Mechanism and kinetics study on removal of Iron from phosphoric acid by cation exchange resin☆
Xinke Leng,Yanjun Zhong,Dehua Xu,Xinlong Wang,Lin Yang*
School of Chemical Engineering,Sichuan University,Chengdu 610065,China
Keywords:Purification of wet-process phosphoric acid Cation exchange resin Kinetics models Diffusion models
ABSTRACT Iron element is one of the main impurities in wet-process phosphoric acid and it has a significant impact on the subsequent phosphorus chemical products.This paper studied the feasibility of using Sinco-430 cation exchange resin for iron removal from phosphoric acid.The specific surface area and the total exchange capacity of resin were 8.91 m2·g-1 and 5.18 mmol·g-1,respectively.The sorption mechanism was determined by FTIR and XPS and the results indicated that iron was combined with--SO3H in resin.The removal process was studied as a function of temperature,H3PO4 content and mass ratio between resin and solution.The unit mass of resin to remove iron was 0.058 g·g-1 resin when the operating parameters were T=50°C,H3PO4 content=27.61 wt%and S/L=0.1,respectively.Kinetics study demonstrated that pseudo-second-order reaction model fits this study best and the calculated activation energy of overall reaction is 29.10 kJ·mol-1.The overall reaction process was mainly controlled by pore diffusion.
1.Introduction
With the consumption of phosphate rock resource,the amount of high-grade phosphate rock decreases year by year and the mediumlow grade phosphate rock has been dominated in China. It is costly to produce phosphoric acid by furnace process using the medium-low grade phosphate rock as the materials.The wet-process of producing phosphoric acid, which uses sulfuric acid as the leaching agent,has been the main technology due to its low cost in recent decades.Nevertheless,the impurities,such as Fe,Al,Mg,Ca,etc.,simultaneously are leached out during the production of wet-process phosphoric acid[1],which leads to a series of problems in the industrial production.For example, with the increasing of Fe,Al, Mg and Ca concentration,the filtration performance of phosphogypsum is inhibited;the scaling in the pipes and equipment is much more serious;it also has significant influence on the production and the quality of the subsequent products[2,3], and so on.These problems can be solved through wet-process phosphoric acid purification technology,which has been the research focus in recent years.
Extensive purification methods including extraction,recrystallization, precipitation and ion exchange have been conducted in wetprocess phosphoric acid purification. Ye et al. purified wet-process phosphoric acid by liquid-liquid extraction and the removal rate of iron was 80.67 wt%when the volume ratio between extractant and solution was 10:1[4].Qiu et al.took research on the removal of Mg2+from wet-phosphoric acid via ion exchange method and the removal efficiency of Mg2+reached up to 86.8 wt%[5].In Tang's research on the removal of Al3+from phosphoric acid, it was found that the sorption process is controlled by diffusion [6]. With the development of ion exchange resin,more and more research began to be concentrated on the usage of ion exchange resin for solution purification.For example,Shen et al.used polystyrene-1,3-diamimourea chelating resin to remove copper selectively[7].Wang et al.used magnetic ion exchange resin for water treatment and the results indicated that 17α-ethinylestradiol could be removed with a much higher removal than other micropollutants reported[8].Aydin et al.studied the removal of Fe3+from saturated boric acid solution by Amberlite IR-120 resin and the results showed the high feasibility of using cation exchange resin for acid solution purification[9].
Ion exchange technique has been proven to be a practical solution purification method because of its several advantages, for instance,financial efficient,environmentally friendly,operable and higher selective for some metal ions.However,the studies on the removal of iron from phosphoric acid were rarely presented. This paper proposed a new technology of using the Sinco-430 cation ion exchange resin for iron removal from wet-process phosphoric acid. The physical and chemical properties of the resin were characterized and the mechanism of iron removal process was explored.In this technological process,the effect of the key processing parameters including mass ratio between resin and solution (S/L), H3PO4content and temperature (T) on the iron removal was studied systematically,and the optimum parameter constitute was provided. Finally, the reaction and diffusion kinetics were discussed in detail.
2.Materials and Methods
2.1.Materials
Sinco-430 ion exchange resin,which was purchased from Wuhan Aidiya Technology Co.,Ltd.,Hubei China,is a kind of strong acidic cation exchange resin.The solution used to study the reaction kinetics was prepared by dissolving iron powder in phosphoric acid in order to simulate the removal process of iron in wet-process phosphoric acid.The wet-process phosphoric acid(WPA)used in application was from Kailin Limited Liability Company,Guizhou China and its components are shown in Table 1.

