Methane Storage and Synthesis of HKUST-1 Prepared with Different Solvent
Gong Xiaojie; Zhang Dan; Duan Linhai; Meng Xiuhong; Lin Wenjie
(1. School of Chemistry and Materials Science, Liaoning Shihua University, Fushun 113001;2. School of Chemical Engineering, Guangdong University of Petrochemical Technology, Maoming 525000;3. Zhenghe Group Co., Ltd., Dongying 257000)
Abstract: The metal-organic framework HKUST-1 was prepared by different solvents. Several analytical tools such as scanning electron microscope (SEM), X-ray diffraction (XRD), N2 adsorption-desorption have been used to characterize the as-synthesized samples. Meanwhile, the solubility of organic ligands in solvents and the CH4 adsorption capacity of samples were also detected. The results showed that HKUST-1 prepared with N,N-dimethylformamide (DMF) used as solvent exhibited excellent CH4 adsorption performance. Further studies have shown that the improved CH4 adsorption properties could be attributed to higher solubility of the ligand 1,3,5-benzenetricarboxylic acid in DMF and larger specific surface areas. This work can provide a novel sight for preparing highly efficient CH4 adsorbent materials by improving the solubility of ligand.
Key words: HKUST-1; solubility; methane storage
1 Introduction
Metal-organic frameworks (MOFs) formed by polydentate organic linkers and metal ions or metal clusters have attracted considerable attention because of their potential applications in gas storage[1-2], drug delivery[3], sensing[4], adsorption[5-7],photocatalysis[8-10], and catalysis[11-17]. Many research efforts have considered the applications of MOFs materials in energy storage, including hydrogen[18]and methane[19]adsorption. HKUST-1, a typical metalorganic framework, is composed of two octahedrally coordinated Cu atoms and eight oxygen atoms of 1,3,5-benzenetricarboxylic acid (BTC) in the structural unit[20-21]. HKUST-1 has already demonstrated its potential for CH4adsorption due to its high surface area, uniform pore size, chemical stability, and accessible unsaturated Cu2+[22]. HKUST-1 was prepared via solvothermal reaction of copper (Ⅱ) nitrate and BTC in solvent. Therefore, solvents have an important effect on the structure and properties of HKUST-1.Hao's group used different-size solvents to synthesize MOFs. The results showed that solvents as spacetemplates can dramatically in fluence the pore size and topological structure of MOFs[23]. Zhang's group used a mixture of water with ethanol as solvents for the synthesis of HKUST-1 and they have found that the size and porosity properties of HKUST-1 nanoparticles can be easily tuned by changing the composition of the mixed solvents[24]. A large number of solvents have been used for the preparation of HKUST-1, so the influence of solvents on the cost and properties of the product HKUST-1 is significant. However, the in fluence of solubility of organic ligands in solvents on the structures and properties of MOFs has rarely been reported.
In this paper, HKUST-1 was synthesized using different solvents (water, methanol, ethanol, ethylene glycol,isopropanol, isobutanol, andN,N-dimethylformamide).The solubility of organic ligands in solvents was examined, and the structure and CH4adsorption capacity of HKUST-1 prepared with corresponding solvents were also detected. The in fluence of solubility of organic ligands in solvents on the structure and properties of the prepared HKUST-1 was discussed.
2 Experimental
2.1 Materials
All the chemicals were purchased from commercial sources, and used without further purification.
2.2 Synthesis procedure
1,3,5-Benzenetricarboxylic acid (2.5 g) and Cu(NO3)2·3H2O (5.0 g) were dissolved in the solvent(water: 29 mL; methanol: 65 mL; ethanol: 94.3 mL;ethylene glycol: 90.1 mL; isopropanol: 124 mL;isobutanol: 149.1 mL;N,N-dimethylformamide: 125 mL),respectively. Subsequently, the reaction mixture after being stirred for 30 min was transferred into a Teflonlined stainless steel autoclave and heated at 348 K for 24 h. The obtained product was marked as HK-1 (N,N-dimethylformamide), HK-2 (methanol), HK-3 (ethanol),HK-4 (ethylene glycol), HK-5 (isopropanol), HK-6(isobutanol), and HK -7 (water), respectively.
2.3 Characterization
The powder X-ray diffraction measurements were recorded with a scanning angle (2θ) ranging from 5° to 40° at room temperature on a Rigaku D/Max-2500 diffractometer with Cu-Kα (λ= 0.15418 nm) radiation. The SEM images were taken by a JSM-7500F field emission scanning electron microscope. The nitrogen adsorption-desorption isotherms were measured on an ASAP 2020 surface area analyzer (Micrometrics, USA) at 77 K. Methane adsorption isotherm was characterized by using a HPVA-100 high pressure volumetric adsorption measurement instrument.
