Selective synthesis of triacetin from glycerol catalyzed by HZSM-5/MCM-41 micro/mesoporous molecular sieve☆
Jiangyong Liu*,Zihao WangYunlin SunRuiqi Jian,Panming JianDan Wang*
1 School of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou,225002,China
2 School of Medicine,Stanford University,Stanford,CA 94304,USA
3 State Key Laboratory of Organic-Inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
Keywords:Mesoscale Porous materials Molecular sieve Triacetin Glycerol esterification
ABSTRACT
1.Introduction
Glycerol,the main byproduct in the biodiesel,has resulted in a drastic surplus in the chemical market due to the ever-increasing production and demand of biodiesel[1-4].Green chemical engineering including the design of chemical processes [5] and efficient catalysts [6] for green product and green process, has been regarded as one of the most efficient means of achieving sustainable development in chemical industry[7].To date,extensive research efforts have been focused on the methods to transform glycerol into high value-added chemicals[8-11].Among them,the acid-catalyzed esterification of glycerol with acetic acid (EGAA) for glycerol conversion into monoacetyl glycerol(monoacetin, MAG), diacetyl glycerol (diacetin, DAG) and triacetyl glycerol (triacetin, TAG) has aroused much attention both in the academic community and industrial field(Fig.1)[1,2,11-13].
Although various catalysts,especially the heterogeneous acid catalysts, have been applied in the EGAA reaction, the low selectivity of the desired products(DAG and TAG)remains the greatest challenge in this reaction[2,14].In addition,the recovery of the key derivatives is complicated since the products have comparable boiling points[15].
Among the three glycerol esters, TAG, as an important fuel additive,antimicrobial and emulsifying agent in pharmaceuticals and cigarette filters[16],however,is the most difficult to produce[1,17].Microporous molecular sieves with unique pore structure,acidity feature and high stability [18-20], were employed in the EGAA reaction [21,22].However,the reaction performances of these catalysts were very poor with the selectivity of TAG generally lower than 30%, which can be attributed to the diffusion difficulty of the acetylated esters within the microporous channels. Even loading the ultra-stable Y zeolite with dodecamolybdophosphoric acid, the result was still unsatisfactory[23].Although the mesoporous molecular sieves with well-defined mesoporous structure,large pore size and high surface area[24-26],they were usually used as the catalyst supports in the EGAA reaction due to the lacking of necessary acidic sites.For example,12-tungstophosphoric acid/MCM-41[27],molybdophosphoric acid/SBA-15[28],and SO3H-SBA-15[29] have showed improved catalytic performance as compared with the microporous molecular sieves. However, these catalysts cannot achieve a high catalytic activity and TAG selectivity at the same time.
In this article,we report the synthesis of well-defined HZSM-5/MCM-41 micro/mesoporous molecular sieve(H-ZM) composites which can serve as efficient catalyst for the selective synthesis of TAG via the EGAA process. The H-ZM catalysts combine the advantages of HZSM-5 and MCM-41,providing suitable acidic property,excellent diffusion efficiency and good stability,resulting in high catalytic performance.The effects of influencing factors including reaction time, catalyst amount and n(AcOH)/n(glycerol)on the catalytic performance were systematically investigated.

Fig.1.Reaction scheme for the catalytic esterification of glycerol with acetic acid.
2.Experimental
2.1.Preparation of the catalysts
All the reagents were used as received without further purification.In a typical experiment,2.0 g ZSM-5 powder and 15 ml NaOH aqueous solution(0.5 mol·L-1)were added to a 100 ml beaker under stirring for 1 h. Then 15 ml Na2SiO3solution (0.25 mol·L-1) and 17 g Cetyltrimethylammonium bromide (CTAB) solution (5.9 wt%) were added to the suspension with stirring for another 1 h.Thereafter,the suspension was transferred into a Teflon-lined stainless-steel autoclave and heated at 120°C for 48 h.After cooling to room temperature,the pH of the reaction mixture was adjusted to 8.5 with l.0 mol·L-1sulfuric acid solution.Afterwards,the mixture was allowed for crystallization with another 24 h.After crystallization,the sample was obtained by filtration and washed thoroughly with deionized water and ethanol.The solid product was dried at 100 °C for 6 h and calcined at 550 °C for 5 h, and thus the ZSM-5/MCM-41 (ZM) composite molecular sieve was obtained. For the synthesis of proton-type HZSM-5/MCM-41(H-ZM)composite molecular sieve,4.5 g ZM sample was firstly added to 100 ml hydrochloric acid(0.1 mol·L-1)in a 250 ml beaker,and the ion-exchange process was conducted for 8 h.And then the suspension was filtrated and washed with deionized water until the pH is neutral.The obtained product was then dried at 100°C for 6 h before subjecting it for calcination at 550 °C for 2 h. The ion-exchange process was repeated for three times,and then the H-ZM micro/mesoporous molecular sieve was finally obtained.