Table 1 Components of WPA used in application
2.2.Methods
2.2.1.Total exchange capacity
Mass total exchange capacity was calculated as Eq.(1)(GB 8144-1987).Where QT(mmol·g-1)is the exchange capacity of resin;V2(ml)is the volume of HCl used in blank experiment;V1(ml)is the volume of HCl used for titration;cHCl(mol·L-1)is the concentration of HCl solution;M(g)is the mass of resin and X2(wt%)is the moisture content of resin.

2.2.2.Automatic Mercury Porosimeter(MIP)
MIP(Pore Master 33,Quantachrome,USA)was adopted to measure the specific surface area of resin. The measuring range of particle diameter and pore diameter of the instrument were 10-15 mm and 0.003-1000 μm,respectively.The operating pressure was 227.53 MPa.
2.2.3.Thermogravimetric(TG)
TG (439F3, Netzsch, Germany) was adopted to characterize the thermo stability of resin.The measurement was executed in at a temperature range from 25°C to 800°C with heating rate of 10°C·min-1and nitrogen atmosphere was employed to avoid oxidation of resin.
2.2.4.Fourier Transform Infrared(FTIR)
FTIR(Nicolet 6700 spectrophotometer,USA)spectra of the resin was obtained as following steps within the range of 500-4000 cm-1.The resin powder and KBr with the mass ratio of 1:100 were mixed uniformly and then prepared into a tablet of the mixture.
2.2.5.X-ray Photoelectron Spectroscopy(XPS)
XPS was performed using KRATOS Axis Ultra(Kratos Analytical,UK).An incident monochromated X-ray beam from the Al target(150 W)was focused on a 0.7 mm×0.3 mm area of the surface of the sample 45°to the sample surface.The electron energy analyzer was operated with a pass energy of 80 eV enabling high resolution of the spectra to be obtained.The step size of 1 eV was employed and each peak was scanned twice. Binding energies were calibrated relative to the C 1s peak(284.6 eV).
2.2.6.Inductively Coupled Plasma Optical Emission Spectrometer(ICP-OES)
ICP-OES (Perkin Elmer Optima 7000DV) was used to determine the iron concentration of sample.The calibration range was between 0.2 mg·L-1and 10 mg·L-1.Samples over the range of the standards were diluted to be within range.The content of iron can be calculated as Eq.(2),where X(mg·L-1)is the test result of ICP-OES,m(g)is the mass of sample used for preparation and 0.0025 means that the sample was diluted 2500 times.

2.2.7.Kinetics experiment
The ionic form of strong acidic cation exchange resin was Na+and it was acidized before being used to adsorb iron in phosphoric acid.The acidification changed the ionic into H+and the H+exchanged with iron much more easily. Fill the ion exchange column with resin and use flowing sulfuric acid (5 wt%) at the rate of 6 ml·min-1to wash the resin for about 12 h. Finally, wash the resin by deionized water until the pH value of washing water is about 7 and store the resin in water[10].
As shown in Fig.1,the study was carried out in a batch reactor whose diameter and height are 8 cm and 12 cm,respectively[11].The circulating water heated the system by a jacket to make sure the uniformity of temperature. The thermometer was used to observe the temperature and the micro sampler was used to take samples without significant effect on resin-to-solution ratio.

Fig.1.Equipment used in kinetics experiment.
The phosphoric acid was diluted several times depending on the mass balance of H3PO4and iron powder was excessively added to the acid to prepare saturated iron solution.The effect of agitation speed on reaction rate of iron removal process was studied first of all in order to eliminate the influence of external diffusion in the following experiments.The agitation speed was set as 0 r·min-1,200 r·min-1,400 r·min-1, 500 r·min-1, 600 r·min-1and 800 r·min-1and the other parameters were kept constant (T = 50 °C, H3PO4content =27.61 wt% and S/L = 0.3). The operating parameters of different experimental conditions were shown in Table 2 and the stirring speedwas kept at 400 r·min-1to ensure the uniformity of concentration everywhere.

