茶多酚对动脉粥样硬化的预防作用与机理研究进展
张姝萍,王岳飞,徐平
茶多酚对动脉粥样硬化的预防作用与机理研究进展
张姝萍,王岳飞,徐平*
浙江大学茶学系,浙江 杭州 310058
动脉粥样硬化是多种心血管疾病的重要病理基础,对心脑血管的损害可累及全身各个器官。茶多酚能够通过抗炎、调节血脂水平、抑制LDL氧化修饰、改善内皮功能、保持斑块稳定性等不同途径有效预防动脉粥样硬化。本文就近年来茶多酚对动脉粥样硬化的预防功能与机理方面的研究进行综述。
动脉粥样硬化;茶多酚;心血管;抗炎;平滑肌细胞增殖
心血管疾病(Cardiovascular disease,CVD)是全球的头号死因[1]。2017年世界卫生组织报道,CVD是严重威胁人类健康的重大疾病,死亡人数约占全球死亡总数的31%[2]。《中国心血管病报告2017》显示,我国CVD死亡占居民总死亡原因的首位,高于肿瘤和其他疾病,每5例死亡中就有2例死于CVD[3]。我国CVD患病率仍在持续上升,其中以冠状动脉粥样硬化性心脏病占比最高。
动脉粥样硬化(Atherosclerosis,AS)是心血管疾病的重要病理基础(图1)[4-6]。AS形成机制极其复杂,一直是心血管疾病研究的热点。AS为多因素所致,以动脉壁内脂质沉着,形成粥糜状的病灶以及纤维增生,使管壁硬化为特征[5]。虽然每年关于AS的论文超过13 000篇,但目前依然未能找到有效的预防机制以及确切的诊治方法。一般认为,AS的发生机制涉及到血管内皮受损、氧自由基损伤、脂质沉着、血小板黏附聚集、炎性细胞浸润、血管平滑肌细胞内钙超负荷、病理性血管新生与局部血栓形成等多项环节[7]。目前AS的治疗药物主要为他汀类药物(调脂药物)、替格瑞洛(抗血小板药物)与CCB(钙拮抗剂)等。AS的成因复杂,药物防治难度大,且大多具有副作用,因此早期预防和控制非常重要。
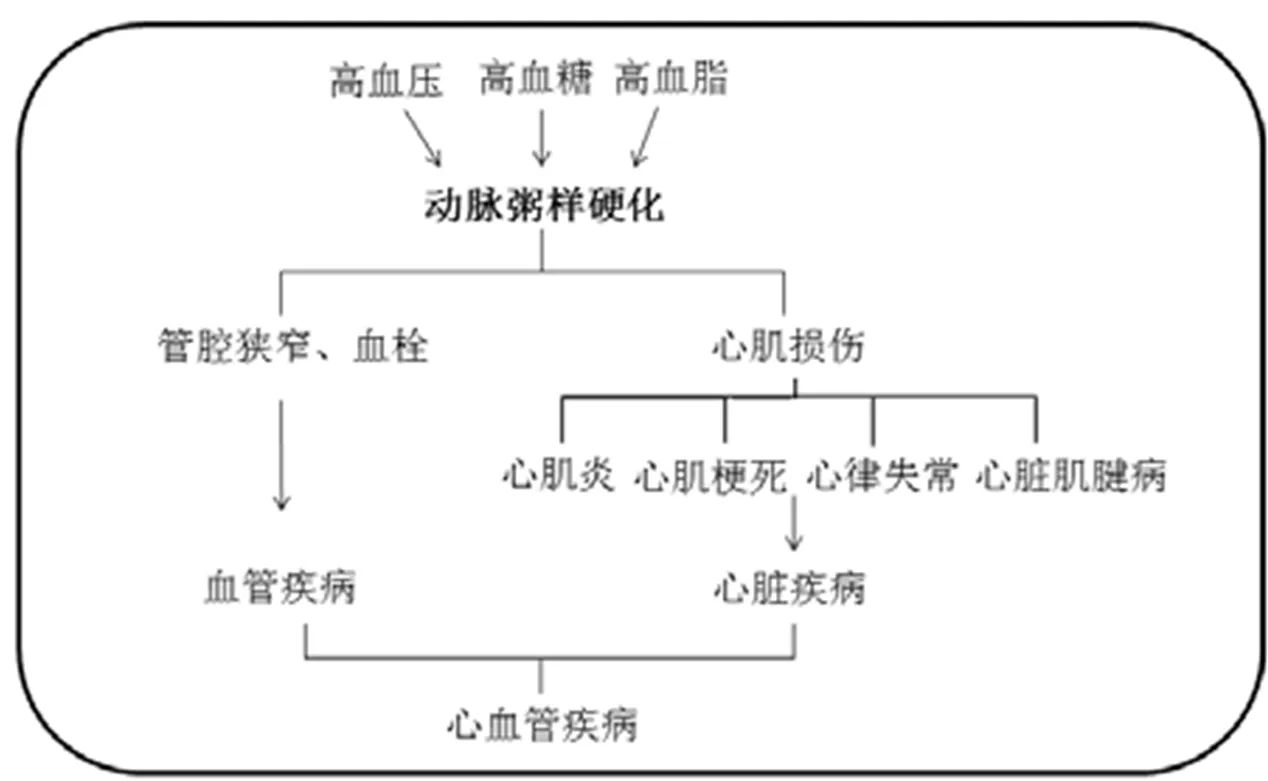
图1 动脉粥样硬化在心血管疾病中的作用[4-6]
茶多酚是茶叶中最重要的活性物质之一,具有抗氧化、抗炎、抗菌和抗癌等多种功效。其中茶多酚在预防动脉粥样硬化,保护心血管方面的作用一直备受重视,在体外研究和动物模型中得到证实。本文综述了茶多酚的抗动脉粥样硬化作用及其机理的研究进展。
1 茶多酚对AS的预防作用
1.1 流行病学
由于老龄化与饮食不均衡等因素,AS的患者数量逐年增加。近年来关于茶对人体心血管疾病改善功能的记录见表1,结果均显示茶叶消费量与心血管疾病防治具有相关性。但不同茶类的作用效果存在差异,针对不同性别人群的作用效果也存在差异。新近荷兰一项调查研究表明,每日饮用2~3杯绿茶可以显著降低男性的CVD患病风险,但在女性中未观察到相关性[21]。2016年日本的一项调查结果也显示,饮用绿茶与以AS为病理基础的冠心病发病率降低有关,且在60岁以上、超重或患有糖尿病的男性中两者相关性更高[22]。
1.2 体外试验
大量临床资料及试验数据证明了血浆中的低密度脂蛋白(Low density lipoprotein,LDL)发生氧化,生成氧化型低密度脂蛋白(Oxidized low density lipoprotein,ox-LDL)是导致AS的主要危险因素[23]。在AS形成早期,血液中的单核细胞进入内皮下间隙,在内膜下被激活后分化成为巨噬细胞,且通过清道夫受体无反馈性吞噬大量ox-LDL,致使细胞内脂质堆积,形成泡沫细胞。其大致过程如图2[24]所示。
通常选取U937单核细胞模型和人血管内皮细胞模型模仿AS早期单核细胞进入内皮下间隙的过程。肿瘤坏死因子(Tumor necrosis factor alpha,TNF-)作为一种强效的促炎因子,可通过多种信号通路介导炎症反应,是AS形成过程中的重要因素[25]。因此TNF-常在体外试验中用于刺激细胞诱导炎症。
在U937单核细胞模型中,EGCG(10 μmol·L-1,30 μmol·L-1)可以抑制由TNF-诱导的炎症因子表达上调,抑制U937单核细胞黏附于人ISO-HAS内皮细胞,同时下调ISO-HAS细胞凋亡和自噬[23]。Li等[26]发现EGCG(1~10 μmol·L-1)可以剂量依赖性地下调U937单核细胞中由血管紧张素II(Angiotensin II,AngII)和白细胞介素-6(Interleukin-6,IL-6)诱导产生的C反应蛋白(C-reaction protein,CRP),而CRP作为炎性细胞因子直接参与粥样斑块形成。
Wang等[27]发现,1~10 μmol·L-1的EGCG处理可以降低内皮素-1(Endothelin-1,ET-1)诱导的血管平滑肌细胞CRP积累,阻断活性氧信号,减轻AS相关炎症反应。Won等[28]发现,绿茶儿茶素(20 μmol·L-1,50 μmol·L-1)可抑制AngII诱导的血管平滑肌细胞增殖,且该抑制作用与MAPK信号通路有关。
1.3 动物试验
