to 1280 °C, a mass lose and the endothermic effects were observed. In the flue gas, CO and CO2increased. SO2also increased however at a low level, which eventually stopped at 1280 °C. Reaction (1) took place and produced CaS (as seen from Fig. 3).
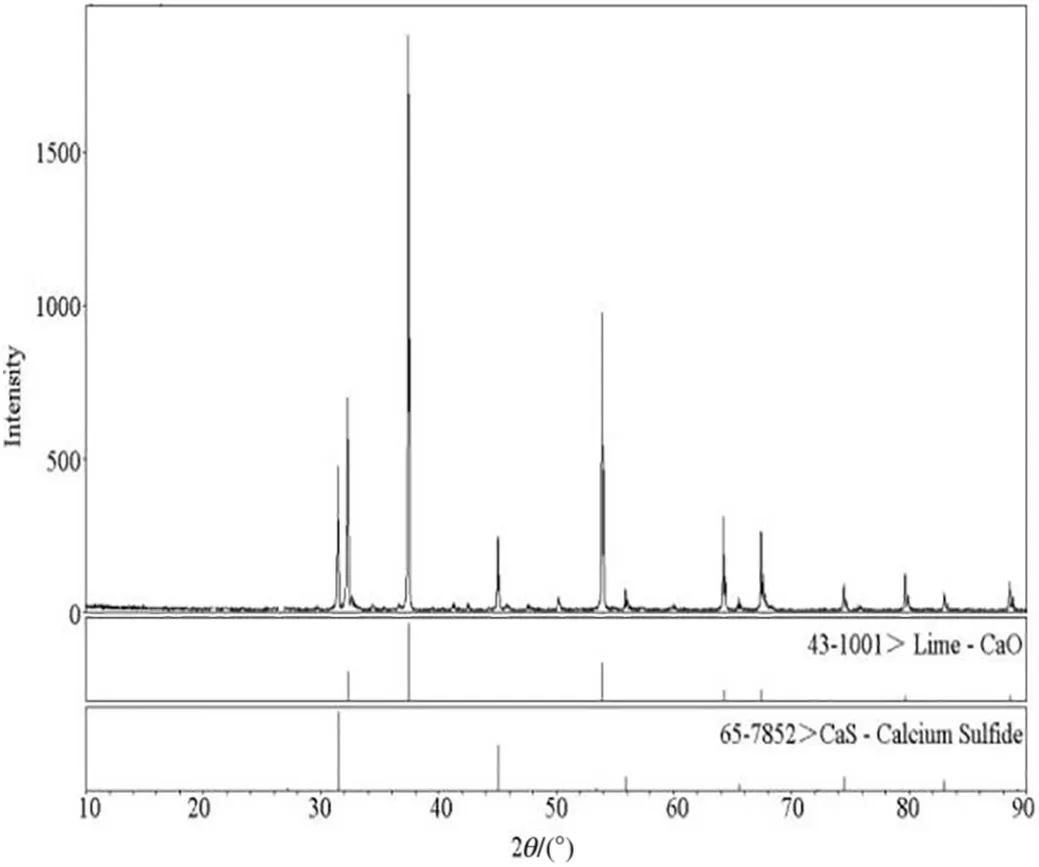
Fig.3.XRD pattern of the residue from the decomposition process of PG.
The XRD pattern of the slag is shown in Fig. 3. The highest decomposition product was CaO, while CaS also existed in the product. As discussed above, CaO was mainly generated through Reactions (7) and (4), while CaS was produced through Reactions(1)-(3), and Reaction (6). Additionally, the results obtained from the experiments were found to be consistent with the theoretical analysis.
3.2.Effect of Al2O3 on decomposition
3.2.1.Theoretical analysis
Fig.4 shows that as the mass ratio of CaSO4:C:Al2O3was increased from 15:1:0 to 10:1:5,the mass of Al2O3also increased.Additionally,the product of Al2O3transformed to Ca3Al2O6and CaAl2O4, due to which some extra amount was added to the system. Furthermore,with the help of Equilib Module, some intermediate products were observed in the process.The following reactions were deduced as the possible reactions when pure gypsum was mixed with coal and Al2O3(See Tables 3).