Table 2 Operating parameters of different experimental conditions

Fig.2.Physical properties testing result of resin[(a)Drying curve of resin at 80°C;(b)dV/d(lg d)versus pore diameter curve].
3.Results and Discussion
3.1.Properties test of Sinco-430 ion exchange resin
The resin was dried at 80°C by oven drying and the mass of resin at different times was recorded as shown in Fig. 2. The content of free water was about 55.31 wt%.According to MIP measurement,specific surface area of resin was 8.91 m2·g-1and the average pore diameter was about 27.02 nm.
As shown in Fig. 3, the TG curve of resin could be divided into three steps,i.e.25-300°C,300-375°C,and above 375°C,which were differently corresponding with the loss of free water, loss of bound water and decomposition of resin,respectively.From this,it may be certainly inferred that the decomposition temperature of resin was about 375°C.
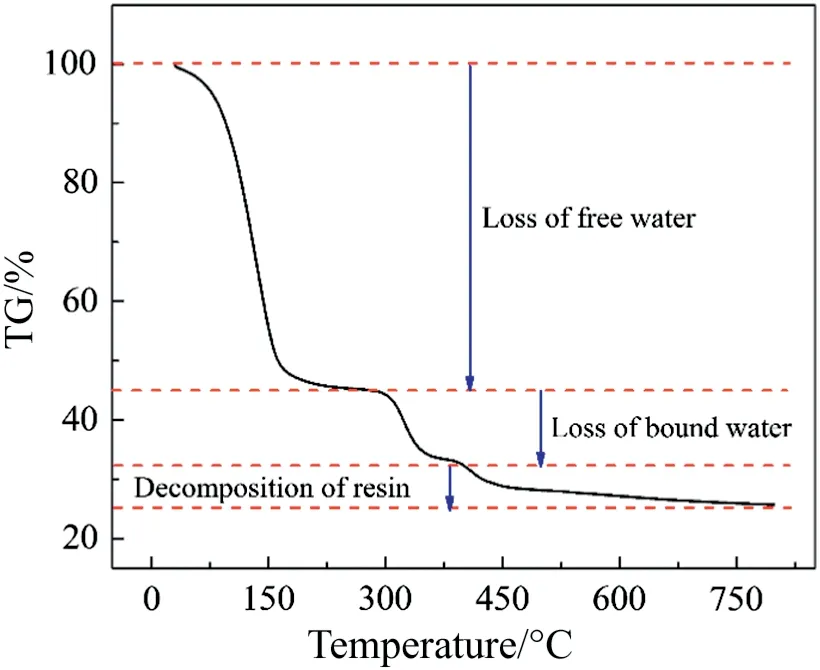
Fig.3.TG curve of the resin.
The value of total exchange capacity was measured three times and calculated according to Eq. (1): QT1= 5.16 mmol·g-1, QT2=5.18 mmol·g-1and QT3=5.20 mmol·g-1.The average total exchange capacity was 5.18 mmol·g-1.Compared with other resins,it could be easily found that the selected resin has a larger exchange capacity,for example,the exchange capacity of IR-120[12],D113 [13],and D152[14]were 5 mmol·g-1,0.02 mmol·g-1,and 3.38 mmol·g-1,respectively. Larger exchange capacity means more potential to purify the solution(Table 3).

