Novel BiVO4@C3N4@GO Composites with Higher Photocatalytic Performance
Liu Yanxiu; Guo Zhi; Wang Lijuan; Lyu Wenxue; Song Hua; Wang Xueqin
(1. Provincial Key Laboratory of Oil & Gas Chemical Technology, College of Chemistry & Chemical Engineering, Northeast Petroleum University, Daqing 163318;2. Shuntian Chemical Group, Linyi 2076001;3.Postdoctoral Center of Northeast Petroleum Univerxity Daqing 163318;4. Daqing Chemical Research Center of Petrochemical Research Institute, Daqing 163714;5. Daqing Petroleum Chemical Engineering Company, Daqing 163714)
Abstract: BiVO4, BiVO4@C3N4 and BiVO4@C3N4@GO composite photo-catalysts were synthesized, and characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), transmission electron microscopy (TEM), ultraviolet-visible diffuse reflectance spectroscopy (UV-vis DRS), Fourier transform infrared spectroscopy (FT-IR), and Brunauer-Emmett-Teller (BET) surface area techniques. The photocatalytic activity was evaluated on the degradation of methylene blue (MB) under visible light irradiation, which denoted that the BiVO4@C3N4@GO ternary composite outperformed the binary composite BiVO4@C3N4 and BiVO4. Then the effects of catalyst dosage, initial pH value,and initial methylene blue concentration on the degradation process were investigated systematically. The improvement of visible-light photocatalytic degradation performance was attributed to the enhanced visible light absorption, larger surface area, higher adsorption ability, and prolonged lifetime of photo-generated electron-hole pairs. The recycle experiments results showed that the BiVO4@C3N4@GO composite had excellent photo-stability for MB photocatalytic degradation.
Key words: BiVO4; C3N4; graphene oxide; visible light; photocatalyst
1 Introduction
Photocatalytic degradation of organic pollutants using semiconductor can lead to a promising approach for wasterwater treatment. Nanosized TiO2has attracted wide attention owing to its excellent physical performance,chemical properties, and wide application prospects in many fields[1-4]. Unfortunately, its application in photocatalysis research is greatly limited because it only responds to UV light. Thus, the exploration of the catalysts with visible light response is necessary in order to use the solar energy.
Monoclinic bismuth vanadate (BiVO4) is capable of water splitting and organic degradation owing to its outstanding visible-light response, chemical stability, and nontoxicity.Many methods (e.g., heterojunction generation and element doping) can be used to improve the photocatalytic ability by modifying the charge transmission, specific surface area,and surface adsorption of BiVO4[5-7]. Designing BiVO4composite catalysts is also a research hotspot. BiVO4was co-modified with metal Ag (electron donor) and the reduced graphene oxide (RGO) by a stepwise chemical approach coupled with photo-deposition and could be used in the visible-light-driven BrO3-reduction[8]. Specifically,RGO could promote the BrO3-adsorption onto the catalyst surface to decelerate the photo-induced electron-hole recombination[8]. Monoclinic BiVO4prepared by an easy sol-gel method was slightly more visible-light-responsive than pure BiVO4[9]. ZnIn2S4-g-C3N4/BiVO4nanocomposites showed higher visible-light photocatalytic decomposition efficiency (VL-PDE), which was attributed to the Z-scheme charge carrier transition mechanism, as the reactive radicals were found to be h+, ·OH and ·O2-[10]. In fact, a more efficient charge transfer system was formed from two or more visible-light responsive components.
In this work, BiVO4was synthesized by a fast low-temperature solid method and was used to prepare the binary composite BiVO4@C3N4and the ternary composite BiVO4@C3N4@GO.Then their structures and functions were comprehensively characterized. The capability of BiVO4@C3N4@GO in heterogeneous photocatalytic reaction was tested using methylene blue (MB) as a control pollutant. The effects of catalyst dosage, initial pH value, and initial MB concentration on MB degradation were explored systematically.
