Effect of the Morphology of ZnO Support on the Desulfurization and Regeneration Performance of Ni/ZnO Catalyst
Kang Lei; Wang Haiyan,; Han Bing; Li Shuanglin; Sun Na; Yang Zhanxu(. College of Chemical Engineering, China University of Petroleum, Qingdao 665555;. College of Chemistry, Chemical Engineering and Environmental Engineering,Liaoning Shihua University, Fushun 300)
Abstract: The Ni/ZnO desulfurization catalyst has been highly valued for its high desulfurization rate and low octane value loss. However, during the process of desulfurization, ZnO is prone to deactivation and the active component Ni is susceptible to agglomeration, which can affect the performance of the catalyst. In order to solve these problems, the modification of ZnO support has been extensively studied. The granular, short rod-shaped and nanowire-structured ZnO were synthesized by controlling the concentration of NaOH, and the desulfurization catalyst was prepared with ZnO serving as the support after loading of metallic Ni. The catalyst was characterized by X-ray diffraction, N2 adsorption-desorption, SEM, TEM and other analytical methods. The desulfurization performance of the catalyst was investigated with n-heptane - thiophene used as model compounds. Test results showed that the morphology and size of ZnO support has great in fluence on the desulfurization performance of the catalyst. Desulfurization catalyst prepared with nanowire-structured ZnO support has the best desulfurization performance, with its desulfurization rate reaching 98.2%. The result was achieved mainly due to the nanowire structure of ZnO support which could effectively restrain the agglomeration of metallic Ni on the surface and reduce the particle size of the active component of metallic Ni so as to improve its dispersion on the surface of the support. In addition,the nanowire structure can reduce the diffusion resistance of thiophene in the reaction process and provide a channel conducive to sulfur transfer and diffusion, which makes it perform well in the desulphurization reaction process and regeneration process.
Key words: ZnO; morphology; nanowires; desulphurization; regeneration
1 Introduction
Sulfide in fuel oil has posed a serious threat to human health and the environment, and the production of ultralow sulfur fuel oil has aroused an increased attention[1].The traditional hydrodesulfurization technology is one of the extensively used desulfurization methods in the industry[2-3], but this technology suffers from harsh reaction conditions, which need a lot of hydrogen source. It also needs substantial equipment investment and causes high operating cost and significant octane value loss[4]. Therefore, the focus of study has generally been transferring to some kinds of non-hydrogenation technologies such as the adsorptive desulfurization techniques that are characteristic of low energy consumption[5-7]. So far, different adsorbents have been studied in order to obtain better adsorptive desulfurization effect, in which ZnO has been garnering more attention in recent years due to the outstanding physical and chemical properties[8].
Tawara's group[9]was the first to report the performance of a Ni/ZnO catalyst as an adsorptive HDS catalyst.ConocoPhilips has developed the S Zorb reactive absorbent desulfurizer on this basis[10]. Ni atom in the desulfurizer is the active center of desulfurization, which can promote the breaking of the C-S bond of sulfide in the reactant to produce NiS. The ZnO support can transfer S in NiS to generate ZnS[11], and the active component Ni can complete its self-regeneration at the same time.Therefore, a proper ZnO support can ensure that the active nickel phase is present during deep desulfurization through the following regenerative step:
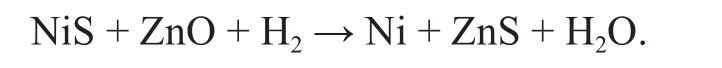
In addition, because the overall gas-solid reaction is often controlled by diffusion[12]and the morphology of adsorbent can affect the diffusion resistance of thiophene, the study on how to control the morphology[13], crystal structure[14],size and dimension of ZnO support in a reasonable manner so as to achieve purposefully regulating the desulfurization and regeneration performance of the catalyst has become a new challenge. This paper has studied the effect of crystal structures, surface structures, and pore structures of ZnO supports of different morphology on their desulfurization and regeneration performance by preparation of catalysts using ZnO supports of different morphology after loading of metallic Ni.
2 Experimental
2.1 Materials and reagents
Chemical reagents included nickel nitrate hexahydrate(Ni(NO3)2·6H2O), zinc acetate dihydrate (Zn(AC)2·2H2O),sodium hydroxide (NaOH), PEG-20000, glucose,n-heptane (C7H16), thiophene (C4H4S) and ethanol(C2H5OH). All chemicals used in the experiments were analytically pure reagents.
2.2 Preparation of ZnO supports of different morphology
0.8 g of Zn(AC)2·2H2O and 1.607 g of PEG-20000 were put into 100 mL of C2H5OH under stirring at 70 °C for 2 h. NaOH was added into the above solution at a concentration of 0.05 mol/L, 0.1 mol/L,and 0.15 mol/L, respectively, under continued stirring for 8 h. Then, the solution was transferred into a hydrothermal reactor with a capacity of 100 mL for carrying out a hydrothermal reaction at 120 °C for 12 h, eventually resulting in white solid products which were centrifuged, washed with distilled water and then dried at 60 °C for 8 h and calcined at 500 °C for 3 h to get three samples labeled as a1, b1and c1,respectively.
2.3 Preparation of catalyst
Ni(NO3)2was loaded onto the three ZnO support samples through the impregnation method with the NiO content equating to 5%, followed by drying at 120 °C for 12 h and then calcining at 500 °C for 3 h, resulting in the generated hydrodesulfurization catalysts labeled as a1s, b1s, and c1s.
2.4 Desulfurization and regeneration experiments
The desulfurization experiments using the catalyst samples were carried out in a fixed-bed reactor. After the catalyst was pressed, the 20—40 mesh particles were screened and the catalyst loading was equal to 10 mL. Model gasoline prepared by the analytical reagents consisting ofn-heptane and thiophene was used as the raw material with a sulfur content of 100 mg/L.
The prepared catalyst was reduced for 3 h at a reduction temperature of 350 °C and a reduction pressure of 0.6 MPa. The desulfurization experiments was carried out with simulated oil serving as the raw material under conditions covering a hourly space velocity of speed of 5 h-1, a reaction pressure of 0.6 MPa, and a reaction temperature of 300 °C. The regeneration process of the catalyst after reaction was performed under conditions covering a reaction temperature of 550 °C, an air flow of 160 mL/min and calcination time of 5 h.
2.5 Characterization
X-ray diffraction patterns of the samples were recorded using a Rigaku's D/max-RB X X-ray diffractometer.The micro-structure was characterized by a JEOL JEM-2100F high resolution transmission electron microscope(TEM). The surface morphology was characterized by a Hitachi SU8010 cold field emission scanning electron microscope (SEM). The BET surface areas of the catalyst were measured by a Quantachrome Autosorb-IQ2-MP automatic physical static analyzer .
2.6 Calculation
2.6.1 Calculation of crystal lattice parameters of adsorbent
The particle size is calculated by the Scherrer equation.
The crystal cell parameters are calculated by the Bragg equation.
2.6.2 Calculation of sulfur capacity of adsorbents
The adsorption curve could be obtained by recording the sulfur mass concentration of the oil flowing out of the reactor for a certain period of time. The sulfur capacity of the adsorbent was calculated by the formula (1).