Table 3 Experimental data of total exchange capacity
3.2.Mechanism analysis of iron removal process
3.2.1.FTIR analysis
The FTIR spectra of the resin treated by different methods were shown in Fig.4.The band at 2927 cm-1was due to the stretching vibration of O--H. The intensity bands at 1126 and 1006 cm-1were due to the stretching vibration of S--O and SO, respectively. The band at 1036 cm-1belonged to the P--O of phosphoric acid remaining inside the resin.The appearance of peaks at 1162-1174 cm-1meant that--SO3H exists inside the resin[15,16].By comparison,there was a red shift of--SO3H after iron sorption.The reason might be that when the iron combined with the functional groups of the resin, pushing electron effect made the reduction. In other words, the sorption process was chemical sorption and the functional group of this kind of resin was--SO3H.
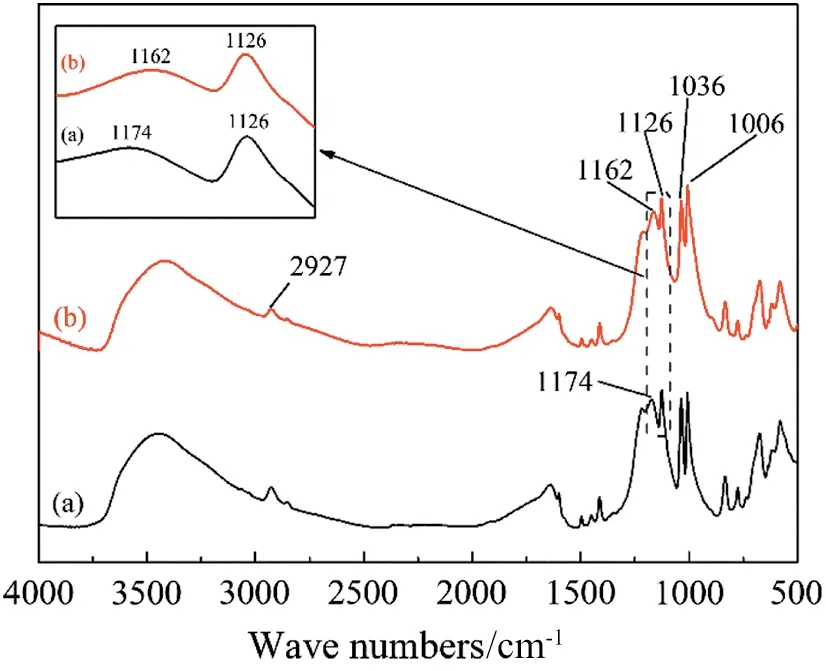
Fig.4.FTIR spectra of resin before and after sorption(a—after acidification,b—after iron sorption).
3.2.2.XPS analysis
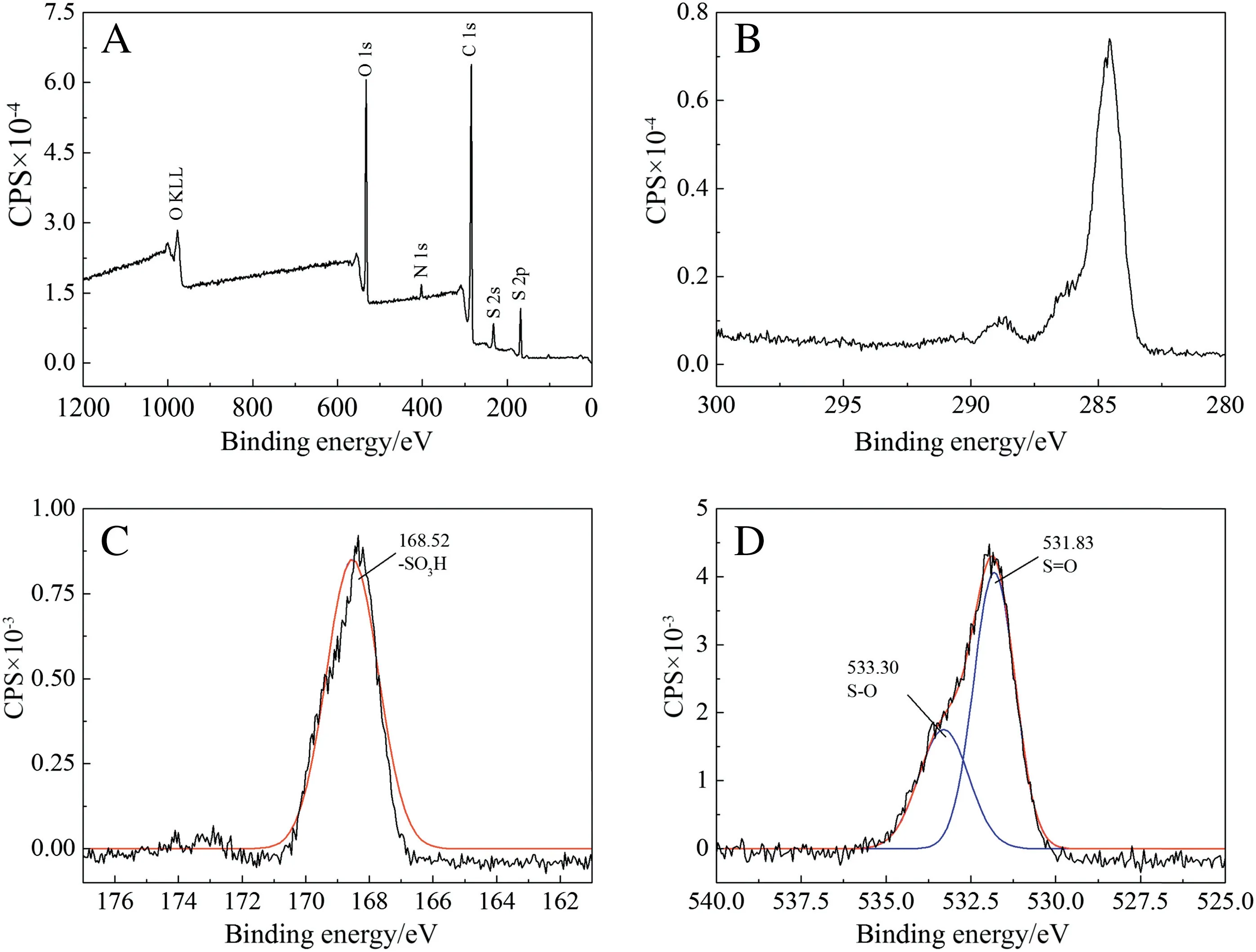
Fig.5.XPS spectra of resin before iron sorption[(a)full spectrum,(b)C 1s,(c)S 2p,(d)O 1s].
The XPS full spectrum of the resin was shown in Fig. 5(a). The unimodal spectra of sulfur(S 2p)and oxygen(O 1s)were shown in Fig.5(c)and(d).As shown in Fig.5,S had one kind of binding energy as a result of--SO3H. And O 1s had two kinds of binding energy for locating in different places of molecular structure (S--O, S=O)[17,18]. In addition, the integral area ratio between S=O and S--O was about 2:1. Such result proved the existence form of oxygen in this kind of resin.
On the other side,the XPS full spectrum of resin after iron sorption was shown in Fig. 6 (a). It could be easily found that iron exists in resin.However,the existence of S and O had been changed as shown in Fig. 6(c) and (d). There were two peaks in S 2p and three peaks in O 1s because of the combination of iron and--SO3H.The binding energy at 169.16 eV and 168.00 eV for S 2p were corresponding to Fe-SO3H and--SO3H, respectively. The binding energy of three peaks for O 1s were 532.34 eV, 532.91 eV and 531.51 eV, which can be assigned to Fe--O--S, S--O and S=O, respectively. The emerging peaks of O(532.91 eV)was caused by exchange of iron and H+.