2 Experimental
2.1 Synthesis of Photocatalysts
2.1.1 Synthesis of BiVO4
BiVO4was prepared by a solid method using 5 mmol of bismuth nitrate (Bi(NO3)3·5H2O) and 5 mmol of ammonium metavanadate (NH4VO3). Firstly, bismuth nitrate was ground into powder in an agate mortar. Then NH4VO3was added and ground gradually until the formation of a reddish-brown slurry, which was transferred to an alumina crucible and dried at 110 °C for 10 h. The resulting sample was taken out, cooled down naturally to room temperature and washed with distilled water and ethanol several times. Finally, BiVO4was obtained after drying at 60 °C for 6 h.
2.1.2 Synthesis of g-C3N4
g-C3N4was produced simply by calcining the melamine powder. Typically, an appropriate amount of melamine firstly was put into a semi-closed alumina crucible with a cover. Then the crucible placed in a muffle furnace was heated at a temperature increase rate of 3 °C/min to 250 °C and kept for 2 h, and then was heated at a temperature increase rate of 10 °C/min to 550 °C and kept for 3 h for further removal of the ammonate. The final products were ground into fine powder in an agate mortar for further use.
2.1.3 Synthesis of BiVO4@C3N4
Firstly, 0.5 g of g-C3N4powder was dispersed in 100 mL of methanol under sonication for 60 min. The resulting suspension was added with 0.5 g of BiVO4under rigorous stirring until the removal of methanol. The products were oven-dried at 60 °C for 12 h.
2.1.4 Synthesis of BiVO4@C3N4@graphene oxide (GO)
A suspension composed of g-C3N4and BiVO4was prepared similar to the procedure mentioned in Section 2.1.3, and then 2.5 mL of 2% graphene oxide were added under ultrasonic stirring for 30 min. The suspension was kept under stirring rigorously until methanol was completely volatilized. The products were oven-dried at 60 °C for 12 h.
2.2 Physicochemical characterization
The powder X-ray diffraction patterns were recorded on a Rigaku D/max-2200 PC diffractometer (40 kV, 100 mA),using nickel- filtered CuKα radiation at λ=0.15405 nm.The Brunauer-Emmett-Teller surface area was determined by nitrogen adsorption at 77 K using a Micrometrics ASAP 2020 instrument (USA). Prior to N2physisorption, the adsorption samples were degassed at 120 °C for 4 h. The microstructure was characterized by a JSM-6360LA highresolution scanning electron microscope (Hitachi, Japan)and a Tecnai G2F20 transmission electron microscope. The ultraviolet-visible diffuse reflectance spectra (UV-vis DRS)were recorded on a Shimadzu UV-2550 spectrophotometer with an integrating sphere using BaSO4as the reference. The Fourier-transform infrared (FT-IR) spectrum of catalysts was recorded by FT-IR spectroscopy (PeproTech Inc., USA).
2.3 Photocatalytic activity detection
The photocatalytic activity was assessed by the visiblelight-driven degradation of methyl blue (MB) in a selfmade reactor, which was kept at a constant temperature of 25 °C. Typically, a certain amount of photocatalyst (0.01 g,0.02 g, 0.03 g, and 0.04 g) was dispersed in 100 mL of a 10 mg/L MB solution, and then was sonicated for 5 min,followed by magnetic stirring for 30 min in dark to ensure the adsorption-desorption balance. A 500 W Xenon lamp with a 420 nm cut-off filter which could only permit visible light above 420 nm was used. During the irradiation, about 2 mL of suspension were collected every 10 min and were centrifugalized to remove the photocatalyst particles. The absorbance of methylene blue was measured at 664 nm using a UV-vis spectrophotometer (UV1900/UV1901PCS).And the concentration of remnant methylene blue was estimated from the absorbance. The degradation rate of MB at a specified time (t) was adopted to evaluate the degradation efficiency.