In this formula (1),Scwas the sulfur capacity of the adsorbent (mg/g);c0was the sulfur mass concentration of the simulated oil flowing into the reactor (mg/L);v0was the flow velocity of the simulated oil (L/h);xiwas the average desulfurization rate between two samplings(%);tiwas the time intervals between two samplings (h);mwas the mass of the adsorbent (g). The sulfur mass concentration of the oil flowing out of the reactor was measured at regular intervals.
3 Results and Discussion
3.1 Characterization of ZnO supports
Figure 1 showed the SEM images of the three ZnO support samples, namely a1, b1and c1, synthesized with different NaOH concentrations. It can be seen from Figure 1a that the sample a1was of a granular structure in different sizes with its length ranging from 100 nm to 200 nm and its width from 50 nm to 100 nm, with its edge being in an irregular polygon form. With the increase of alkali concentration in the solution, as shown in Figure 1b, the sample presented a one-dimensional nanowire structure, about 3—5 µm in length and about 50—100 nm in diameter. When the alkali concentration continued to increase to 1.5 mol/L, the sample presented a rod-liked structure, about 1—2 µm in length and about 300 - 500 nm in diameter. It showed that the morphology and particle size of ZnO both changed with an increasing alkali concentration in the solution. The sample at first changed from the granular shape to nanowires and then to short rod-liked shape, and its length-diameter ratio increased at first and then decreased.
It could occur mainly because in the alkali solution, the formation of ZnO was divided into three stages, namely:dissolution, nucleation, and growth[15]:
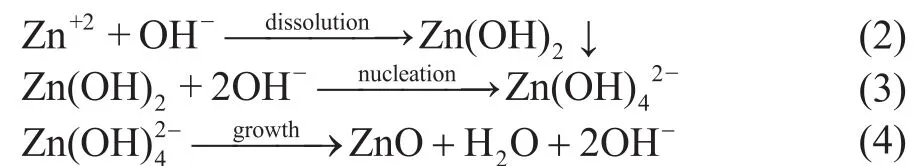
During the reaction, OH-ions could be used as both reactant and product to participate in the whole process of ZnO synthesis. Therefore, the concentration of OH-ions determined the nucleation and growth rate of ZnO.When the concentration of NaOH was low in the reaction, the formation of ZnO was mainly controlled by nucleation, and only approximately equiaxed grains can be obtained. However, with an increase in NaOH concentration, the growth process controlled the ZnO synthesis.During the growth process, the negative electricity of the polarity growth unit [Zn(OH)4]2-led to different growth rates of all ZnO crystal face, and the growth rates of different crystal face were:v(001)>v(101)>v(100)[16], which could further prompt the ZnO crystals growth along the c axis to form the nanowire structure. However, as the alkali concentration in the solution continued to increase, ZnO would dissolve because it is a amphoteric compound, which could result in the formation of a shorter rod-liked structure.