3.2.3.Effect of agitation speed on reaction rate of iron removal process
The experiments were all taken in 5 min to contrast the reaction rate of different agitation speeds. As shown in Fig. 7, when the agitation speed was over 400 r·min-1,the removal rate of iron stayed nearly constant 68 wt%.In other words,the influence of external diffusion could be eliminated when the agitation speed reached 400 r·min-1.Thus,the agitation speed was constantly kept 400 r·min-1in the following experiments and the effect of agitation speed on reaction rate of iron removal process didn't need to be considered anymore.
3.2.4.Effect of temperature on iron removal capacity
To investigate the effect of temperature on the iron sorption capacity, the temperature of circulating water was set at 30 °C, 45 °C,55 °C, and 70 °C, respectively, with keeping the other parameters constant (S/L = 0.3, H3PO4content = 27.61%, and the original iron content of the system was about 1.9 wt%).Fig.8 displayed iron sorption capacity under different temperature. It took about 7 min to reach equilibrium. The equilibrium iron content was 0.56 wt%, 0.56 wt%,0.54 wt% and 0.48 wt% under 30 °C, 45 °C, 55 °C, and 70 °C, respectively, which suggested that the sorption reaction is an endothermic reaction because the increase of temperature moved the reaction equilibrium to the adsorption direction. However, the sorption may be a diffusion-controlled process based on the little influence of temperature on the reaction rate and the rapid increase of reaction rate with the increase of agitation speed according to the results shown in Fig. 7.
3.2.5.Effect of H3PO4content on iron removal capacity
The effect of H3PO4content was studied by adopting phosphoric acid solution with different H3PO4content,i.e.,13.80 wt%,20.71 wt%,27.61 wt%,34.51 wt% and 41.41 wt%,respectively.Other parameters were kept constant, with S/L = 0.3 and temperature = 50 °C. As shown in Fig. 9, the original iron content of the system was 2.4 wt%,1.5 wt%, 1.9 wt%,1.5 wt% and 1.8 wt%, respectively.With the increase of H3PO4content, the dissolution rate of iron kept changing. When H3PO4content is 27.61%, most of iron could be removed. On this basis, 27.61% of H3PO4content was considered to be the optimized one.
3.2.6. Effect of mass ratio between resin and solution on iron removal capacity