Fig.2.Small-angle XRD patterns(a),wide-angle XRD patterns(b)and N2 adsorption-desorption isotherms(c)of the samples.
2.2.Characterizations
The powder X-ray diffraction(XRD)was recorded on a Bruker D8 Advance diffractometer using Cu Kαradiation (λ = 0.154056 nm).The Fourier-transform infrared(FT-IR)spectra of the samples within the spectral range 4000-400 cm-1were obtained on a Bruker Tensor 27 spectrometer.The morphology and microstructure of the samples were investigated with scanning electron microscope(SEM,Hitachi S-4800)and transmission electron microscope(TEM,Philip Tecnai 12).The N2isothermal adsorption-desorption measurement was conducted with a Micromeritics ASAP 2020 system. Prior to the test, the samples were outgassed at 200°C for 5 h.The surface area of the samples was determined by the Brunauer-Emmett-Teller(BET)method.The mesopore volume was calculated at a relative pressure(P/P0)of about 0.99,where P and P0are the measured and equilibrium pressures,respectively.The mesopore size was measured with the Barrett-Joyner-Halenda(BJH)method.In addition,the micropore size was calculated by the Horvath-Kawazoe(HK)method,and the micropore volume was measured with the t-plot method.The NH3-TPD experiment was performed on a Finetec FINESORB-3010 apparatus equipped with a thermal conductivity detector.Prior to the NH3adsorption,the samples were pretreated at 200°C for 2 h in a flow of Ar and then cooled down to 100°C.Afterwards,ammonia was introduced and maintained for 1 h.Finally,the desorption step was conducted in Ar with a heating rate of 10°C·min-1.
2.3.Catalytic analysis of the H-ZM
For the selective synthesis of TAG from the EGAA process,in a typical process, the catalyst, glycerol and acetic acid were added to a threenecked, round-bottomed flask quipped with a mechanical stirrer, a thermometer and a distillation column.During the reaction,the formed water was constantly removed to positively shift the chemical equilibrium.The esterification process was monitored by analyzing the product with gas chromatography using a flame ionization detector(FID)equipped with a SE-30 capillary column.
3.Results and Discussions

Fig. 3. Representative SEM image (a) and TEM image (b) of H-ZM, and the NH3-TPD profiles(c)of the samples.
Fig. 2a shows the small-angle XRD patterns of the ZM and H-ZM samples.It can be observed that similar to the characteristic diffraction peaks of MCM-41, a strong diffraction peak at about 2.1°, and three lower intensity diffraction peaks at the higher diffraction angles present for both of the samples.These diffraction peaks correspond to the(100),(110),(200)and(210)crystal planes,indicating that the samples have a highly ordered hexagonal symmetry structure[30].The wide-angle XRD patterns of ZM and H-ZM(Fig.2(b))show that the samples exhibit characteristic diffraction peaks of ZSM-5,but the peak intensities of the composite molecular sieves are lower than that of the pure ZSM-5 sample.This suggests that the ZM and H-ZM samples are composite materials with both the mesoporous structure of MCM-41 and microporous structure of ZSM-5.Additional information about the composite structure of ZM and H-ZM can be reflected from the FT-IR spectra of the samples(Fig.S1),where the band at about 550 cm-1attributed to the asymmetric stretching vibration of SiO4tetrahedra is an important feature of ZSM-5 as a typical MFI type zeolite[31].The pure MCM-41,however,has no band at 550 cm-1,indicating that the soluble aluminosilicate species containing the fragments of the ZSM-5 structure had finally entered the mesoporous structure of MCM-41 in ZM and H-ZM.The N2adsorption-desorption isotherms of ZM and H-ZM(Fig.2(c)and Table 1)show that the samples present fast adsorption at P/P0of 0-0.2,and have a small hysteresis loop at P/P0of 0.32-0.42 and a larger one at higher P/P0, which is another evidence of the micro/mesoporous structure of ZM and H-ZM and is in accordance with the XRD and FT-IR results.