Figure 1 SEM analysis of three ZnO supports
Figure 2 shows the XRD patterns of three samples a1, b1and c1. It is observed that the three samples showed typical diffraction patterns of ZnO (in compliance with the JCPDS standards card)[17]. However, the characteristic diffraction peaks of the three samples were different in intensity, but they were mainly composed of (101) surface.The (101) surface was composed of O2-, which was a negative polarity surface[18].

Figure 2 XRD patterns of three ZnO supports
It can be seen from Figure 2 that the sample b1with nanowire structure had the most (101) polarity surface,and more polarity surfaces were favorable to the catalytic performance of the catalyst[19]. At the same time,Casarin, et al.[20]have found that S2+is preferred to be dissociatively adsorbed on the ZnO (101) surface.
The cell parameters of the three samples with different morphology were calculated, with the results shown in Table 1. It can be seen from Table 1 that the lattice constant of the nanowire sample b1was the smallest,whereas the smaller particle size will increase the rate of sulfur transfer in the ZnO adsorbent[21-22]; and the crystal cell parameters of the nanowire sample b1is the biggest. Rodrgueztalavera R., et al.[23]considered that the larger the lattice expansion, the greater the lattice distortion and strain energy of the adsorbed O2-on the surface. In order to compensate for the lattice stress,oxygen atoms in the ZnO lattice near the surface can easily escape from the lattice to generate a large number of oxygen holes on the surface of the particles, and then increase the reactive point, leading to the improvements in the catalytic efficiency[24].

Table 1 Crystal lattice parameters of three ZnO supports
The nitrogen adsorption-desorption isotherms of the samples a1, b1and c1are shown in Figure 3. Figure 3 shows that in the process of adsorption, the nitrogen adsorption quantity of the three samples followed the same rule with the change in adsorption pressure. The N2adsorption quantity was less under low relative pressure with no obvious inflection point, but when the relative pressure was higher, the N2adsorption quantity of the three samples increased sharply. According to the classification of the IUPAC, all of the three samples presented a typical type Ш isotherm. However, the nitrogen adsorption quantity of the three samples was different during the adsorption process. The nanowire sample had the largest adsorption quantity of N2. The specific surface area of the samples calculated by the BET method and the pore volume and average pore diameter calculated by the BJH method are listed in Table 2. It can be seen from Table 2 that the specific surface areas of samples a1and c1were 20.96 m2/g and 25.05 m2/g, respectively, both of which were significantly lower than that of the sample b1. The specific surface area of the sample b1reached 31.32 m2/g,and its total pore volume and pore diameter also increased. Large specific surface area and pore volume facilitated the adsorption, diffusion and desorption of molecules and was beneficial to the dispersion of active metal components[25]. At the same time, the proportion of surface active metal components was increased with an increasing specific surface area(Table 3), which was conducive to the desulfurization reaction.