Fig.6.XPS spectra of resin after iron sorption[(a)full spectrum,(c)C 1s,(c)S 2p,(d)O 1s]Influence of operating parameters on iron removal capacity.
The effect of mass ratio between the resin and the solution was studied by changing the amount of resin.The mass of phosphoric acid solution with an original iron content of 1.8 wt% was a constant(150 g),while the mass of resin was set at 15 g,45 g and 75 g,corresponding to mass ratio between resin and solution (S/L) are 0.1, 0.3 and 0.5, respectively. Other parameters including H3PO4content and temperature were kept constant(H3PO4content=27.61 wt%,temperature=50°C).As shown in Fig.10,it took about 4 min to reach equilibrium for three mass ratios and a unit mass of resin to remove iron was 0.058 g·g-1resin (S/L = 0.1), 0.043 g·g-1resin (S/L = 0.3) and 0.033 g·g-1resin(S/L=0.5),respectively.These implied that S/L decreasing had no significant influence on equilibrium time but could raise unit mass of resin to remove iron.
Above all,a series of optimal parameters was obtained,T=50°C,H3PO4=27.61 wt%and S/L=0.1.To further confirm the purification effect,WPA was also taken to carry out the experiment adopting the optimal operating parameters.The removal rate in the simulated and actual systems was compared in Table 4.The relatively lower iron removal rate in WPA could be due to the competition from Mg and other elements[19].The activation energy of Mg removal and the ironic radius of Mg may be both smaller than those of iron.