载脂蛋白(Apolipoprotion E)基因敲除小鼠(ApoE-/-小鼠)可在正常饲料喂养下产生严重的高胆固醇血症,自发形成AS斑块,且斑块分布和病理特征与人类AS斑块相似,是研究AS病程及干预效果的理想动物模型[29]。Chyu等[30]每天给ApoE-/-小鼠注射10 mg·kg-1的EGCG溶液(处理时间分别设21 d与42 d),结果发现,21 d EGCG处理组小鼠颈总动脉外膜损伤致内膜病变而引起的AS斑块面积比对照组小55%,42 d EGCG处理组AS斑块面积比对照组小73%。Mika等[31]连续12周用高脂饲料与绿茶儿茶素(90 mg·kg-1)同时喂养ApoE-/-小鼠,结果显示,在整个主动脉与主动脉根部截面上,AS斑块面积分别减少28%与45%。Hayek等[32]给ApoE-/-小鼠喂食富含儿茶素的水(50 μg·d-1),6周后,儿茶素处理组小鼠主动脉AS斑块面积与对照组相比小39%,并且儿茶素组小鼠体内LDL的氧化敏感性有所降低。Morrison等[33]按照西式饮食方式,在雌性ApoE-/-leiden小鼠的饮食中补充1%的胆固醇,4周之后将小鼠分为两组,保持高胆固醇饮食并给其中一组喂食儿茶素,另一组则为对照组。20周后,儿茶素组小鼠每日摄入儿茶素量为(110±11) mg·kg-1(儿茶素/小鼠体重),且该组小鼠平均AS病变面积相比对照组减小27%。在雄性ApoE-/-小鼠模型中,连续6周含0.02%的儿茶素的饮食可使小鼠平均AS病变面积减小32%[34]。
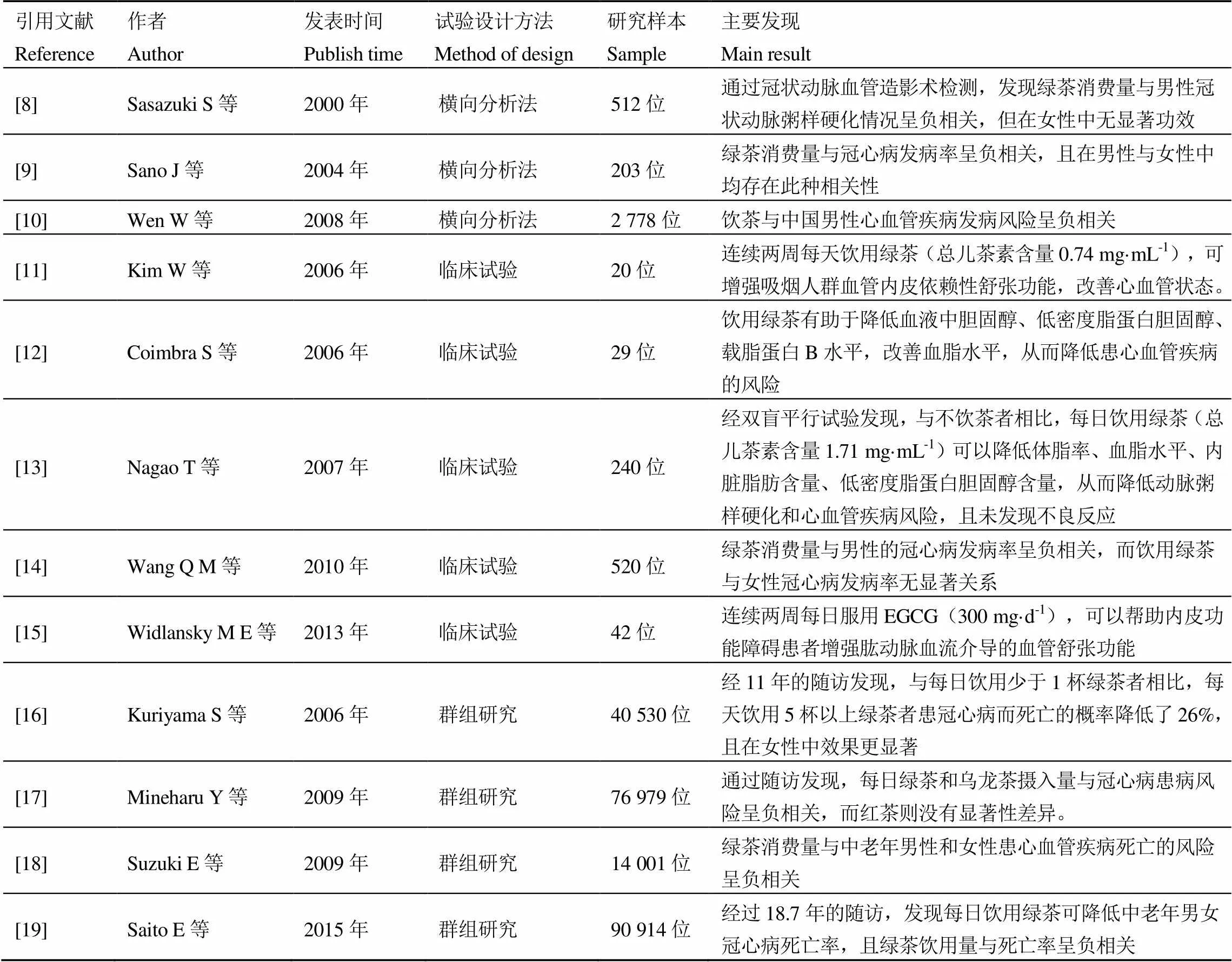
表1 茶叶消费与心血管疾病的关系研究
注:一般来说,冲泡后的绿茶与乌龙茶每杯(237 mL)中EGCG含量为126.5~548.5 μg·mL-1,红茶中EGCG含量为0~295.3 μg·mL-1 [20]
Note: Generally speaking, the content of EGCG in each cup (237 mL) of brewed green tea and oolong tea is 126.5-548.5 μg·mL-1, and that in black tea is 0-295.3 μg·mL-1[20]
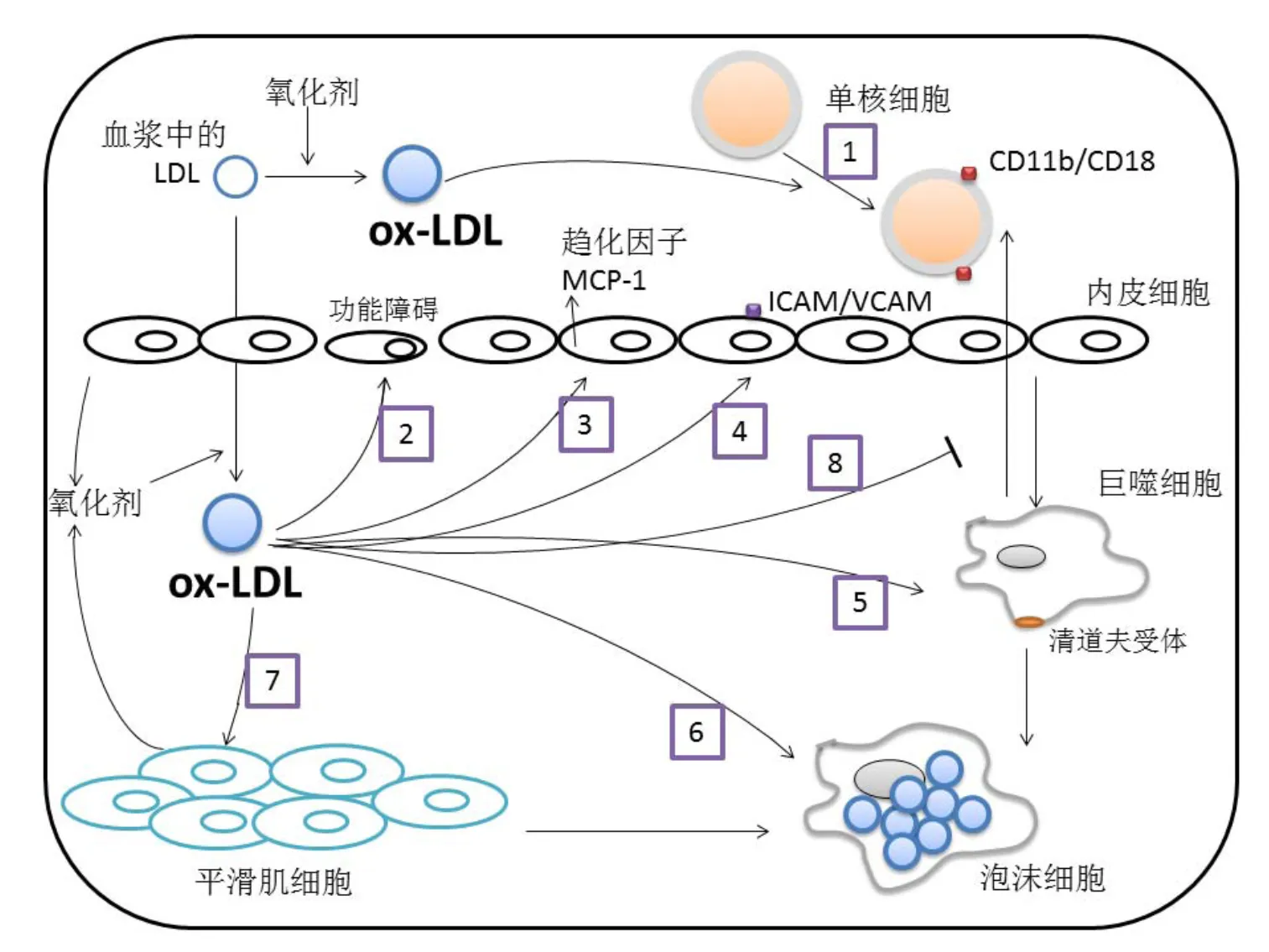
注:(1)血流中的ox-LDL刺激单核细胞上调CD11b/CD18粘附分子的表达,(2)平滑肌细胞和内皮细胞产生活性氧,LDL进入内皮之后,在活性氧的作用下被氧化成为ox-LDL。ox-LDL可诱导内皮功能障碍,(3)ox-LDL诱导促炎细胞因子的表达,如MCP-1,(4)ox-LDL诱导生长因子和几种细胞表面粘附分子的表达,细胞表面粘附分子促使单核细胞和T淋巴细胞进入内膜,(5、6)ox-LDL激活单核细胞向巨噬细胞的发育,巨噬细胞通过清道夫受体无反馈性吞噬大量ox-LDL,(7)ox-LDL刺激细胞增殖和平滑肌细胞迁移,(8)ox-LDL抑制巨噬细胞的迁移。图中箭头表示激活或诱导,竖线表示抑制或阻碍
2 作用机理
2.1 抗炎作用
2.1.1 下调AS相关炎症因子
大量试验研究与临床观察证实,受损内皮细胞分泌多种炎症因子以及生长因子,诱导单核-巨噬细胞通过免疫球蛋白超家族、整合素家族与内皮细胞进行黏附,进而进入到内皮细胞下,是AS的诱因[26,35-36]。Ross[7]和Libby[4]的炎症学说认为,某些脂类如溶血磷脂、氧化固醇等作为信号分子,与内皮细胞的受体结合后可以激活许多促炎细胞因子的基因表达,如单核细胞趋化蛋白-l(Monocyte chemoattractant protein-1,MCP-1)、血管-细胞粘附分子(Vascular cell adhesion molecular-1,VCAM-1)、细胞间粘附分子(Intercellular adhesion molecular-1,ICAM-1)、内皮细胞白细胞粘附分子(E-selectin)等。当巨噬细胞被激活时,会分泌致炎因子如白细胞介素-1(Interleukin-1,IL-1)、IL-6、TNF-与CRP等,这些炎症因子直接参与粥样斑块形成[26]。茶多酚能够下调AS相关炎症因子的表达。
VCAM-1和ICAM-1在白细胞粘附于血管内皮细胞表面并进入内皮下,以及在平滑肌细胞增殖中起到重要作用[35]。Chae等[37]通过体外研究发现,10~50 μmol·L-1EGCG处理可显著抑制人脐静脉血管内皮细胞(Human umbilical vein endothelial cells,HUVEC)中由AngII诱导的VCAM-1与ICAM-1相关mRNA合成,以及减少HUVEC细胞膜上的VCAM-1和ICAM-1分子。Ludwig等[38]发现,EGCG(10~100 μmol·L-1)可剂量依赖性地抑制由IL-1诱导的HUVEC细胞内VCAM-1表达,并抑制TNF-诱导的THP-1单核细胞粘附于血管内皮细胞。
MCP-1是一种对于单核细胞、嗜碱性粒细胞、记忆性T细胞有效的化学吸引蛋白质[39],血清中MCP-1含量升高是冠状动脉疾病风险的炎症标志物之一[36]。早期研究显示,在人类[40]和灵长类动物[41]中,可以观察到AS斑块中MCP-1相关mRNA的表达有所增加,表明了MCP-1在动脉壁中介导单核细胞浸润的潜在作用。Wang等[42]研究表明,EGCG(10、25、50 μmol·L-1)在HUVEC细胞中抑制TNF-诱导的MCP-1产生,且抑制效果呈现剂量依赖关系。Ahn等[39]发现,EGCG(10、30、50 μmol·L-1)可剂量依赖性地抑制牛冠状动脉微血管内皮细胞内Akt的磷酸化以及TNF-受体1(TNF-receptor 1,TNFR1)上的TNF因子活化,从而减少MCP-1的产生。