Figure 3 Nitrogen adsorption-desorption isotherms of three ZnO supports

Table 2 BET surface area, pore volume and average pore diameter of three ZnO supports
3.2 Desulfurization of three Ni/ZnO catalysts
Ni(NO3)2was loaded onto the three ZnO supports in order to prepare the desulfurization catalysts a1s, b1s, and c1s.The TEM images of the three catalysts before reaction are shown in Figure 4. It can be seen from Figure 4 that after being impregnated with NiO, the morphology of three supports had no obvious change, but the dispersion of the active component Ni on the surface was very different. For the catalyst a1swith a granular structure and the catalyst c1swith a short rod-liked structure, the NiO particles on the ZnO supports were larger and presented a massive gathering. For the catalyst b1swith the nanowire structure, the NiO on the surface of the ZnO support was small and was dispersed evenly. The proportion of the surface atoms is shown in Table 3 by XPS analysis.Upon combining with the BET data, it could be easily realized that the surface Ni atoms were increased with the increase in the specific surface area of ZnO support. It might occur because the surface exposure of nanowires in the ZnO support could help to provide high-quality surface for the deposition and stabilization of the NiO during the impregnation, calcination,and reduction steps. It could effectively improve the performance of the catalyst.

Table 3 Atomic proportion on the surface of three catalysts
The desulfurization performance of the three desulfurizing agents is shown in Figure 5. The desulfurization rates of a1sand c1swere 93.5% and 94.3%, respectively,but the desulfurization rate of b1sreached 98.2%, so the desulfurization performance of the catalyst with nanowire ZnO support was obviously higher than that of the catalysts with the granular and rod-shaped ZnO supports. The morphology of ZnO support played a vital role in the desulfurization process, because the adsorptive desulfurization processes usually could occur on the “adsorption sites” of materials, in which the low coordinated steps, edges, terraces, kinks, and/or corner atoms are often the favorable activation sites[26-27]. Upon being viewed from the point of morphology regulation,the establishment of a nanowire structure can provide numerous edge sites. Therefore, the desulfurization performance of the catalyst with nano-linear ZnO support was better.

Figure 4 TEM images of the three catalysts

Figure 5 Desulfurization of the three catalysts
Figure 6 shows the morphology and crystal structures of the three samples after deactivation. It can be seen from the scanning electron microscope (SEM) images in Figure 6 that the catalyst a1sshowed obvious aggregation after deactivation, and the rod-liked structure of the catalyst c1sbecame shorter and an obvious agglomeration occurred after deactivation, while the catalyst b1swith ZnO nanowires support still retained its original structure.This was mainly caused by the fact that the ZnO supports of the catalysts a1sand c1swere small in size and the surface energy was very high, which was conducive to agglomeration. However, the surface energy of the catalyst b1swas reduced due to its ZnO support which was made of the one-dimensional nanowires structure,so that it was able to prevent the surface agglomeration effectively to retain its original morphology.
It can be seen from the XRD patterns of Figure 6 that after reaction, all the three catalysts had ZnS diffraction peaks appearing at 2θ= 27.0° and 2θ= 28.6° and also showed Ni0diffraction peaks appearing at 2θ= 43.0°.In addition, no H2S was detected in the exhaust gas.This result was consistent with the reaction mechanism mentioned by Petzold[4]. In this mechanism, the activated Ni0can promote the generation of NiS after thiophene decomposition, while the ZnO support with a proper morphology can promote the transformation of NiS into Ni0. Meanwhile, the sulfur diffusion becomes a rate determining step, and the morphology of nanowires can overcome the diffusion resistance in the reaction and improve the reaction rate.