The degradation rate of MB is calculated as follows:
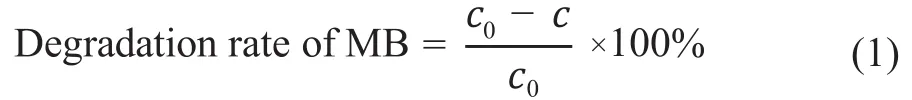
wherec0is the initial MB level in water andcis the MB level after photocatalytic degradation which is based on the linear association between the absorbance and the concentration described by the Lambert-Beer law.
3 Results and Discussion
3.1 Characterization of photocatalysts
3.1.1 Powder XRD analyses
The XRD patterns could uncover the crystalline properties(Figure 1). BiVO4showed the XRD peaks at 18.9°, 28.8°,30.6°, and 34.7°, which were ascribed to monoclinic BiVO4crystals (JCPDS-00-14-0688), with the peak at 24.4° corresponding to tetragonal BiVO4crystals(JCPDS-00-14-0133). BiVO4@C3N4showed a peak at 2θ= 27.52°, which was attributed to the (002) plane of the graphite-like structure of C3N4, and peaks at 2θ= 18.9°,28.8°, 30.6°, and 34.7° corresponded to the characteristic monoclinic BiVO4crystals[11]. BiVO4@C3N4@GO showed the co-existence of BiVO4and g-C3N4phases, albeit without characteristic peak of GO, which might occur, because the GO quantity was too samll as compared to the other two components. A few reports about BiVO4prepared by the hydrothermal method and the sol-gel method refer to the monoclinic crystals[12-14], and the crystals of BiVO4are retained in the BiVO4@C3N4composites[15]. Zhu[16]prepared the monoclitic and tetragonal BiVO4crystals through the microwave hydrothermal method. The method for preparation of BiVO4described in this article is the low temperature solidphase method, and the monoclinic and tetragonal phases coexist under some conditions adopted by this method[17].
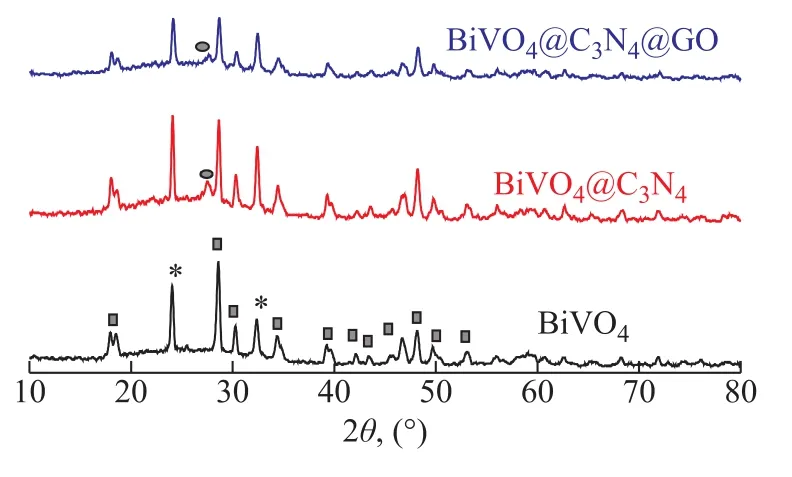
Figure 1 XRD patterns of catalysts
3.1.2 SEM analyses
Figure 2 shows the morphology of g-C3N4, BiVO4,BiVO4@C3N4, and BiVO4@C3N4@GO. Clearly, the single grains of all the samples are irregularly-shaped. Most of g-C3N4was strip-like and sheet-like and could form a complex intersecting network. BiVO4particles were nearly spherical which were made up of numerous small particles with grain size ranging from 3 μm to 10 μm. For the BiVO4@C3N4composite, g-C3N4was distributed on the surface of BiVO4. The form of BiVO4@C3N4@GO ternary composite was a schistose aggregation, and its grain size distribution was more uniform than pure BiVO4and BiVO4@C3N4. The limitation of solid-phase method for BiVO4synthesis was related with the large particles dimension, which could hinder the photocatalytic performance. During the bonding process, the ultrasonic shaking used thereby could reduce the size of composite and improve its photocatalytic performance[15].