2.4 Solubility of 1, 3, 5-benzenetricarboxylic acid
The solubility of 1,3,5-benzenetricarboxylic acid in different solvents was measured by a synthetic method with a laser monitoring observation technique[25-26].The solution was placed in a jacketed glass vessel, the temperature of which was controlled through a water bath. The temperature was monitored by a mercuryin-glass thermometer with an uncertainty of ±0.01 K.During the experiment, a certain amount of solvents was introduced into the vessel, and 1,3,5-benzenetricarboxylic acid was added after being weighed. The solution was stirred continuously at the required temperature,
and the dissolution of 1,3,5-benzenetricarboxylic acid was examined by a laser beam penetrating the vessel.1,3,5-Benzenetricarboxylic acid was added slowly until it could not be dissolved completely, and the intensity of the laser beam penetrating the vessel reached a minimum,indicating that the solution was saturated. The total amount of added 1,3,5-benzenetricarboxylic acid was recorded. Thus, the molar fraction of the solubilityx1can be calculated by Equation (1).

in whichm1represents the mass of 1,3,5-benzenetricarboxylic acid, andm2represents the mass of organic solvents, whileM1,M2are the molecular weight of 1,3,5-benzenetricarboxylic acid and organic solvent,respectively. The relative standard uncertainty was less than 2% which was calculated from the standard deviations of repeated experimental measurements.
2.5 Crystallinity calculation
The relative crystallinity of the sample is calculated by self-reference. The peak area of the main diffraction peak is taken as the standard of XRD patterns. The crystallinity is defined to 100 % with HK-1 as the standard sample.The relative crystallinity of other samples is obtained by comparing with HK-1.
3 Results and Discussion
3.1 XRD characterization
The crystal structure of samples prepared using different solvents was investigated. As shown in Figure 1, the XRD patterns of HK-1 (N,N-dimethylformamide), HK-2(methanol), HK-3 (ethanol) were in good agreement with the simulated pattern of HKUST-1. Meanwhile, some peaks disappeared in the XRD pattern of HK-5 (isopropanol) and the intensity of some peaks descended obviously for HK-6(isobutanol). HK-4 (ethylene glycol) was a blue transparent liquid and HK-7 (water) was a blue sky-colored paste(Figure 2). These results indicated that HKUST-1 could be synthesized successfully usingN,N-dimethylformamide,methanol, and ethanol serving as the solvents. However, the crystal structure of HK-5 and HK-6 showed poor quality upon using isopropanol or isobutanol as the solvents. In contrast HKUST-1 could not be prepared using water or ethylene glycol as the solvents under the experimental conditions.
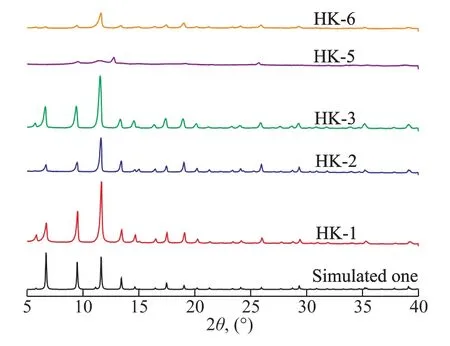
Figure 1 XRD patterns of HK samples

Figure 2 Images of HK-4 and HK-7
3.2 SEM analyses
To reveal the influence of solvents on the structure of the as-synthesized samples, the morphological images of samples were characterized by a scanning electron microscope (SEM). It can be seen from Figure 3 that the morphology of samples was different. HK-1 presented an octahedral shape (Figure 3a), while HK-2 exhibited a soft block shape (Figure 3b). HK-3 showed a block shape(Figure 3c). HK-5 and HK-6 prepared by isopropanol and isobutanol, respectively, showed a rod-like shape (Figure 3d, Figure 3e). In addition to the rod-like morphology,HK-6 displayed a block shape.