CRP作为炎性细胞因子直接参与粥样斑块形成。Ramesh等[43]用高脂饮食建立AS大鼠模型,研究EGCG的抗AS作用。结果表明,给AS大鼠腹腔注射EGCG(100 mg·kg-1)两周,可显著降低CRP的表达,降低炎症血液学参数(红细胞沉降率、白细胞总数等),说明EGCG的抗AS功效与抗炎作用密切相关。
2.1.2 调节信号转导通路
茶多酚可以影响细胞内多条与AS相关的炎性信号通路,目前研究表明它主要可以作用于核因子活化B细胞κ轻链增强子(Nuclear factor kappa-light-chain-enhancer of activated B cells,NF-κB)信号通路、丝裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)/NF-κB信号通路、NF-κB/Notch信号通路、微小RNA(MicroRNA,miRNA)信号通路等。
大量研究证实,NF-κB信号通路是调控转录多种炎性因子的中心环节和共同通路,与AS的发生发展密切相关[37,44]。茶多酚不是NF-κB信号通路的特异性抑制剂,但能部分抑制NF-κB信号通路,显著下调炎症因子包括黏附分子、细胞因子和基质金属蛋白酶的表达,发挥抗AS的作用[23]。Wang等[42]发现,EGCG在HUVEC细胞中阻断67 kDa层粘连蛋白受体介导的NF-κB信号通路,从而抑制HUVEC细胞产生炎症因子MCP-1。除了EGCG对NF-κB信号通路活化有良好的抑制作用之外[42,45],ECG也能显著抑制由TNF-α诱导的NF-κB通路活化,抑制促炎因子的分泌,抑制炎症进程[46]。
MAPK信号通路在调节细胞增殖、分化、转化以及凋亡方面起到重要作用[47-49]。MAPK通路中的3种激酶在内皮的损伤和保护中起关键作用[47,49],包括细胞外信号调节激酶(Extracellular signal-regulated kinases,ERK),c-Jun氨基末端激酶(c-Jun-N-terminal kinase,JNK)和p38蛋白激酶(p38 MAPK)。ERK一般被有丝分裂原和生长因子激活,介导细胞增殖;p38 MAPK和 JNK激酶的激活则源于应激反应,介导细胞应激和凋亡[47,49],其中p38 MAPK与NF-κB之间形成的相互激活网络能调控多种炎症介质的基因表达,促进AS的发生发展。Yang等[50]发现,在Ang II诱导的HUVEC细胞模型中,EGCG(5~25 μmol·L-1)可以抑制Ang II诱导的p38 MAPK和JNK1/2激酶活化,减小Ang II引起的内皮功能障碍。Chae等[37]发现EGCG(10~50 μmol·L-1)对抗HUVEC炎症和黏附反应是通过抑制p38 MAPK信号通路实现的,EGCG抑制了MAPK通路中p38 MAPK和ERK的磷酸化。
许多研究表明Notch信号通路在AS的炎症反应中起到重要作用[51-53]。研究显示,在受损的心肌和血管中,Notch的配体和受体数量都有所增加[54-56]。Xie等[57]用尿酸刺激建立HUVEC体外炎症模型,发现用EGCG(20 μmol·L-1)进行预处理能有效抑制细胞中Notch信号通路,并显著抑制炎症因子MCP-1、IL-6和TNF-的分泌,减少炎症因子对内皮细胞的损伤。
miRNA是一类广泛存在于动植物体内的非编码单链小分子RNA,通过与特异性靶信使结合,在转录后水平抑制基因表达。miRNA广泛参与细胞分化、生长发育、增殖与凋亡等各种过程[58-59]。研究发现,多种miRNA与AS的进程高度相关[60-61]。而低剂量的茶多酚(10 μmol·L-1)处理48 h即能显著影响miRNA信号通路,调控多种炎症相关miRNA的表达[62]。杨红霞等[63]发现,EGCG(25 μmol·L-1,50 μmol·L-1)通过抑制THP-1巨噬细胞源性泡沫细胞中的miR-33a信号通路,进而调控ABCA1(介导细胞胆固醇流出的主要膜转运体)表达,促进巨噬细胞内胆固醇流出。
2.2 调节脂质代谢异常
2.2.1 调节血脂水平
血脂异常是AS重要诱导因素,调脂治疗可预防AS[4,64]。血浆内高水平的低密度脂蛋白胆固醇(Low-density lipoprotein cholesterol,LDL-C)是AS的重要诱因[65]。在动物试验[66-68]与人体试验[69-72]中茶多酚的调节血脂作用均有报道。Ikeda等[73]发现不同单体组合的儿茶素对大鼠肠道吸收胆固醇有抑制作用,其中以ECG和EGCG的组合效果最佳。Nagao等[16]将270名志愿者分成两组,其中一组每天饮用340 mL富含绿茶儿茶素(1 714 μg·mL-1)的饮品,另一组每天饮用相同体积的对照饮品(含绿茶儿茶素283 μg·mL-1),连续12周后发现,绿茶儿茶素可以有效降低血压和血脂,同时降低血液内LDL含量。Imbe等[74]让志愿者连续12 d每日饮用600 mL绿茶提取物溶液(含儿茶素1 012.5 μg·mL-1),结果显示,受试者总胆固醇和LDL-C水平均显著降低。
茶多酚还可以加速胆固醇的代谢及促进胆固醇的排泄。连续6周给小鼠喂饲EGCG(0.2%体重、1%体重)可显著抑制胆固醇合成相关基因的表达,上调肝细胞中控制将胆固醇转变为胆汁酸的CYP7A1基因的mRNA表达,加速小鼠体内胆固醇代谢[75]。Hsu等[76]发现,当人摄入高脂饮食时,饮用富含茶多酚(276.7 μg·mL-1)的乌龙茶可以增加脂类物质的排泄量。Muramatsu等[77]用高胆固醇食物喂养大鼠建立高血脂模型,然后将绿茶儿茶素(含EGCG,EGC,ECG和EC)添加进入大鼠食物(10~20 g·kg-1),发现绿茶儿茶素可以显著增加大鼠粪便中总脂肪与总胆固醇含量。