3.1.3 TEM analyses
To further obtain the microscopic structure information,the transmission electron microscopy (TEM) analysis was carried out. It can be seen from Figure 3 that the microscopic shape of BiVO4particles, g-C3N4, and composites was in agreement with the SEM results.GO and g-C3N4sheets had a good interfacial contact with BiVO4, and there was an overlap between g-C3N4nanosheets and GO sheets.
3.1.4 FT-IR analyses
The FT-IR spectra of BiVO4, BiVO4@C3N4, and BiVO4@C3N4@GO are shown in Figure 4. The bands of BiVO4at 476 cm-1and 819 cm-1were the characteristic symmetric and asymmetric stretching vibrations of VO43-in monoclinic BiVO4, and a strong band at 686 cm-1was ascribed to the bending vibration of Bi-O. BiVO4@C3N4showed the peaks at 1 637 cm-1and 1 571 cm-1(stretching vibrations of C=N); at 1 458 cm-1, 1 407 cm-1, 1 318 cm-11 238 cm-1, and 1 207 cm-1(stretching vibrations of C-N in C-N heterocycles); at 807 cm-1and 889 cm-1(breathing of triazine units); and at 3 156 cm-1(stretching vibration of N-H or the surface-adsorbed water)[18]. The spectra of BiVO4@C3N4@GO and BiVO4@C3N4were not largely different because of the low dosage of GO (2%).
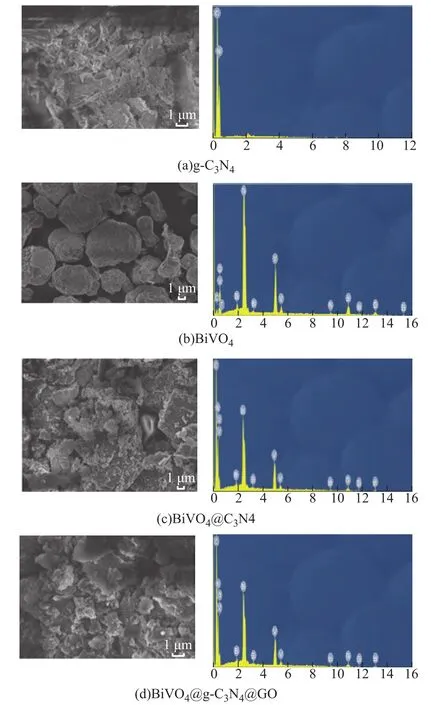
Figure 2 SEM images of catalysts
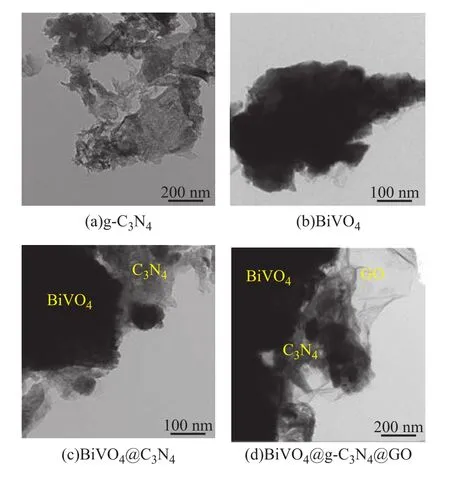
Figure 3 TEM images of catalysts

Figure 4 FT-IR spectra of catalysts
3.1.4 UV-vis DRS analyses