3.3 Porosity properties
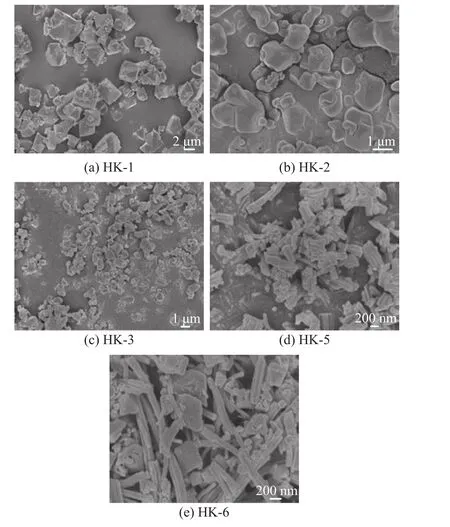
Figure 3 SEM images of samples
The pore structure properties of the samples have an important effect on the CH4adsorption capacity. The N2adsorption-desorption isotherms of samples are shown in Figure 4. HK-1, HK-2, and HK-3 displayed the type-Ⅰisotherms clearly, demonstrating their typical microporous networks. In contrast HK-5,HK-6 showed the type-Ⅳisotherms according to the IUPAC classification, corresponding to mesoporous and microporous materials (curves d and e). The BET surface area and total pore volume of the samples are listed in Table 1. The specific surface area of HK-1, HK-2,and HK-3 was 1 319 m2/g, 1 379 m2/g, and 1 693 m2/g,respectively. However, the specific surface area of HK-5, and HK-6 was only 293 m2/g and 480 m2/g,respectively.

Figure 4 N2 adsorption -desorption isotherms of samples
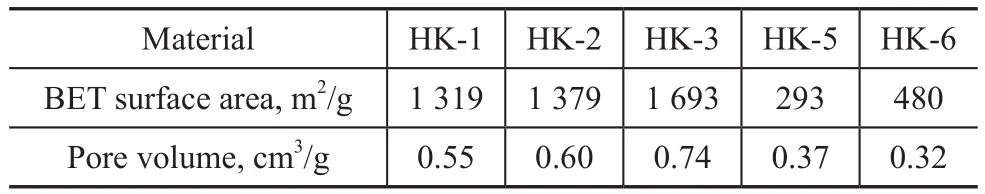
Table 1 Pore characteristics of samples
3.4 CH4 adsorption capacity
The CH4adsorption isotherms of the samples are shown in Figure 5 within the pressure range of 0-35 bar at 298 K. The CH4adsorption capacity of HK-1, HK-2, and HK-3 increased with a rising pressure in the range of 0-35 bar. When it reached a certain pressure value, the adsorption capacity of CH4increased slowly and then tended to saturation. This result indicated that the CH4adsorption on HK-1, HK-2,and HK-3 was a microporous adsorption. However, the CH4adsorption capacity of HK-5 and HK-6 was significantly lower than that of HK-1, HK-2, and HK-3. Because HK-5 and HK-6 contained mesoporous structure, it is unfavorable for them to adsorb CH4. Under the same pressure, the amount of CH4adsorbed on the samples decreased in the following order: HK-1> HK-3> HK-2> HK-6> HK-5. Smaller pores were more efficient in CH4adsorption because CH4molecules could interact with more surface atoms of the HK samples[27]. The CH4adsorption capacity of HK-1 (DMF)was high enough to reach 219.85 cm3/g (or 9.81 mmol/g). In contrast the CH4adsorption capacity of HK-5 (isopropanol)was lower, which was equal to merely 31.3 cm3/g (or 1.40 mmol/g). Therefore, the solvent had a significant effect on the CH4adsorption capacity.

Figure 5 CH4 adsorption of samples at 298 K
3.5 Solubility
The solubility of organic ligands in solvents had a certain effect on the crystalline quality of the as-synthesized materials. The solubility of 1,3,5-benzenetricarboxylic acid in methanol, ethanol, isopropanol and isobutanol was determined in a temperature range of 298—360 K, as shown in Figure 6.The solubility of 1,3,5-benzenetricarboxylic acid increased with a rising temperature. The organic ligand 1,3,5-benzenetricarboxylic acid was completely dissolved inN,N-dimethylformamide, while water and ethylene glycol used as the solvents could not help to synthesize HKUST-1. Therefore the solubility of 1,3,5-benzenetricarboxylic acid in the solvent decreased in the following order: DMF> ethanol> methanol>isobutanol > isopropanol at the same temperature.