胰胆固醇脂酶(Pancreatic cholesterol esterase,PCE)是小肠内胆固醇吸收的关键酶,胰脂肪酶(Pancreatic lipase,PL)是肠道内脂肪吸收的关键酶[78]。PCE与PL作为脂质代谢的重要酶,在AS发生发展过程中起着重要作用。Jeon等[79]发现,儿茶素聚合物(125 μmol·L-1)可以显著抑制PCE和PL的活性。Nakai等[80]从茶叶中分离出54种多酚类物质,并检测这些多酚物质对PL的抑制作用,发现其中EGCG的抑制作用最佳。
2.2.2 抑制LDL的氧化修饰
低密度脂蛋白(Low-density lipoprotein,LDL)是血浆中主要的携带胆固醇的脂蛋白,它由25%的载脂蛋白B-100(Apolipoprotein- B100,apoB100)和约75%的脂质组成[81]。根据AS的氧化应激理论,活性氧(Reactive oxygen species,ROS)导致LDL氧化,生成ox-LDL[82]。氧化产物ox-LDL被单核-巨噬细胞吞噬,载脂的巨噬细胞转化为泡沫细胞并累积,进一步导致动脉粥样硬化[83-84]。
LDL氧化涉及脂质过氧化与载脂蛋白改性两个方面[85],防止LDL氧化是防治AS的有效方法[84]。越来越多的科学研究表明,以茶多酚为代表的膳食多酚有助于抑制LDL氧化,预防冠心病[86-90]。
茶多酚可以抑制LDL上的apoB100改性[91]。Goto等[92]发现,EGCG(25 μmol·L-1)通过上调LDL受体水平和降低apoB100水平改善胆固醇代谢。刘波静[93]分别用茶多酚(200 mg·kg-l·d-l)和吉非罗齐(200 mg·kg-l·d-l,高血脂症常用药物)与高脂饲料一同喂养小鼠,发现茶多酚和吉非罗齐都具有升高血清apoA1,降低apoBl00和apoBl00/apoAl比值的效果,二者相比茶多酚的作用更为明显。
茶多酚可以通过清除自由基抑制脂质过氧化[91]。自由基可以通过Fen-ton反应和Haber-weiss反应介导ROS的生成,而ROS与AS发病进程密不可分。ROS与生物膜的磷脂、酶和膜受体相关的多不饱和脂肪酸的侧链及核酸等大分子物质起脂质过氧化反应,形成脂质过氧化产物(Lipid Peroxide,LPO)。此外,ROS在ox-LDL介导的内皮细胞毒性中起重要作用,特别是通过激活caspase级联和细胞凋亡引起内皮细胞损伤[94-96]。茶多酚结构组成中的芳香环能和羟基结合,中和自由基,抑制LDL的氧化修饰,从而预防AS[97-98]。Choi等[99]发现,EGCG(25 μmol·L-1)可以抑制ox-LDL诱导的ROS产生,从而保护人血管内皮细胞。Li等[100]研究表明,EGCG(10~100 μmol·L-1)降低AngII诱导的NADPH氧化酶表达,减少ROS的产生,同时抑制AngII诱导的NF-κB和蛋白因子-1(Activator protein-1,AP-1)的激活。
过渡元素中金属元素如铁、铜等是许多自由基产生过程的催化剂[101]。茶多酚具有邻苯二酚结构,有较强的络合金属离子的性能[102]。Cu2+促进脂质过氧化,催化脂质过氧化物的分解,生成脂烷氧基和过氧自由基[103]。Fe2+同样介导脂质过氧化,也是·OH等自由基产生的媒介物。Potapovich等[104]的研究表明,茶多酚可螯合巨噬细胞中的Cu2+、Fe2+等过渡金属离子,使之失活,从而影响LDL氧化。
细胞内存在自身抗氧化防御系统,能及时清除体内过剩的自由基,维持其动态平衡。这些抗氧化防御系统主要包括抗氧化酶(超氧化物歧化酶SOD、过氧化氢酶CAT、谷胱甘肽过氧化物酶GSH-Px、谷胱甘肽硫转移酶 GSTs等)和一些低分子化合物(VE、VC以及谷胱甘肽等),它们可以增强机体抗氧化防御能力,抑制脂质过氧化水平。而茶多酚作为中间抗氧化剂,可与以上物质进行协同抗氧化,从而更好地发挥防御系统的功能,保持体内自由基的动态平衡。赵秀兰等[105]用高脂饲料喂养试验家兔建立AS模型,并用含茶多酚(0.2%、0.4%)的饮用水与高脂饲料一同进行喂养。结果显示,茶多酚组的家兔体内SOD与GSH-Px活力升高,血清中脂质过氧化产物MDA的含量降低,AS发展受到抑制,表明茶多酚可以稳固机体的氧化还原平衡并抑制LDL的氧化。Jayashree等[106]发现,连续一周给小鼠喂食绿茶提取物(50 mg·kg-1,100 mg·kg-1,200 mg·kg-1)可以抑制由喂食Patulin(2 mg·kg-1)导致的SOD、CAT、GSH-Px活力减弱,从而减小氧化损伤。此外,茶多酚也可以促进VC、VE等胞内低分子抗氧化剂修复再生和循环利用,从而抑制LDL氧化[107-108]。
2.3 改善内皮功能
2.3.1 抑制内皮细胞凋亡
血管内皮细胞(Endothelial cells,ECs)凋亡是AS病理发生的早期事件,可促进粥样硬化病变形成、斑块侵蚀和急性冠脉综合征(Acute Coronary Syndromes,ACS)发展[109]。ECs损伤和AS前期形成的多种危险因素可诱导ECs凋亡。茶多酚可通过抑制ECs凋亡延缓AS病变进程,这种对ECs凋亡的抑制作用与其抑制caspase家族的激活与超常表达相关。