Pure BiVO4displayed the absorption edges at around 580 nm (Figure 5). The BiVO4@C3N4and BiVO4@C3N4@GO heterojunction photocatalysts exhibited the absorption edges at 560 nm and 570 nm. The band gap (Eg, eV) of a catalyst was calculated as follows:

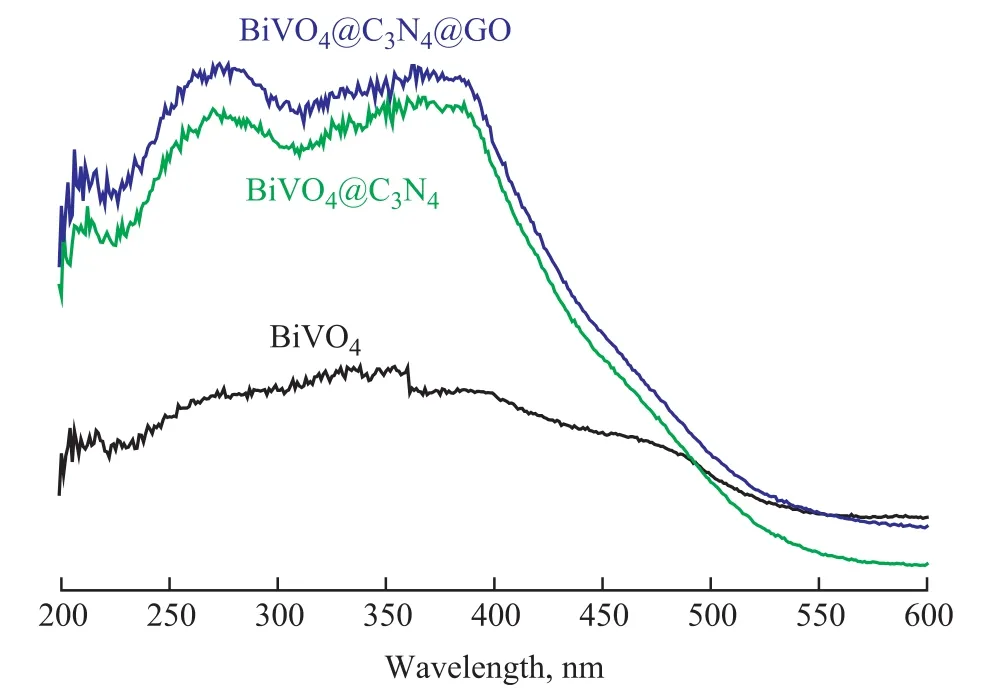
Figure 5 UV-vis DRS spectra for catalysts
wherehis the Planck's constant (4.135 7×10-15eV·s),cis the light speed (2.998×108m/s), and λ is the wavelength(nm). The band gaps of BiVO4, BiVO4@C3N4, and BiVO4@C3N4@GO were estimated to be 2.14 eV, 2.21 eV,and 2.18 eV, respectively. There were certain responses in the ultraviolet and visible regions for pure-BiVO4,BiVO4@C3N4, and BiVO4@C3N4@GO, and the intensity of light absorption of composites was enhanced greatly,denoting that the utilization of visible light was improved,which was in agreement with the previous report[19].As shown in Figure 6, the TG curves indicate that BiVO4had good oxidation resistance in air atmosphere. The weight loss of BiVO4was just 8.6% at a temperature of up to 750 °C, which would boil down the absorbed water, the organic residual solvent, and the unreacted raw materials of unburned BiVO4. BiVO4@g-C3N4binary composite started to decompose at 400 °C and lost 55% of weight at 520 °C, and there were nearly no decomposition occurring at temperature ranging from 520 °C to 750 °C.The weight loss of BiVO4@g-C3N4@GO composite was close to that of the binary composite material.