The SEM image of H-ZM(Fig.3(a))shows that the sample is composed of uniform irregular aggregates with the size of about 100 nm,and no typical morphology of ZSM-5 zeolite can be observed,which further confirms that the H-ZM sample is a composite molecular sieve but not a simple mechanical mixture of ZSM-5 and MCM-41. The TEM image of H-ZM(Fig.3(b))also illustrates a highly ordered mesopore organization of the sample,and is in line with the small-angle XRD result.The acidic property of ZM and H-ZM were investigated by the NH3-TPD experiment(Fig.3(c)).The NH3-TPD profile of ZM shows only one peak,corresponding to the desorption of NH3from the weak acidic sites,while the intensity of this peak is significantly improved in H-ZM.Inaddition,another desorption peak centered at about 500°C ascribed to the strong acidic sites appears in H-ZM,indicating more and stronger acidic sites were generated for H-ZM after the ion-exchange process of ZM.
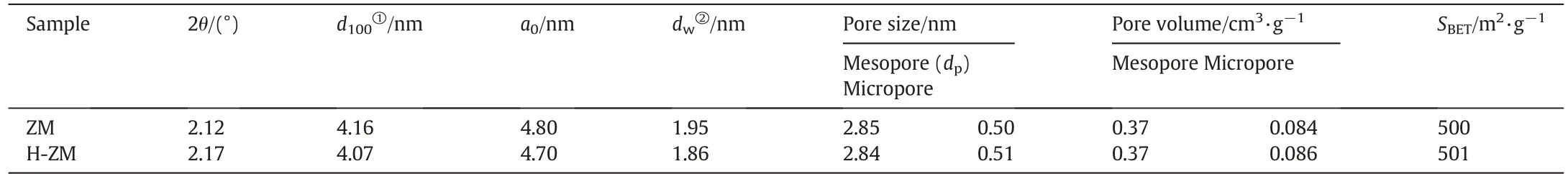
Table 1 Texture parameters of the samples
The acidic H-ZM catalyst was employed for the selective synthesis of TAG from the EGAA process,and the effects of influencing factors including reaction time, catalyst amount and n(AcOH)/n(glycerol) on the catalytic performance were systematically investigated.In the absence of catalyst, although the conversion of glycerol reached 47.7%after 24 h,the undesired MAG accounted for 82.1%of the product and only very little of TAG was generated(Table 2).Fig.4a shows the effect of reaction time on the catalytic performance,suggesting that the glycerol conversion reached 93%within 2 h and 100%in 10 h.For the glycerol esterification process, acetic acid could selectively attach any hydroxyl groups (—OH) in the glycerol backbone or any--OH from the partially reacted glycerides.At the initial stage,glycerol was converted mostly to MAG and DAG.As the reaction progressed,MAG and DAG were gradually transformed into TAG (Fig. S2) that is the mostdifficult to produce in the three-step reaction of glycerol esterification due to the steric hindrance effect[32].The TAG selectivity reached as high as 88.9%in 32 h and 91.3%in 48 h with 100%glycerol conversion,which is much better than the recent reports with the TAG selectivity of 71% (Keggin-type heteropolyacid catalysts) [12], 42% (Fe4(SiW12O40)3catalyst)[33],57%(Sulphonated hydrothermal carbon catalyst)[34]and 29.2%(SO42-/γ-Al2O3catalyst)[35].