Caspase家族是细胞凋亡过程中的关键元件,其激活与超常表达均会引起细胞凋亡。茶多酚作为一种caspase抑制剂可以有效抑制ox-LDL介导的ECs凋亡[110-111]。Jeong等[112]发现,茶多酚抑制ox-LDL介导的血管内皮细胞凋亡是通过阻碍内皮细胞凋亡信号级联实现的。
细胞凋亡相关基因bcl-2家族通常可以抑制细胞凋亡,但是其组分bax基因却起促进凋亡的作用[113]。ox-LDL使促进凋亡的bax基因过度表达,且减少bcl-2和bcl-XL的表达[111]。在H2O2诱导凋亡的鼠平滑肌细胞模型中, 10~150 μmol·L-1的EGCG预处理可显著增加bcl-2基因的表达水平,降低bax基因的表达,同时降低由H2O2刺激引起的caspase-3,caspase-8与caspase-9水平升高[114]。
2.3.2 调控NO产生,改善内皮依赖性舒血管作用
内皮型一氧化氮合酶(Endothelial NO synthase,eNOS)减少是动脉粥样硬化过程中主要的代谢紊乱现象之一[115],它对血管通透性与血管功能起着重要作用。蛋白激酶Akt和磷酸肌醇3激酶PI3K可以使其激活[116]。一氧化氮(Nitric oxide,NO)是一类内皮衍生因子,对血管通透性和炎症状态非常重要,在内皮中受一氧化氮合酶(Endothelial NOS,eNOS)调节[117]。NO与AS病程中的许多关键因素有关,例如LDL氧化、内皮白细胞粘附以及血管平滑肌细胞增殖等。研究表明,在具有AS症状的内皮细胞中,eNOS相关mRNA水平出现下调。Xuan等[68]发现,EGCG(10 mg·L-1)通过激活PI3K/Akt信号通路,上调血管内皮细胞NO水平,降低细胞内caspase-3水平,保护内皮细胞。还有研究表明,EGCG通过PI3K/Akt信号通路增加血管内皮细胞内eNOS的产生量,使细胞内NO量增加,并提高胰岛素敏感性,从而改善内皮功能[96,118]。Liu等[119]发现,Hcy可以抑制HUVEC细胞产生eNOS,而EGCG(3~30 μmol·L-1)可以显著改善这一现象,同时EGCG抑制细胞内的促凋亡因子caspase-3和caspase-9的表达。EGCG保护内皮功能的示意图见图3。
2.4 保持斑块稳定性
2.4.1 调节关键酶活性
稳定性差的AS斑块易发生破裂,导致急性心血管事件,这是冠心病的主要死因[120]。脂质核心的成分及纤维帽的厚度是决定AS斑块是否稳定的关键因素。AS斑块中的细胞外基质过度降解会使斑块的胶原含量减少,纤维帽变薄,从而导致斑块不稳定。基质金属蛋白酶(Matrix metalloproteinase,MMP)是一类具有降解细胞外基质活性的蛋白酶超家族,其中的成员MMP-9与AS斑块的不稳定性尤其相关[121-122]。MMPs组织抑制因子(Tissue inhibitor of metalloproteinase,TIMP)是内源性的MMP-9拮抗剂,能够抑制MMP-9的活化,减弱其降解细胞外基质的能力。
茶多酚能抑制MMPs的表达,增加TIMP的表达,从而抑制细胞外基质过度降解,维持斑块稳定。在体外试验中,EGCG(1~10 μmol·L-1)可以抑制人主动脉血管平滑肌细胞中MMP-2的活化,并上调MMP-2的组织抑制剂TIMP-2蛋白的表达[123]。Bolduc等[124]使用含儿茶素(30 mg·kg-1·d-1)的饮用水喂养自发性动脉粥样硬化(ATX)LDLr-/-:hApoB+/+小鼠3个月,发现儿茶素可以抑制ATX小鼠体内由ROS诱导的MMP-9活化以及血管平滑肌细胞增殖。Cheng等[125]用含绿茶提取物(1 mg·mL-1)的饮用水(42.3±9.4 mL·d-1)喂养雄性Wister大鼠,一周后损伤大鼠左颈总动脉,然后继续用相同的饮用水喂养大鼠两周,结果发现绿茶提取物组的大鼠左颈总动脉内膜增生显著减少,左颈总动脉内膜面积比对照组小30%,且绿茶提取物抑制了大鼠受损动脉中MMP-2的活化,上调了TIMP-2的表达。
2.4.2抗血小板活性
茶多酚具有抗血小板与抗血栓形成的作用[126-127]。通过抗血小板治疗可以减少局部血栓形成、抑制血管炎症,从而稳定易损的AS斑块。正常血小板具有四种功能,即活化、黏附、聚集和分泌。在一些刺激因素(如凝血酶、肾上腺素、血栓素A2)的作用下,血小板糖蛋白GPⅡb/Ⅲa与纤维蛋白原结合,促血小板聚集,导致血栓形成以及纤维蛋白的沉积。活化后的血小板可释放生长因子,诱导血管平滑肌细胞迁移与增殖。所以抑制血小板活化在AS防治中非常重要。
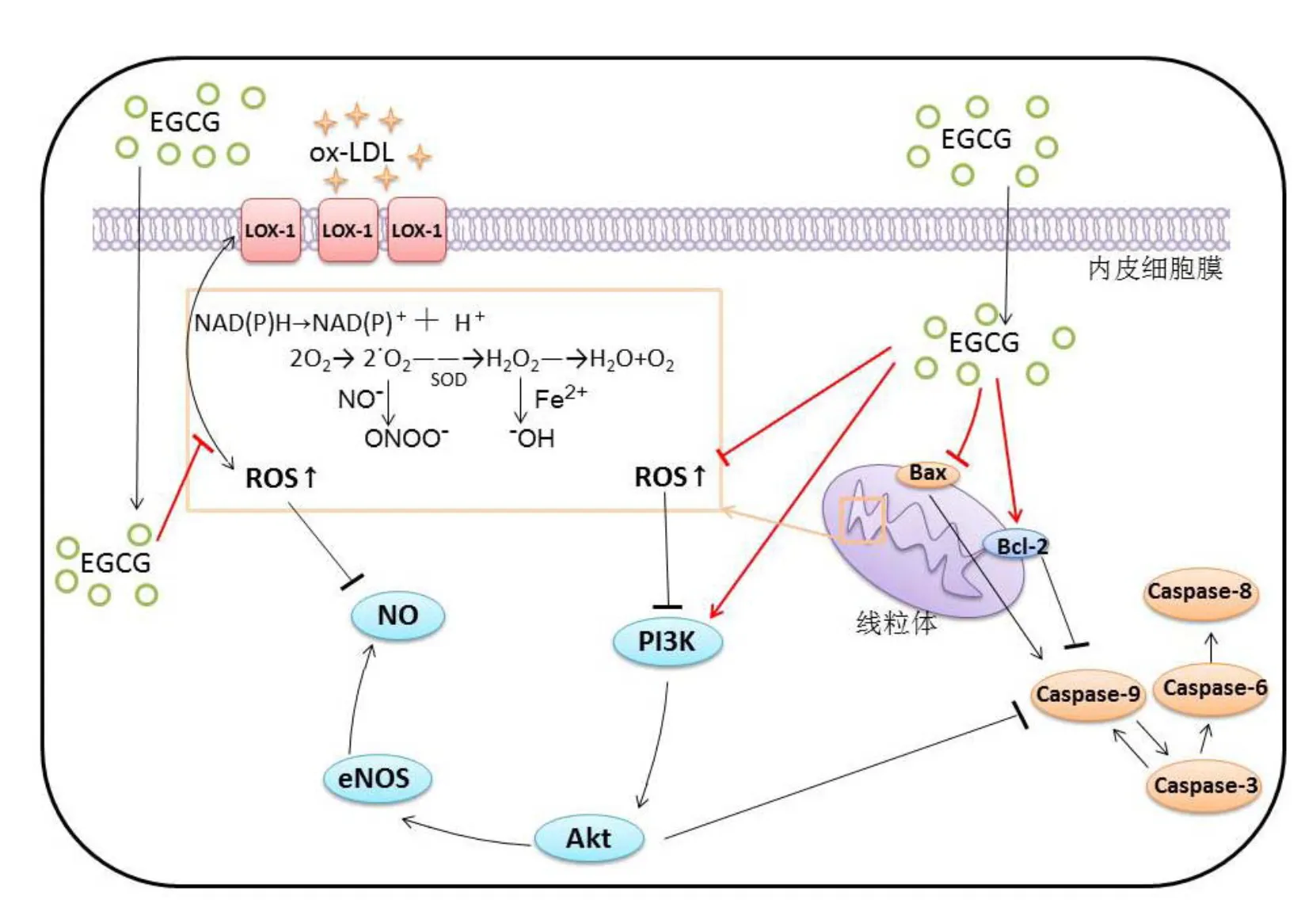
注:LOX-1为低密度脂蛋白受体-1;图中箭头表示激活或诱导,竖线表示抑制或阻碍