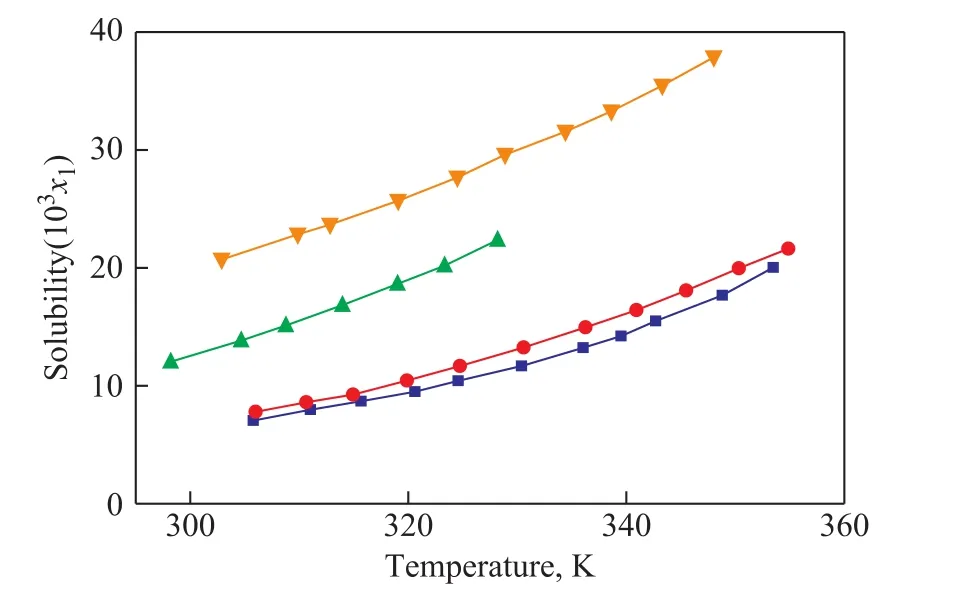
Figure 6 Solubility (x1) of 1,3,5-benzenetricarboxylic acid in pure solvents
3.6 Mechanism
This study found out that HKUST-1 could not be prepared using water or ethylene glycol as the solvent. Water or ethylene glycol possessed strong polarity and can form hydrogen bonds with organic ligand easily, which could hinder the coordination of the central metal ion Cu2+with 1,3,5-benzenetricarboxylic acid[27]. Therefore, water or ethylene glycol could not be used as the solvents to synthesize HKUST-1. The other solvents in this paper included:N,N-dimethylformamide, ethanol, methanol,isobutanol, and isopropanol, the polarity of which was weakened, so that it was not easy to form hydrogen bonds with the organic ligand 1,3,5-benzenetricarboxylic acid. The solubility of 1,3,5-benzenetricarboxylic acid in methanol,ethanol, isopropanol and isobutanol was determined in a temperature range of 298—360 K, as shown in Figure 6.Because 1,3,5-benzenetricarboxylic acid could completely dissolve inN,N-dimethylformamide, the order of solubility of 1,3,5-benzenetricarboxylic acid in the solvent decreased in the following order:N,N-dimethylformamide>ethanol> methanol> isobutanol> isopropanol at the same temperature. It was found that the order of solubility of 1,3,5-benzenetricarboxylic acid was consistent with the order of the CH4adsorption capacity of the samples prepared with the corresponding solvent. The greater solubility of 1,3,5-benzenetricarboxylic acid in the solvent could facilitate the coordination of the carboxylate groups of 1,3,5-benzenetricarboxylic acid to Cu2+ions. This inclusion would be favorable to the formation of a large number of HKUST-1 nuclei, which were conducive to the formation of higher quality crystal phase of the products. Table 2 shows the crystallinity of the products. The order of crystallinity of samples is similar to the order of the CH4adsorption capacity of samples. Therefore, the CH4adsorption capacity of the HK samples is not only related to the pore structure, but also to the crystallinity of the products.

Table 2 Crystallinity of samples
4 Conclusions
HKUST-1 had been synthesized by using different solvents. HK-1, HK-2, HK-3 showed microporous structures by usingN,N-dimethylformamide, methanol,and ethanol as the solvent, respectively. HK-5, HK-6 possessed larger pores because of the larger molecular sizes of solvents (isobutanol, isopropanol). It was demonstrated that the CH4adsorption capacity of HK-1,HK-2, and HK-3 was better than HK-5 and HK-6 owing to the suitable pore structure. Moreover, the order of solubility of 1,3,5-benzenetricarboxylic acid was consistent with the order of the CH4adsorption capacity of the samples prepared by the corresponding solvent.This work can provide a new insight into the solvent effect on the MOFs formation and application.
Acknowledge:This work was financially supported by the National Natural Science Foundation of China(21476101), the Liaoning Provincial Education Department Foundation (L2015304), and the funds of Liaoning Shihua University (2019XJJ-005).
- 中国炼油与石油化工的其它文章
- Effect of the Morphology of ZnO Support on the Desulfurization and Regeneration Performance of Ni/ZnO Catalyst
- Optimization of Dividing Wall Column with Heat Transfer Process Across the Wall for Feed Properties Variation
- Novel BiVO4@C3N4@GO Composites with Higher Photocatalytic Performance
- Research on Hydrogenolysis of Glycerol to 1, 2-Propylene Glycol by Using Supported Raney-Cu/Al2O3
- Synthesis of Nanosized SSZ-13 Zeolite and Performance of Its Mixed Matrix Membrane for CO2/CH4 Separation
- Intelligent Transformation and Upgrading of Oil Refining& Petrochemical Industries in China: Investigation &Application