3.2.2.Laboratory experiments
As shown in Figs. 5 and 6, some big difference between the processes carried out with and without Al2O3.The XRD results (Fig. 7)show that the main ingredient in PG's decomposition product was calcium sulphoaluminate (Ca4Al6O12(SO4)), however no calcium sulfate, calcium oxide or calcium sulfide characteristic peaks were observed in the residue. So, Al2O3changed the decomposition route of CaSO4, which is in good agreement with the theoretical results. However, there was some difference between the experimental and theoretical analyses. One of the differences is that sulphoaluminate was present in the experiments, whereas Ca3Al2O6and CaAl2O4were present in the theory. It was due to the consideration of thermodynamics for the reaction of CaSO4with coal in the theoretical analysis, while several other factors had a combined effect in the experiment.
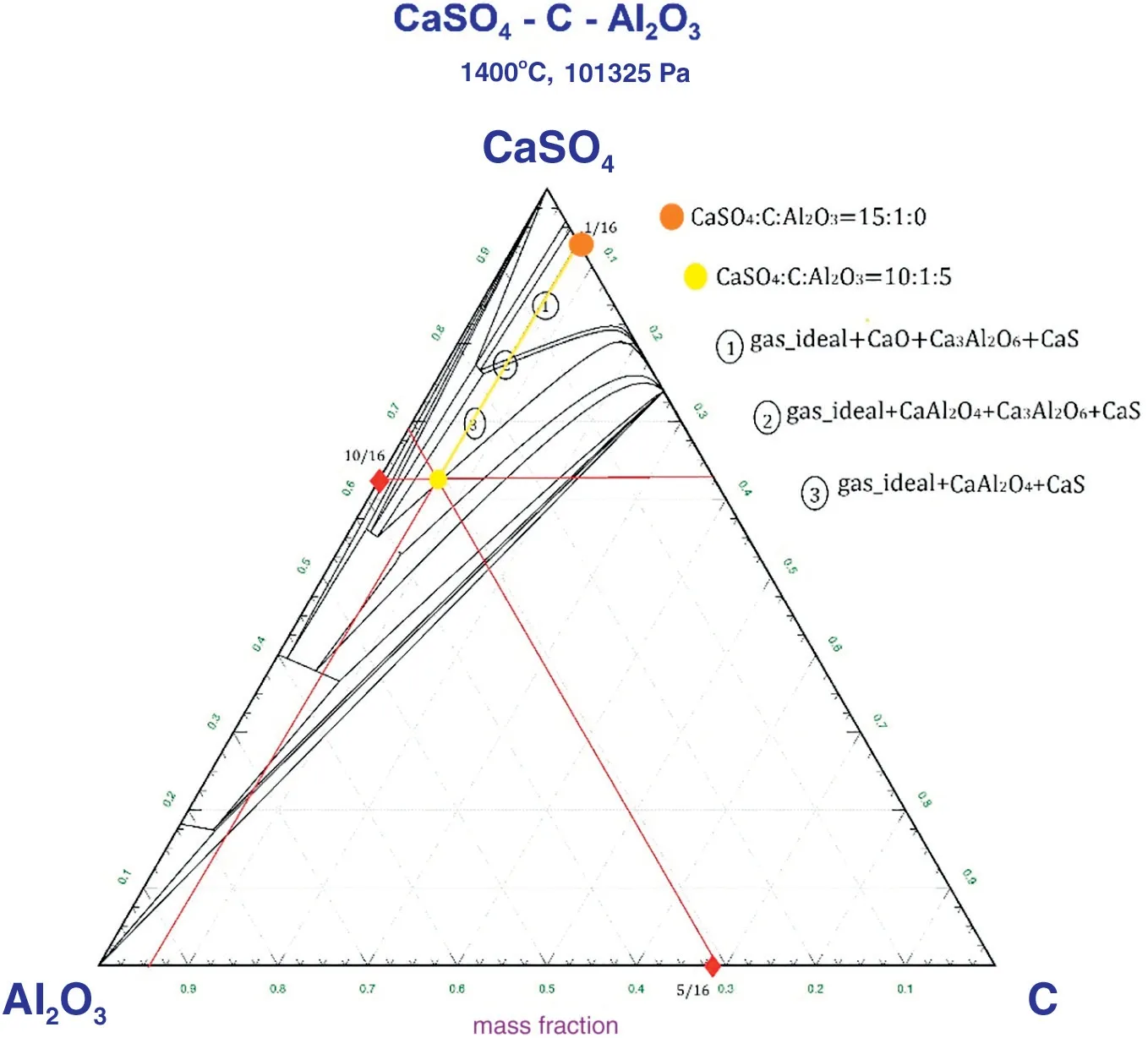
Fig.4.Phase diagram of CaSO4-C-Al2O3 at 1400°C.