Figure 6 SEM micrographs and XRD patterns of three spent catalysts
3.3 Regeneration performance of three catalysts
The three catalyst samples were regenerated under conditions covering a reaction temperature of 550°C, an air flow of 160 mL/min, and a calcination duration of 5 h. The morphology and crystal structures of the three catalysts after the first regeneration are shown in Figure 7.It can be seen from Figure 8 that the corresponding ZnS diffraction peaks of the three samples disappeared after regeneration, which showed that ZnS was completely transformed into ZnO. As shown by the SEM images,after the regeneration reaction, the granular catalyst a1sshowed more obvious agglomeration, and the catalyst c1shad some rod-like structure which could keep the original shape but most of the rod-like structure was assembled into blocks, whereas the morphology of the catalyst b1sdid not change significantly. This could occur because the b1Scatalyst exhibited a one-dimensional nanowire structure, which retained the small size effect of nanoparticles (nanometers in diameter) to achieve high catalytic activity. At the same time, the macroscopic properties of bulk materials (microns in length)[28]were introduced to effectively avoid the agglomeration of zinc oxide carriers during the catalytic process.
The desulfurization performance of the three catalysts after the first regeneration reaction is shown in Figure 8.After the first regeneration, the desulfurization rate of the catalyst b1swas stable and remained at about 97%, the desulfurization rate of the catalyst a1sdecreased to 90% due to the aggregation, and the desulfurization rate of the catalyst c1sdecreased to 88%.This result was mainly attributed to the aggregation of ZnO supports of the catalysts a1sand c1s, which would destroy the original morphology and could increase the diffusion resistance of sulfide in the reaction process,meanwhile the aggregation of the catalysts might lead to an increase in the crystalline size of ZnO supports,which could significantly decrease the desulfurization ability[29]. It indicated that the morphology of ZnO had a significant in fluence on the regeneration performance of desulfurizer.