Figure 6 Thermogravimetric analysis of catalysts
3.1.5 Surface area analyses
The surface areas of the catalysts were detected by N2adsorption assays. The BET surface area of BiVO4was small (2.57 m2/g), and it was enlarged to 8.43 m2/g with g-C3N4loading, while the BET surface area of BiVO4@C3N4@GO further increased to 8.58 m2/g, indicating that the binary and ternary composites offered more active sites to improve the MB adsorption and thereby the visible light catalytic degradation efficiency (VL-PDE).
3.2 VL-PDE
3.2.1 Comparison experiments
The VL-PDEs of the samples are shown in Figure 7.When no catalyst was added, only 3% of MB were degraded after 60 min, indicating that the MB solution was very stable under visible light irradiation. On the contrary, the degradation rate of MB reached 43.2%,52.2%, and 70.2% by using BiVO4, BiVO4@C3N4, and BiVO4@C3N4@GO, respectively. The higher VL-PDEs of the binary and ternary composites might be explained by two reasons, namely: (1) Their larger surface areas offered more active sites for MB adsorption; (2) A heterojunction was formed and subsequent electron-hole recombination rate by efficient charge carrier transfer at the interface was decelerated[20-22]. As for the BiVO4@C3N4@GO ternary composite, graphene was more capable of quenching the long wavelength emission, indicating that graphene could receive the photogenerated electrons to accelerate the charge separation[23].

Figure 7 Comparison on MB photodegradation by BiVO4,BiVO4@C3N4 and BiVO4@C3N4@GO
3.2.2 Effect of catalyst dosage
Catalyst dosage is a major indicator of large-scale production from the economic perspective. The MB degradation rates versus the dosage of BiVO4@C3N4@GO are shown in Figure 8. Clearly, the MB decomposition rate firstly rose when the catylyst amount increased from 0.1 to 0.3 g/L and then declined when the catalyst dosage increased from 0.3 g/L to 0.4 g/L, which revealed that the optimal catalyst dosage was 0.3 g/L. The reason might be that the excessive solid would make the reaction system more turbid and thus could reduce the light penetration through the MB solution[24].
3.2.3 Effect of initial pH value
The effect of initial pH value on MB decomposition over BiVO4@C3N4@GO is illustrated in Figure 9. The MB decomposition rate highly relied on the pH value and reached a maximum at a pH value of 9.0. Acidic and strongly alkaline conditions were both unfavorable to MB removal, as excessive H+ions or OH-ions would hinder the photocatalytic decomposition. Weakly basic conditions(pH=9) boosted the reaction to produce according to Eq. 4.Moreover, the newly formed active species (·OH) could also accelerate the oxidation and the MB decomposition.Lower pH value could reverse the chemical reaction to form according to Eq. 3 and the MB decomposition rate declined according to Eq. 6. However, highly alkaline solution would depress the MB adsorption on the catalyst surface, which might explain the low degradation efficiency obtained under a strongly alkaline environment.


Figure 8 Effect of catalyst dosage (under conditions covering an initial MB amount of 10 mg/L, a pH value of 7.0,and a temperature of 25 °C)
3.2.4 Effect of initial MB concentration

Figure 9 Effect of pH value (under conditions covering an initial MB amount of 10 mg/L a catalyst dosage of 0.3 g/L,and a temperature of 25 °C)
The effect of initial MB concentration on MB degradation by BiVO4@C3N4@GO is exhibited in Figure 10. The MB degradation efficiency was roughly stable in the presence of 5 mg/L to 20 mg/L of MB, but significantly declined when the MB concentration increased to 30 mg/L. The significant decline of photocatalytic activity should be attributed to the lower photon adsorption on the catalyst surface, while a further unsatisfatory MB degradation rate was caused by the less electron generation due to the lower photon entry.