Table 3 Thermodynamic parameters of the reactions involved in the decomposition process of PG with Al2O3

Fig.5.TG-DTA curves of gypsum and coal with the addition of Al2O3.

Fig.6.Gas components of gypsum mixed with coal with the addition of Al2O3.
After adding Al2O3,the temperature during the big mass loss of PG was decreased from 1170°C to 1100°C.The temperature drop slowed down after 1360°C.Fig.6 shows that when the temperature was increased from 1270°C to 1350°C,an obvious endothermic peak was observed.As for the CO2,its concentration was always kept at a very high value within 760-1100 °C. Subsequently, it began to decline. When the temperature reached 1180°C,the concentration of CO2began to slowly rise again.For the concentration of SO2,there were two peaks at 1060 °C and 1270 °C. The endothermic peak was caused by the calcium sulfate-type polycrystalline transformation at 1220°C,which suggested that a significant amount of calcium sulfate cannot be decomposed at this temperature.According to the reduction decomposition of pure gypsum(as shown in Fig.6),small amount of CO was produced,while Reaction(2)was the main reaction in this process.The production and consumption of CaS was mainly via Reactions(3)and(7).The production of CaS was accompanied by an endothermic phenomenon and the formation of CO2. However, for consumption of CaS, the concentration of SO2increased along with a heat absorption phenomenon.Additionally, Reaction (7) has a much higher molar enthalpy than Reaction (3). In other words, when the temperature was high, as long as CaS existed, it would easily be transformed into CaO via Reaction (7). In addition, the transformation was accompanied by endothermic phenomenon along with the production of CO2and SO2.After 1270 °C, Reactions (7) and (3) cannot happen, which meant that CaS did not exist in the solid phase.When the temperature ranged from 1270 °C to 1360 °C, SO2was decreased, while there was almost no CO present in the system.However, the amount of CO2slightly increased. It can be seen that Reaction (4) still took place. At higher temperatures, the decrease in temperature slowed down significantly and no gas-release was observed.

Fig. 7. XRD pattern of residue from the decomposition of gypsum and coal with the addition of Al2O3.

Table 4 Thermodynamic parameters of reactions involved in the decomposition process of PG with Fe2O3
The experiment carried out by Li showed that calcium sulphoaluminate can usually be generated through two kinds of reaction[35],which can be represented by following reaction equations.
The product of calcarea carbonica was mainly formed through Reaction(5).Due to the same ratio of raw materials for Reactions(4)and(5),the enthalpy of Reaction(4)is much smaller than Reaction(5)at the same temperature.Thus,calcium sulfate is more likely to react with coke to generate CaO(Reaction(4))instead of CaCO3.As shown above,the product of calcium sulphoaluminate was mainly produced through Reaction (15). In addition, before the generation of calcium sulphoaluminate,the molar ratio of calcium oxide and calcium sulfate in the sample was 3:1,respectively.
3.3.Effect of Fe2O3 on decomposition
3.3.1.Theoretical analysis
The ternary phase diagram of CaSO4-C-Fe2O3showed that when the mass ratio of CaSO4:C:Fe2O3was increased from 15:1:0 to 10:1:5,the mass of Fe2O3increased. Additionally, the products of FeS, Ca2Fe2O5and FeS were obtained.With the help of Equilib Module,some intermediate products(FeS2and Fe3O4)were observed under these conditions.The following reactions were deduced as the possible reactions taking place in the system in which pure gypsum was mixed with coal along with Fe2O3(See Table 4).