茶多酚具有抗血栓与抗凝血作用。研究发现,茶多酚可以抑制胶原、ADP、肾上腺素与钙离子载体等不同刺激因素诱导的离体血小板聚集[128-130]。体内研究表明,灌胃或通过饮食摄入茶多酚可不同程度地抑制血小板聚集及血小板活性因子的合成活性[128,131-132]。Chen等[131]以0.5、1 g·kg-1·d-1EGC灌胃小鼠30 d,发现EGC显著抑制小鼠的血小板聚集,抑制率分别达18.4%和25.6%,高剂量组小鼠中内源性的活化凝血激酶时间也显著延长。Choi等[132]发现,摄入儿茶素可显著抑制慢性镉中毒大鼠的血小板血栓素A2增加,从而达到抑制血小板聚集,调节炎症的作用。试验表明,在人体内茶多酚也存在抗血小板活化的作用。在一项针对75名健康男性的试验中,受试者每日服用250 mL红茶提取物溶液(儿茶素含量为268.8 μg·mL-1),6周后其血液中单核细胞-血小板聚集体以及嗜中性粒细胞-血小板聚集体均有减少,说明长期饮茶或可减弱血小板活化反应[133]。
然而,也有研究指出,饮茶对冠状动脉疾病患者的血小板聚集活性无显著作用。Duffy等[134]将49名冠状动脉疾病的患者随机分成两组,一组每日服用900 mL红茶(儿茶素含量约130 μg·mL-1),另一组则饮用同量的水。8周后,2组患者的体外血小板聚集活性无明显差异。但因本试验中的患者同时在服用阿司匹林,故在不服用阿司匹林药物的情况下,红茶对血小板聚集活性的影响仍未明晰。
2.4.3 对抗病理性血管生成
目前多数学者认为,血管生成分为生理性和病理性血管生成两部分[135]。病理性血管新生是很多炎性疾病的特征,这些新生血管可以诱发AS斑块的破裂。抗血管生成疗法可以稳定易损AS斑块,抑制血管进一步变窄和炎症发展[136]。
血管平滑肌细胞(Vascular smooth muscle cells,VSMCs)是AS斑块主要的细胞成分,VSMCs的病理性增生是AS发展过程中的重要步骤。众多研究表明,一些生长因子,比如PDGF,VEGF与Ang II等均可以促进VSMCs的生长,在AS的病程中发挥重要作用[6,136]。在正常血管和早期出现粥样斑块的血管中,诱导平滑肌细胞凋亡可以抑制血管损伤后的病理性内膜增生[135]。Ahn等[137]使用EGCG(1~100 μmol·L-1)对大鼠主动脉平滑肌细胞进行预处理,随后用PDGF-BB和FCS诱导细胞病理性生长,结果发现,与对照组相比,EGCG预处理可以显著抑制细胞中血管内皮生长因子如ERK1/2、VEGF-R1和VEGF-R2的磷酸化,从而抑制平滑肌细胞的病理性增生。Lin等[138]发现,EGCG(50 mg·kg-1)可以显著抑制由Ang II诱导的平滑肌细胞的迁移与增殖。EGCG抑制VSMCs病理性增殖与迁移的功效的原理,可能是调节NF-κB信号通路,影响胞内p53活化[139],以及降低血小板衍生生长因子受体b(PDGF-Rb)及其下游信号靶点ERK1/2的活性[137]。Kavantzas等[140]用高脂喂养A、B两组雄性新西兰白兔,同时在B组的饮食中添加2.5%(︰)的绿茶,17周后发现,B组白兔VEGF阳性染色泡沫细胞和平滑肌细胞数量显著小于A组,且B组的斑块面积相比A组(对照组)减小大约30%。
3 总结与展望
茶多酚能够作用于AS形成和进展过程的各个环节,降低动脉粥样硬化危险,主要作用包括:下调AS相关炎症因子,调节细胞信号通路,调节脂质代谢异常,改善内皮功能,调节斑块稳定相关酶活性,抗血小板活性,对抗病理性血管生成等。
目前,针对动脉粥样硬化,以茶多酚为活性成分或食品功能因子研发的产品较少。可将茶多酚作为多种预防心血管疾病的保健食品的基料,用于开发具有预防保健活性的食品、饮料等,发挥茶多酚对AS的预防功效。
茶多酚作为一种天然产物,安全性强,毒副作用弱,且水溶性较好,将茶多酚用作AS辅助药物的研究具有科学意义和应用价值。但是,在将茶多酚应用到AS临床研究的过程中,依然存在一些需要解决的问题。其一是茶多酚对于AS的改善功能在不同年龄、不同性别的人群是否都有效仍存在争议,其二是人体吸收代谢的复杂性使到达血管的茶多酚含量及活性都受到了很大的影响。
就流行病学中的研究出现的结论不统一,可能是研究对象的日常生活方式不同而导致的,比如是否吸烟、日常饮食习惯是否良好等。关于流行病学调查由于混杂因素而出现的不一致结果,可以进一步加强临床试验以作补充。
此外,由于试验设计不同,体外研究与体内环境之间的差异,以及动物试验与在人体内功效的差异,使得体外研究、动物试验与人体试验研究结论不完全一致。人体试验时一般采用口服的方法,生物利用度是影响茶多酚抗AS作用的重要因素。因此可以考虑采用茶多酚改性的方法来增强茶多酚的吸收与在人体中的活性,助其更好地发挥作用。此外需要注意的是,人体吸收代谢是一个复杂的过程,短期摄入茶多酚与长期摄入茶多酚产生的预防AS功效有所不同,当长期摄入茶多酚时,其在人体内的代谢与积累情况还有待进一步深入研究。
[1] World Health Organization. Cardiovascular diseases (CVDs) [OL]. (2017-05-17)[2018-07-04]. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
[2] World Health Organization. World Health Statistics 2017: Monitoring health for the SDGs [OL]. (2017-05-17)[2018-07-04]. http://www.who.int/ gho/ publications/world_health_statistics/ 2017/en.
[3] 陈伟伟, 高润霖, 刘力生, 等. 《中国心血管病报告 2017》概要[J]. 中国循环杂志, 2018, 33(1): 1-8.