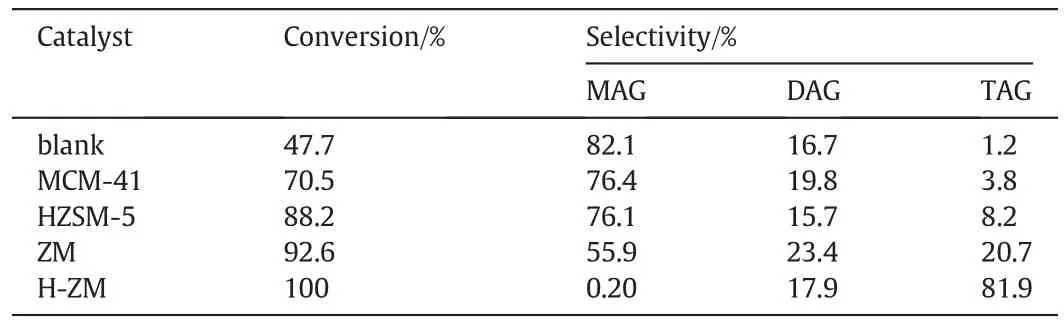
Table 2 Comparison of the catalytic performance of different catalysts①
Previous studies show that the key role of solid acid catalysts in the EGAA process depends on the acidity,texture and surface morphology of the catalyst[12,28,36,37].In addition,the diffusion efficiency of the reactants and products is also of vital importance for the high rate of glycerol conversion and selective formation of DAG and TAG as both products, especially the latter, which is space demanding for the diffusion within the catalyst pores[2,21,38].Table 2 lists the catalytic performances of the MCM-41,HZSM-5 and ZM for comparison,which can well confirm the importance of catalyst acidity and diffusion efficiency in the EGAA process oriented to the production of higher esters.Therefore, the superb catalytic performance of the H-ZM catalyst as compared with its counterparts for the EGAA reaction can be attributed to the synergistic effect regarding suitable acidic property,excellent diffusion efficiency and good stability derived from the composite structure of H-ZM that can effectively integrate the advantages of microporous molecular sieve HZSM-5 and mesoporous molecular sieve MCM-41.These merits of the H-ZM catalyst provide numerous acidic sites,high surface area and confined reaction environment for the direct synthesis of TAG by the EGAA process.Considering that the TAG selectivity had only tiny change after 32 h,we chose 32 h as the optimal reaction time for the investigation of other influencing factors.Fig.4(b)exhibits the effect of catalyst amount(0.5-2.5 g)on the catalytic performance.As observed,the catalyst amount had no effect on the glycerol conversion but significant effect on the TAG selectivity.The highest TAG selectivity was achieved with the H-ZM amount of 1.5 g,indicating that most suitable number of accessible active sites were available for the deepest conversion of glycerol to TAG at this catalyst amount while further increase beyond 1.5 g would accelerate the generation of by-products[39].The effect of n(AcOH)/n(glycerol)(3.0-8.0)on the esterification performance was studied as well,and the results were presented in Fig.4(c).

Fig.4.(a)The effect of reaction time on the catalytic performance of the EGAA process.Reaction condition:0.15 mol glycerol,1.20 mol acetic acid,1.5 g H-ZM,125°C.(b)The effect of catalyst amount on the catalytic performance. Reaction condition: 0.15 mol glycerol,1.20 mol acetic acid,32 h,125°C.(c)The effect of molar ratio of acetic acid and glycerol [n(AcOH)/n(glycerol)]on the catalytic performance.Reaction condition:0.15 mol glycerol,1.5 g H-ZM,32 h,125°C.
Theoretically, at least 3 mol acetic acid is required to react completely with 1 mol glycerol to produce TAG.However,it can be expected that the excessive acetic acid should be used to positively shift the chemical equilibrium and achieve the higher selectivity of TAG[1].Despite the 100%conversion of glycerol,the TAG selectivity constantly increased with increasing the n(AcOH)/n(glycerol). In addition, the TAG selectivity did not apparently increase at molar ratios higher than 6,indicating the occurrence of saturation [27].Therefore,n(AcOH)/n(glycerol)of 6 was selected as the optimal reaction condition.Catalyst stability and the feasibility of reuse are key attractions to robust heterogeneous catalysts and is one of the most important parameters to commercialize the catalyst[40].To that end,the durability test of the H-ZM catalyst was performed and presented in Fig. 5, indicating that the catalytic stability can be well reserved with no obvious decrease in the catalytic activity and TAG selectivity over four cycles.After reaction,the spent H-ZM catalyst still displays a good textural mesoporosity(Fig.S3).

Fig.5.Evaluation of the durability of H-ZM as the catalyst for the EGAA process.