Fig.8.Phase diagram of CaSO4-C-Fe2O3 at 1400°C.

Fig.9.TG-DTA curves of gypsum and coal with the addition of Fe2O3.

3.3.2.Laboratory experiments
Within the temperature range of 1080-1219 °C, a significant mass loss was observed. However, the thermal effect was not obvious during the process. Besides, there was no CO or SO2released in this case. As a result, both endothermic and exothermic reactions existed simultaneously, which could offset the heat effect. Furthermore, Reactions (4), (5), (6) and (16) conformed to above phenomenon. Among these reactions, only the Reactions (6) and (16)were exothermic. Nevertheless, the occurrence of Reaction (6)was highly dependent on the presence of CO. If the concentration was less than 11 × 10-6, Reaction (6) was hard to happen. It means that Reaction (16) must happen under such a condition.However, Reaction (16) needed elemental sulfur as a reactant,and therefore either Reaction (4) or Reaction (5) was bound to happen. It is worth noticing that Reactions (17)-(19) would consume CaCO3. In comparison, Reaction(5) was more likely to occur than Reaction (4) due to the fact that the free energy of Reaction(5) was more than that of Reaction (5) under the same temperature. At 1219°C,the endothermic peak was due to the polycrystalline transition of calcium sulfate. When the temperature ranged from 1236 °C to 1264 °C, there was an endothermic peak and a big mass loss,while no CO was generated.A small amount of SO2was also produced. These results showed that Reaction (17)was the main reaction in this case.Within the temperature range of 1280-1314 °C, an endothermic peak occurred, which was due to Reaction(18). From 1340 °C to 1350 °C, an endothermic peak was observed,which was mainly due to Reaction(19).These results are in accordance with the increase in concentrations of CO and SO2.(See Figs.8-11).
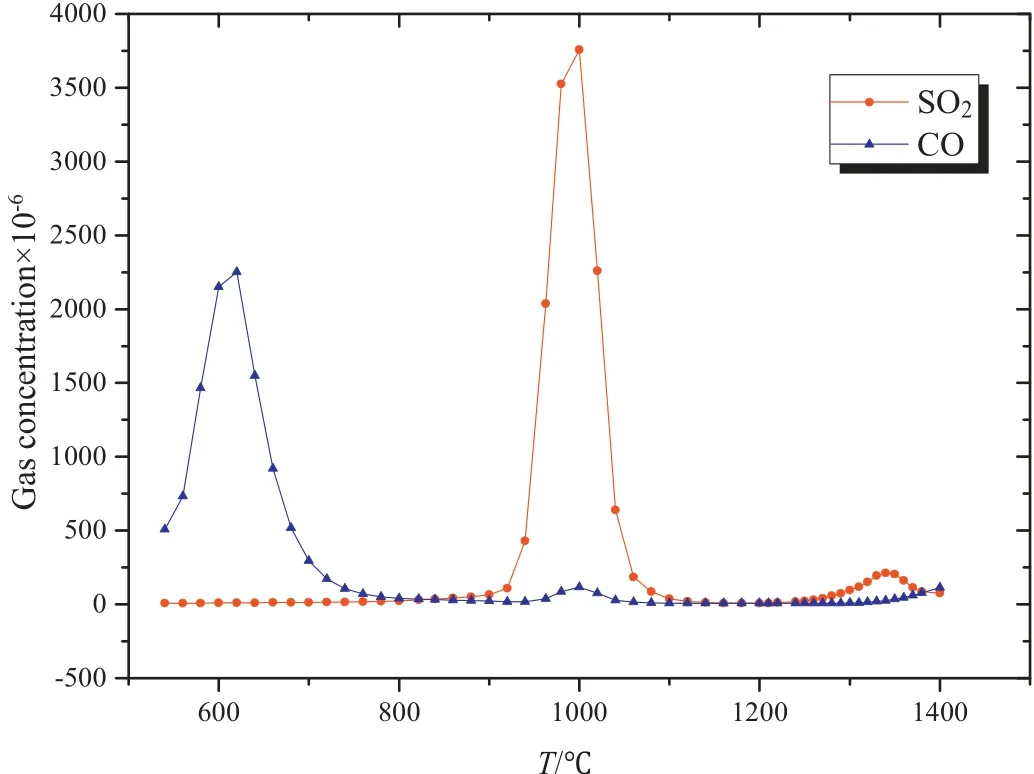
Fig.10.Gas components of gypsum mixed with coal with the addition of Fe2O3.