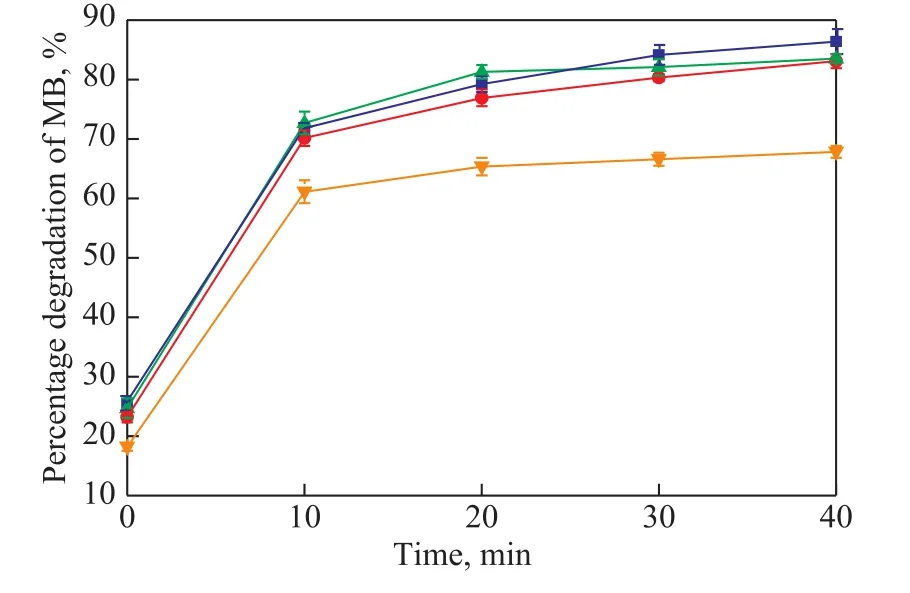
Figure 10 Effect of initial MB concentration (under conditions covering a catalyst dosage of 0.3 g/L, a pH value of 7.0, and a temperature of 25 °C)
3.3 Photocatalytic performance of recycled catalyst

Figure 11 Photocatalytic performance of recycled catalyst(under conditions covering an initial MB concentration of 10 mg/L, a catalyst dosage of 0.3 g/L, a pH=7, and a temperature of 25 °C)
Finally, we have performed the photoactivity test for recycled BiVO4@C3N4@GO composite. Figure 11 displays the data of experiments for degradation of MB over the recycled ternary composite under irradiation of visible light. It is clear to see that during four successive recycles of the composite, no significant photoactivity loss was observed, suggesting that the performance of the recycled ternary composite was stable.
4 Conclusions
BiVO4was synthesized by a fast low-temperature solid method, and its photocatalytic activity was enhanced by being incorporated with g-C3N4and graphene oxide. The pecentage degradation of MB was 43.2%, 52.2% and 70.2% after 60 min of reaction over BiVO4, BiVO4@C3N4, and BiVO4@C3N4@GO, respectively. The enhanced photocatalytic activity of BiVO4@g-C3N4@GO was attributed to the increase in surface area, visible light absorption, and photo-induced electron-hole separation. Under optimal conditions (covering an initial MB concentration of 10 mg/L, a catalyst dosage of 0.3 g/L, a pH value of 9, and a temperature of 25 °C), the MB degradation rate within 40 min reached up to 88.0%.The ternary composite was stable after repeated use for 4 cycles.
Acknowledgements:We are grateful for the tireless efforts of our project team and the tremendous vision and support of the Natural Science Foundation of Heilongjiang Province of China [QC2017005], and the Province Postdoctoral Fund[LBH-Z15032].
- 中国炼油与石油化工的其它文章
- Effect of the Morphology of ZnO Support on the Desulfurization and Regeneration Performance of Ni/ZnO Catalyst
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Methane Storage and Synthesis of HKUST-1 Prepared with Different Solvent
- Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
- Synthesis of Nanosized SSZ-13 Zeolite and Performance of Its Mixed Matrix Membrane for CO2/CH4 Separation
- Intelligent Transformation and Upgrading of Oil Refining& Petrochemical Industries in China: Investigation &Application