Figure 7 SEM micrographs and XRD patterns of three 1st regenerative catalysts

Figure 8 Desulfurization performance of three catalysts after being subject to 1st regeneration reaction
3.4 Multiple regeneration cycles of the catalyst b1s
Figure 9 shows the performance of the catalyst b1s after being subject to multiple regeneration reactions under conditions covering a regeneration temperature of 550 °C,an air flow rate of 160 mL/min, and a calcination duration of 5 h.
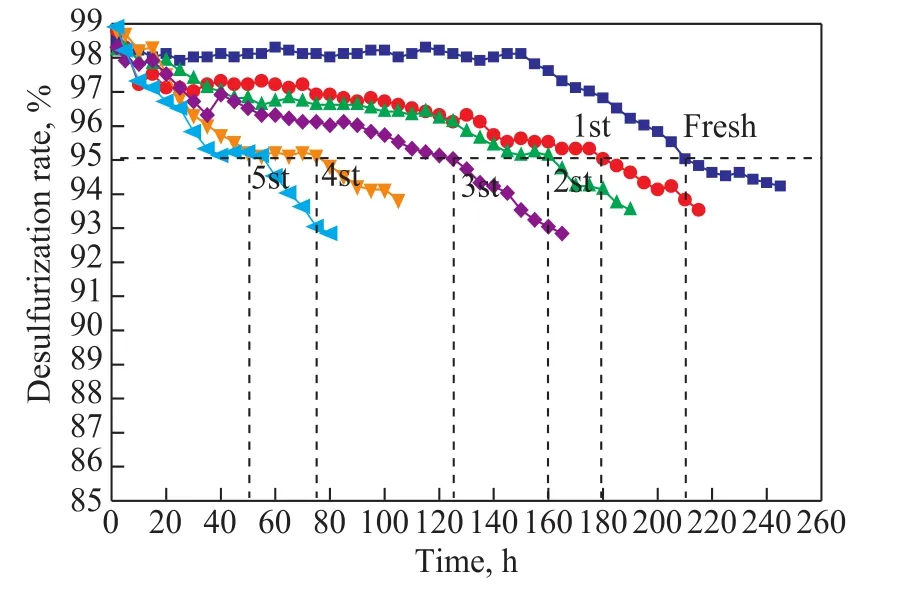
Figure 9 Performance of the catalyst b1s after being subject to multiple regeneration cycles
When the desulfurization performance of the regenerated catalyst was tested, the oil samples were taken every 2 h with the desulfurization rate determined. When the desulfurization rate was less than 95%, the reaction was stopped and then the next cycle of the catalyst regeneration was carried out. The reaction time was recorded and defined as the life of the catalyst, while the sulfur capacity was calculated according to the formula (4).
Since it was required to keep the desulfurization rate at 95% or above, the test results had revealed that after the first regeneration the life of the catalyst b1s was only reduced by 14.29% as compared with that of the fresh desulfurizing agent, and the sulfur capacity was reduced from 211.4 mg/g to 179.9 mg/g. Furthermore, after three cycles of regeneration, the life of the catalyst remained at 125 h and the sulfur capacity remained at 125.8 mg/g,which indicated that the catalyst with ZnO nanowires used as the support had good regeneration performance.However, as the time of regeneration continued to increase, its service life and sulfur capacity gradually decreased. Upon taking into account the SEM images of catalyst after regeneration, Figure 10 shows that after three cycles of regeneration, the catalyst still retained a good nanowire structure, while the length-diameter ratio did not change significantly. However, as the cycles of regeneration increased, the length-diameter ratio decreased and the nanowire structure expanded. After 5 cycles of regeneration, the catalyst showed obvious aggregation, making the desulfurization rate decreased and the catalyst life greatly shortened.
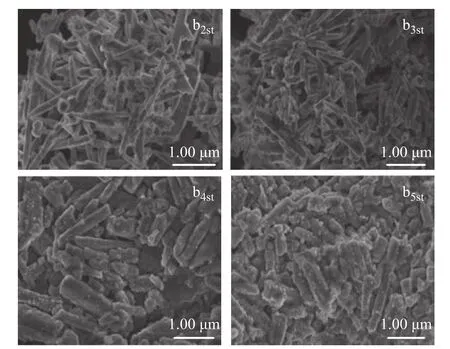
Figure 10 SEM micrographs of multiple regeneration cycles of the catalyst b1s
4 Conclusions
The experiment focused on how to regulate the morphology of ZnO support by changing the alkali concentration. When the alkali concentration increased,both the morphology and particle size of ZnO support changed significantly. The sample at first changed from granular to nanowires, then to short rod-liked shape, and the length-diameter ratio at first increased and then decreased. The morphology of ZnO support had a significant influence on the desulfurization and regeneration performance of Ni/ZnO catalyst. The desulphurization and regeneration performance of the desulfurization catalyst prepared with ZnO nanowires support was the best and its desulphurization rate was over 98%, which was attributed mainly to the nanowire structure of ZnO support that could effectively reduce the particle size of the active component of metallic Ni and facilitate its dispersion, and the nanowire structure provided the channel which was conducive to sulfur transfer and diffusion. Moreover, the ZnO nanowires support had smaller particle size and larger specific surface area, which made the desulfurization performance significantly higher than the Ni/ZnO desulfurization catalyst prepared with granular and rodliked ZnO support. During the regeneration process, the desulfurization catalyst prepared with ZnO nanowire support showed stable desulfurization performance when the regeneration eycles were less than 4. However, after 5 cycles of regeneration, the linear structure of regenerated catalyst was destructed and its life was shortened, which indicated that the morphology of ZnO support was also crucial to the catalyst regeneration performance.
Acknowledgments:The work is financially supported by the National Natural Science Foundation of China (21401093)
- 中国炼油与石油化工的其它文章
- Methane Storage and Synthesis of HKUST-1 Prepared with Different Solvent
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Novel BiVO4@C3N4@GO Composites with Higher Photocatalytic Performance
- Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
- Synthesis of Nanosized SSZ-13 Zeolite and Performance of Its Mixed Matrix Membrane for CO2/CH4 Separation
- Intelligent Transformation and Upgrading of Oil Refining& Petrochemical Industries in China: Investigation &Application