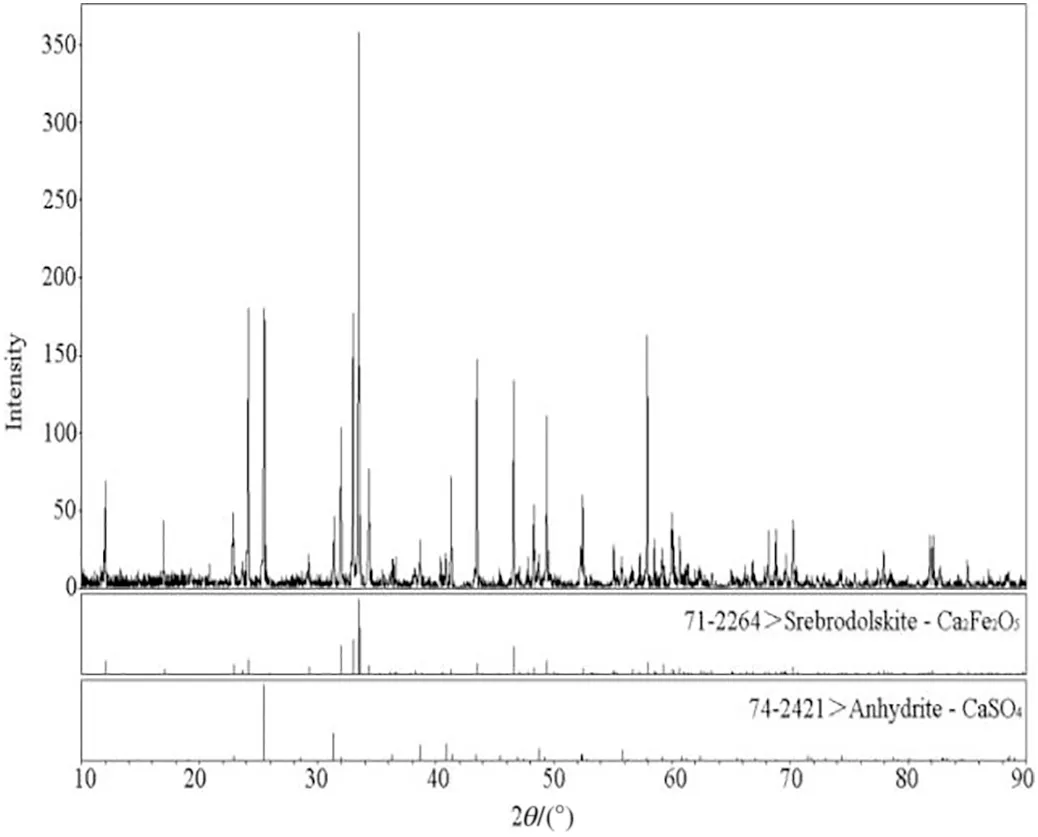
Fig.11.XRD pattern of residue from the decomposition process of gypsum and coal with the addition of Fe2O3.
Based on the above discussion, it can be concluded that experiments confirmed the theoretical analysis and on the whole, the effect of Fe2O3on the PG decomposition were successfully demonstrated.
4.Conclusions
The effects of alumina and iron oxide on the decomposition of phosphogypsum were studied using FactSage and laboratory experiments. Based upon the results, the main conclusions are as follows: when alumina was added, it would reduce the temperature of PG decomposition. A big difference between the actual experiments and theoretical analysis was that no intermediate product was observed in the laboratory experiments. And, when the temperature was below 1400 °C, alumina would react with CaO (the product of PG) to form calcium sulphoaluminate (Ca4Al6O12(SO4)). Additionally, Then, when Fe2O3was added in the reaction,calcium sulfate would react via Reaction (7) to product intermediate product. Calcium carbonate is eventually transformed to Ca2Fe2O5through Reaction (19), which decreases the decomposition temperature of calcium sulfide.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