[4] Libby P. Changing concepts of atherogenesis [J]. Journal of internal medicine, 2000, 247(3): 349-358.
[5] Lusis A J. Atherosclerosis [J]. Nature, 2000, 407(6801): 233-241.
[6] 李露, 吕佳倩, 江承佳, 等. 茶多酚对心血管保护作用的研究进展[J]. 食品科学, 2016, 37(19): 283-288.
[7] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s [J]. Nature, 1993, 362(6423): 801-809.
[8] Sasazuki S, Kodama H, Yoshimasu K, et al. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women [J]. Annals of epidemiology, 2000, 10(6): 401-408.
[9] Sano J, Inami S, Seimiya K, et al. Effects of green tea intake on the development of coronary artery disease [J]. Circulation Journal, 2004, 68(7): 665-670.
[10] Wen W, Xiang Y B, Zheng W, et al. The association of alcohol, tea, and other modifiable lifestyle factors with myocardial infarction and stroke in Chinese men [J]. CVD prevention and control, 2008, 3(3): 133-140.
[11] Kim W, Jeong M H, Cho S H, et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers [J]. Circulation Journal, 2006, 70(8): 1052-1057.
[12] Coimbra S, Santos-Silva A, Rocha-Pereira P, et al. Green tea consumption improves plasma lipid profiles in adults [J]. Nutrition research, 2006, 26(11): 604-607.
[13] Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans [J]. Obesity, 2007, 15(6): 1473-1483.
[14] Wang Q M, Gong Q Y, Yan J J, et al. Association between green tea intake and coronary artery disease in a Chinese population [J]. Circulation Journal, 2010, 74(2): 294-300.
[15] Widlansky M E, Hamburg N M, Anter E, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease [J]. Journal of the American College of Nutrition, 2007, 26(2): 95-102.
[16] Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study [J]. Jama, 2006, 296(10): 1255-1265.
[17] Mineharu Y, Koizumi A, Wada Y, et al. Coffee, green tea, black tea and Oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women [J]. Journal of Epidemiology Community Health, 2010, 65(3): 230-240.
[18] Suzuki E, Yorifuji T, Takao S, et al. Green tea consumption and mortality among Japanese elderly people: the prospective Shizuoka elderly cohort [J]. Annals of epidemiology, 2009, 19(10): 732-739.
[19] Saito E, Inoue M, Sawada N, et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: the Japan public health center-based prospective study (JPHC Study) [J]. Annals of epidemiology, 2015, 25(7): 512-518. e3.
[20] Higdon J V, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions [J]. 2003, 43(1): 89-143.
[21] Van den Brandt P A. Coffee or Tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women [J]. European journal of epidemiology, 2018, 33(2): 183-200.
[22] Tian C, Huang Q, Yang L, et al. Green tea consumption is associated with reduced incident CHD and improved CHD-related biomarkers in the Dongfeng-Tongji cohort [J]. Scientific Reports, 2017. https://doi.org/10.1038/srep45949.
[23] Yamagata K, Xie Y, Suzuki S, et al. Epigallocatechin-3-gallate inhibits VCAM-1 expression and apoptosis induction associated with LC3 expressions in TNFα-stimulated human endothelial cells [J]. Phytomedicine, 2015, 22(4): 431-437.
[24] Naito Y, Yoshikawa T. Green tea and heart health [J]. Journal of cardiovascular pharmacology, 2009, 54(5): 385-390.
[25] Ludwig A, Lorenz M, Grimbo N, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells [J]. Biochemical and biophysical research communications, 2004, 316(3): 659-665.
[26] Li M, Liu J T, Pang X M, et al. Epigallocatechin-3-gallate inhibits angiotensin II and interleukin-6-induced C-reactive protein production in macrophages [J]. Pharmacological Reports, 2012, 64(4): 912-918.
[27] Wang C J, Liu J T, Guo F. (-)-Epigallocatechin gallate inhibits endothelin-1-induced C-reactive protein production in vascular smooth muscle cells [J]. Basic & clinical pharmacology & toxicology, 2010, 107(2): 669-675.
[28] Won S M, Park Y H, Kim H J, et al. Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway [J]. Experimental and Molecular Medicine, 2006, 38(5): 525-534.
[29] Zhang S H, Reddick R L, Piedrahita J A, et al. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E [J]. Science, 1992, 258(5081): 468-471.
[30] Chyu K Y, Babbidge S M, Zhao X, et al. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice [J]. Circulation, 2004, 109(20): 2448-2453.
[31] Mika M, Kostogrys R B, Franczyk-Żarów M, et al. Anti-atherosclerotic activity of catechins depends on their stereoisomerism [J]. Atherosclerosis, 2015, 240(1): 125-130.
[32] Hayek T, Fuhrman B, Vaya J, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 1997, 17(11): 2744-2752.
[33] Morrison M, van der Heijden R, Heeringa P, et al. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB[J]. Atherosclerosis, 2014, 233(1): 149-156.
[34] Auclair S, Milenkovic D, Besson C, et al. Catechin reduces atherosclerotic lesion development in apo E-deficient mice: a transcriptomic study [J]. Atherosclerosis, 2009, 204(2): e21-e27.
[35] Ridker P M, Hennekens C H, Roitman-Johnson B, et al. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men [J]. The Lancet, 1998, 351(9096): 88-92.
[36] Martinovic I, Abegunewardene N, Seul M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease [J]. Circulation Journal, 2005, 69(12): 1484-1489.
[37] Chae Y J, Kim C H, Ha T S, et al. Epigallocatechin-3--gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways [J]. Cellular Physiology and Biochemistry, 2007, 20(6): 859-866.
[38] Ludwig A, Lorenz M, Grimbo N, et al. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells [J]. Biochemical and Biophysical Research Communications, 2004, 316(3): 659-665.
[39] Ahn H Y, Xu Y, Davidge S T. Epigallocatechin-3--gallate inhibits TNF-induced monocyte chemotactic protein-1 production from vascular endothelial cells [J]. Life Sciences, 2008, 82(17/18): 964-968.
[40] Nelken N A, Coughlin S R, Gordon D, et al. Monocyte chemoattractant protein-1 in human atheromatous plaques [J]. The Journal of Clinical Investigation, 1991, 88(4): 1121-1127.
[41] Yu X, Dluz S, Graves D T, et al. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates [J]. Proceedings of the National Academy of Sciences, 1992, 89(15): 6953-6957.
[42] Wang Z M, Gao W, Wang H, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF--induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells [J]. Cellular Physiology and Biochemistry, 2014, 33(5): 1349-1358.
[43] Ramesh E, Geraldine P, Thomas P A. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis [J]. Chemico-Biological Interactions, 2010, 183(1): 125-132.
[44] Das S, Babick A P, Xu Y J, et al. TNF--mediated signal transduction pathway is a major determinant of apoptosis in dilated cardiomyopathy [J]. Journal of Cellular and Molecular Medicine, 2010, 14(7): 1988-1997.
[45] Wheeler D S, Catravas J D, Odoms K, et al. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1β-dependent proinflammatory signal transduction in cultured respiratory epithelial cells [J]. The Journal of Nutrition, 2004, 134(5): 1039-1044.
[46] Kürbitz C, Heise D, Redmer T, et al. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells [J]. Cancer Science, 2011, 102(4): 728-734.
[47] Baines C P, Molkentin J D. Stress signaling pathways that modulate cardiac myocyte apoptosis [J]. Journal of Molecular and Cellular Cardiology, 2005, 38(1): 47-62.
[48] Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt [J]. Journal of Molecular and Cellular Cardiology, 2005, 38(1): 63-71.
[49] Armstrong S C. Protein kinase activation and myocardial ischemia/reperfusion injury [J]. Cardiovascular Research, 2004, 61(3): 427-436.
[50] Yang D, Liu J, Tian C, et al. Epigallocatechin gallate inhibits angiotensin II-induced endothelial barrier dysfunction via inhibition of the p38 MAPK/HSP27 pathway [J]. Acta Pharmacologica Sinica, 2010, 31(10): 1401.
[51] Takeshita K, Satoh M, Ii M, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis [J]. Circulation Research, 2007, 100(1): 70-78.