Fig.7.Removal rate of iron at different agitation speeds in the same time.
3.3.Reaction kinetics and diffusion models
3.3.1.Reaction kinetics
Composite integration rule was used to calculate the average reaction rate according to the data of iron concentration at different contact time.Data calculated by mass balance equations were employed with pseudo-first-order and pseudo-second-order equations. Where k1is the pseudo-first-order reaction rate constant,k2is the pseudo-secondorder reaction rate constant and ctis the concentration of iron at any time.

Fig.8.Effect of temperature on sorption capacity.
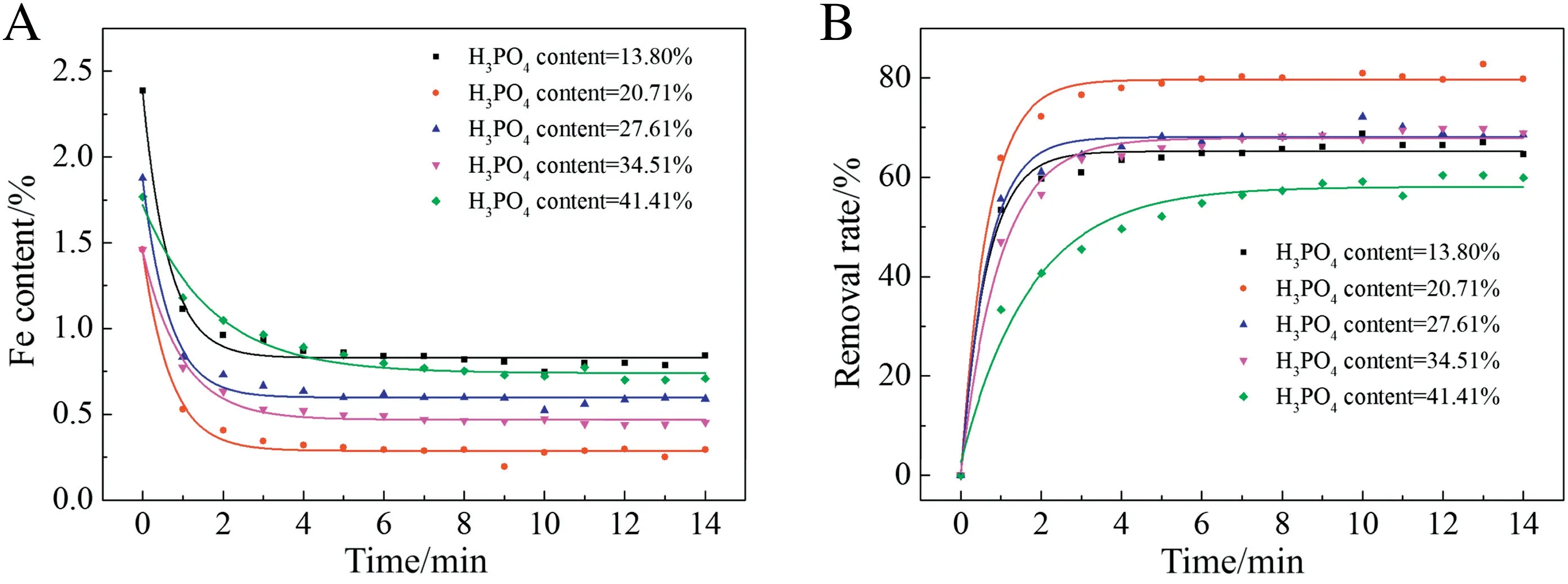
Fig.9.Effect of H3PO4 content on sorption capacity.
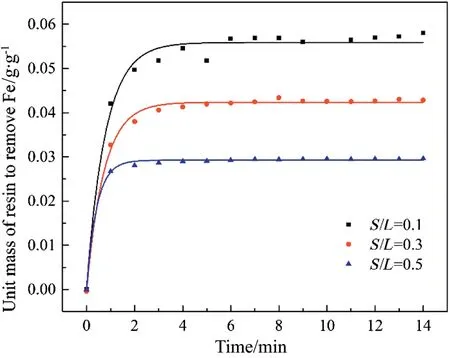
Fig.10.Effect of S/L on sorption capacity.
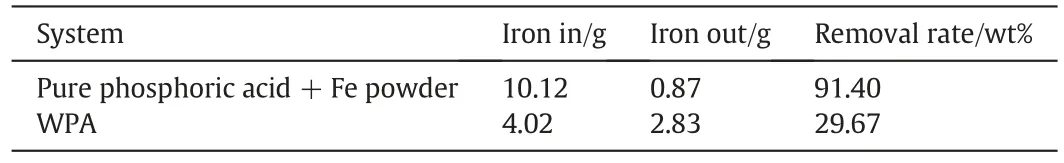
Table 4 Removal rate of iron from pure phosphoric acid and WPA

As shown in Table 5, the regression correlation coefficients of both models were larger than 0.9. However, it was easy to find that the linear regression correlation coefficients of the pseudo-secondorder equation were higher than those of the first-order rate equation and much closer to 1, indicating that the former could describe the sorption reaction better.In addition,the value of k2increased with the increase of temperature.

Table 5 Comparison between pseudo-first-order and pseudo-second-order reaction rate equation
Arrhenius equation [Eq. (6)] could be used to determine the temperature dependence of this reaction. Where A is the Arrhenius factor, Eais the activation energy, R is the ideal gas constant and k2is the pseudo-second-order reaction rate constant. Plot of lnk2versus 1/T is shown in Fig. 11 and the slope of the plot is 3500.40 which is equal to Ea/R. The activation energy of the reaction was calculated as 29.10 kJ·mol-1, lower than 50 kJ·mol-1. It indicated that temperature has little influence on this sorption reaction and the reaction mechanism may be controlled by pore diffusion or film diffusion [20].