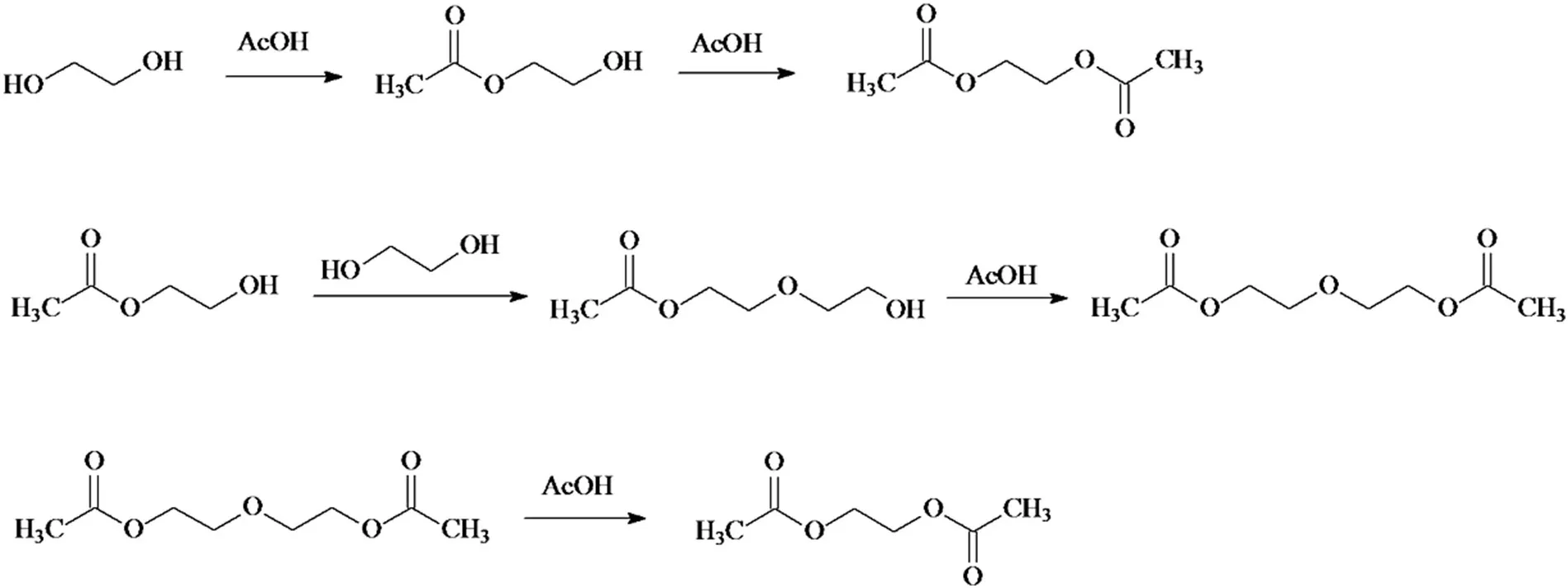
Fig.6.Reaction scheme for the catalytic esterification of ethylene glycol with acetic acid.
Furthermore,we chose the esterification of ethylene glycol with acetic acid(Fig.6)as another esterification reaction to test the catalytic performance of the H-ZM catalyst.The reaction condition is similar with that of the EGAA process except that the reactant composition in the flask is 0.25 mol ethylene glycol,0.75 mol acetic acid and 0.7 g H-ZM.The reaction results(Fig.7)show that a high yield of ethylene glycol diacetate as the target product can be achieved, indicating that the H-ZM catalyst is also effective in this reaction.

Fig.7.The esterification of ethylene glycol with acetic acid using the H-ZM catalyst.
4.Conclusions
In conclusion,the HZSM-5/MCM-41 micro/mesoporous molecular sieve was prepared and employed as an efficient catalyst for the selective synthesis of TAG from the EGAA process.The H-ZM catalyst exhibits remarkable catalytic performance with glycerol conversion of 100%and TAG selectivity of 91.3%,which can be ascribed to the suitable acidic property, excellent diffusion efficiency and good stability due to the combined advantages of microporous molecular sieve HZSM-5 and mesoporous molecular sieve MCM-41.These unique features of the HZM catalyst ensures numerous acidic sites,high surface area and confined reaction environment for the highly selective synthesis of TAG.In addition to the excellent reaction performance,the environmental benignity and cost-effectiveness also make the H-ZM catalyst a promising candidate for the glycerol esterification process and beyond.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2018.09.013.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