Fig.11.Plot of lnk2 versus 1/T.
3.3.2.Diffusion models
Particle diffusion [Eq. (8)], film diffusion [Eq. (9)] and moving boundary diffusion[Eq.(10)]were applied to determine the effect of operating conditions on diffusion [21]. Where F is the ion exchange extent,k is the diffusion rate constant and t is the contact time.
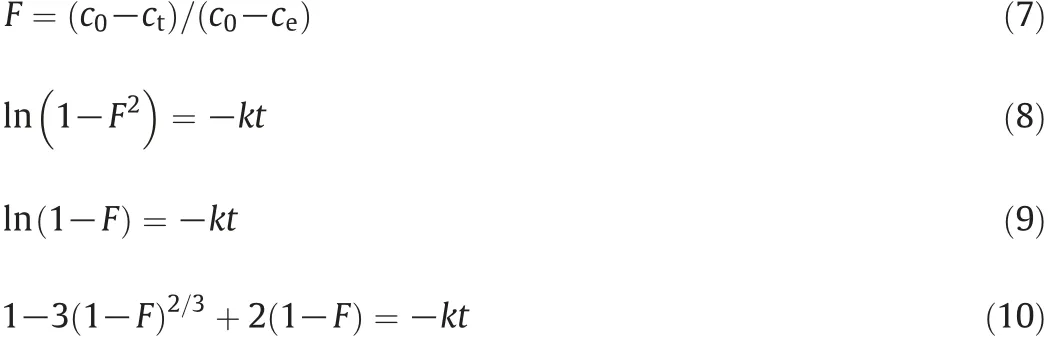
Table 6(Nos.1-4)described the effect of temperature on diffusion process,the linear regression correlation coefficients of particle diffusion and film diffusion were also larger than that of moving boundary diffusion.And temperature also had V-shape influence on the diffusion rate constant of the both models.When the temperature was 55°C,the diffusion rate constant of the both models was the largest.The viscosity of solution decreased with the increase of temperature and this could decrease the effect of diffusion.However,the increase of temperature promoted the ionization of phosphoric acid and made the concentration of H+increase.This leaded to the competitive ion exchange as mentioned above.Especially at 70°C,the competition was much stronger[22,23]. Iron was hard to combine with the exchange sites and the concentration gradient of iron was not obvious.Instead,the effect of diffusion increased.Under the influence of these two factors,55°C may be the best operating temperature of this system.In addition,55°C is also close to the temperature of the wet-process phosphoric acid in the actual production.

Table 6 Effect of different operating parameters on diffusion models
As evidenced in Table 6(Nos.5-9),the linear regression correlation coefficients of particle diffusion and film diffusion were also larger than those of moving boundary diffusion.However,the increase of H3PO4content had V-shape influence on the diffusion rate constant of the both models. When the H3PO4content was 27.61 wt%, the diffusion rate constant of the both models was the largest because the free water inside resin moved to the solution at lower pH and this could reduce the rate of swelling.As a result,the surface area of the resin was more protonated and competitive ion exchange occurred between H+protons and free iron ions for the fixation sites[24,25].
As shown in Table 6(Nos.10-12),the linear regression correlation coefficients of particle diffusion and film diffusion were larger than those of moving boundary diffusion.Typically,in the case that particle diffusion or film diffusion was the controlling step,the diffusion rate constant would be increased with the increase of S/L.In current study,however, the rate of increase change slowdown with the increase of S/L.It indicated that raising the mass ratio between resin and solution can reduce the impact of particle diffusion and film diffusion but cannot eliminate the impact forever.Ions should diffuse in the pore of the resin before they combine with the exchange sites.According to the abovementioned analysis,the iron removal process in phosphoric acid could be described in Fig.12[26].

Fig.12.Mass transfer process of the sorption.
4.Conclusions
In this study,the Sinco-430 cation ion exchange resin was successfully used for the removal of iron from phosphoric acid.The research of sorption mechanism proved that iron was combined with--SO3H in the resin.It was also observed that iron sorption highly depends on the concentration of H3PO4.In addition,mass ratio between resin and solution affects greatly the iron removal while temperature has less effect.The resin removal effect per unit mass was optimal when T=50°C,H3PO4content=27.61 wt%and S/L=0.1,which could reach up 0.058 g Fe/g resin. The kinetics analysis indicated that the sorption reaction was agreed with pseudo-second-order equation well, and the activation energy of reaction was calculated as 29.10 kJ·mol-1, therefore infers from this, pore diffusion is the controlling step of overall reaction.This study can provide significant guidance for the theoretical and applied research on the wet-process phosphoric acid purification via ion exchange method.
 Chinese Journal of Chemical Engineering2019年5期
Chinese Journal of Chemical Engineering2019年5期
- Chinese Journal of Chemical Engineering的其它文章
- Assessment of the TFM in predicting the onset of turbulent fluidization☆
- CFD study on double-to single-loop flow pattern transition and its influence on macro mixing efficiency in fully baffled tank stirred by a Rushton turbine☆
- Simulation of drop breakage in liquid-liquid system by coupling of CFD and PBM:Comparison of breakage kernels and effects of agitator configurations☆
- Heat transfer characteristics of molten plastics in a vertical falling film reactor☆
- Stabilizing silica nanoparticles in high saline water by using polyvinylpyrrolidone for reduction of asphaltene precipitation damage under dynamic condition
- Numerical simulation and experimental study on dissolving characteristics of layered salt rocks