[52] Marchant D J, Boyd J H, Lin D C, et al. Inflammation in myocardial diseases [J]. Circulation Research, 2012, 110(1): 126-144.
[53] Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF [J]. Oxidative Medicine and Cellular Longevity, 2015, 2015: 1-18. DOI: 10.1155/2015/610813.
[54] Croquelois A, Domenighetti A A, Nemir M, et al. Control of the adaptive response of the heart to stress via the Notch1 receptor pathway [J]. Journal of Experimental Medicine, 2008, 205(13): 3173-3185.
[55] Kratsios P, Catela C, Salimova E, et al. Distinct roles for cell-autonomous Notch signaling in cardiomyocytes of the embryonic and adult heart [J]. Circulation Research, 2010, 106(3): 559-572.
[56] Li Y, Takeshita K, Liu P Y, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury [J]. Circulation, 2009, 119(20): 2686-2692.
[57] Xie H, Sun J, Chen Y, et al. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway [J]. Oxidative Medicine and Cellular Longevity, 2015, 2015: 1-10.
[58] Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives [J]. Current Medicinal Chemistry, 2016, 23(36): 4135-4150.
[59] 马兰, 张基昌, 崔燕, 等. MicroRNAs 与冠心病发生发展关系的研究进展[J]. 中国实验诊断学, 2013, 17(7): 1354-1357.
[60] Albinsson S, Suarez Y, Skoura A, et al. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2010, 30(6): 1118-1126.
[61] Cipollone F, Felicioni L, Sarzani R, et al. A unique microRNA signature associated with plaque instability in humans [J]. Stroke, 2011, 42(9): 2556-2563.
[62] Fix L N, Shan M, Efferth T, et al. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60 [J]. Cancer Genomics-Proteomics, 2010, 7(5): 261-277.
[63] 杨红霞, 高亚, 蒋恒波, 等. EGCG通过miR-33a上调ABCA1表达减少巨噬细胞脂质蓄积[J]. 2016, 39(9): 1279-1284.
[64] 江亚文. 动脉粥样硬化的研究进展[J]. 广东医学, 2003, 24(1): 88-90.
[65] Rose G, Shipley M. Plasma cholesterol concentration and death from coronary heart disease: 10 year results of the Whitehall study [J]. The British Medical Journal, 1986, 293(6542): 306-307.
[66] Yang T T C, Koo M W L. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion [J]. Life Sciences, 1999, 66(5): 411-423.
[67] Ikeda I, Kobayashi M, Hamada T, et al. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate [J]. Journal of Agricultural and Food Chemistry, 2003, 51(25): 7303-7307.
[68] Xuan F, Jian J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux [J]. International journal of molecular medicine, 2016, 38(1): 328-336.
[69] Kajimoto O, Kajimoto Y, Yabune M, et al. Tea catechins reduce serum cholesterol levels in mild and borderline hypercholesterolemia patients [J]. Journal of Clinical Biochemistry and Nutrition, 2003, 33(3): 101-111.
[70] Tsubono Y, Tsugane S. Green tea intake in relation to serum lipid levels in middle-aged Japanese men and women [J]. Annals of Epidemiology, 1997, 7(4): 280-284.
[71] Tokunaga S, White I R, Frost C, et al. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan [J]. Annals of Epidemiology, 2002, 12(3): 157-165.
[72] Wu A H, Spicer D, Stanczyk F Z, et al. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormonal levels in healthy postmenopausal women [J]. Cancer Prevention Research, 2012, 5(3): 393-402.
[73] Ikeda I, Imasato Y, Sasaki E, et al. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats [J]. Biochimica et Biophysica Acta, 1992, 1127(2): 141-146.
[74] Imbe H, Sano H, Miyawaki M, et al. “Benifuuki” green tea, containing O-methylated EGCG, reduces serum low-density lipoprotein cholesterol and lectin-like oxidized low-density lipoprotein receptor-1 ligands containing apolipoprotein B: A double-blind, placebo-controlled randomized trial [J]. Journal of Functional Foods, 2016, 25: 25-37.
[75] Jin H H, Yang J L, Chung J H, et al. Hypocholesterolemic effects of green tea in cholesterol-fed rats [J]. Journal of the Korean Society of Food Science and Nutrition, 2004, 33(1): 47-51.
[76] Hsu T F, Kusumoto A, Abe K, et al. Polyphenol-enriched Oolong tea increases fecal lipid excretion [J]. European Journal of Clinical Nutrition, 2006, 60(11): 1330-1336.
[77] Muramatsu K, Fukuyo M, Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats [J]. Journal of Nutritional Science and Vitaminology, 1986, 32(6): 613-622.
[78] Heidrich J E, Contos L M, Hunsaker L A, et al. Inhibition of pancreatic cholesterol esterase reduces cholesterol absorption in the hamster [J]. BMC Pharmacology, 2004, 4(1): 5. https://doi.org/10.1186/1471-2210-4-5.
[79] Jeon S Y, Imm J Y. Lipase inhibition and cholesterol-lowering activities of laccase-catalyzed catechin polymers [J]. Food Science and Biotechnology, 2014, 23(5): 1703-1707.
[80] Nakai M, Fukui Y, Asami S, et al. Inhibitory effects of oolong tea polyphenols on pancreatic lipase[J]. Journal of Agricultural and Food Chemistry, 2005, 53(11): 4593-4598.
[81] Morita S. Metabolism and modification of apolipoprotein B-containing lipoproteins involved in dyslipidemia and atherosclerosis [J]. Biological and Pharmaceutical Bulletin, 2016, 39(1): 1-24.
[82] Frijhoff J, Winyard P G, Zarkovic N, et al. Clinical relevance of biomarkers of oxidative stress [J]. Antioxidants & redox signaling, 2015, 23(14): 1144-1170.
[83] Steinberg D, Parthasarathy S, Carew T E, et al. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity [J]. The New England Journal of Medicine, 1989, 320(14): 915-924.
[84] Steinberg D. The LDL modification hypothesis of atherogenesis: an update [J]. Journal of Lipid Research, 2009, 50(Sup 1): S376-S381
[85] Frei B, Gaziano J M. Content of antioxidants, preformed lipid hydroperoxides, and cholesterol as predictors of the susceptibility of human LDL to metal ion-dependent and-independent oxidation [J]. Journal of Lipid Research, 1993, 34(12): 2135-2145.
[86] Koo S I, Noh S K. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect [J]. The Journal of nutritional biochemistry, 2007, 18(3): 179-183.
[87] Chen G, Wang H, Zhang X, et al. Nutraceuticals and functional foods in the management of hyperlipidemia [J]. Critical reviews in food science and nutrition, 2014, 54(9): 1180-1201.
[88] Annuzzi G, Bozzetto L, Costabile G, et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial [J]. The American journal of clinical nutrition, 2013, 99(3): 463-471.
[89] Yao Z, Zhang L, Ji G. Efficacy of polyphenolic ingredients of Chinese herbs in treating dyslipidemia of metabolic syndromes [J]. Journal of integrative medicine, 2014, 12(3): 135-146.
[90] Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling [J]. Oxidative Medicine and Cellular Longevity, 2015, 2015: 1-15. http://dx.doi.org/10.1155/2015/504253.
[91] Sukhbold E, Sekimoto S, Watanabe E, et al. Effects of oolonghomobisflavan a on oxidation of low-density lipoprotein [J]. Bioscience, Biotechnology, and Biochemistry, 2017, 81(8): 1569-1575.
[92] Goto T, Saito Y, Morikawa K, et al. Epigallocatechin gallate changes mRNA expression level of genes involved in cholesterol metabolism in hepatocytes [J]. British Journal of Nutrition, 2012, 107(6): 769-773.
[93] 刘波静. 茶多酚对动物血清血脂和载脂蛋白水平的影响和抗氧化作用[J]. 茶叶科学, 2000, 20(1): 67-70.
[94] Li P F, Dietz R, Von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell [J]. FEBS letters, 1997, 404(2/3): 249-252.
[95] Li P F, Dietz R, Von Harsdorf R. Differential effect of hydrogen peroxide and superoxide anion on apoptosis and proliferation of vascular smooth muscle cells [J]. Circulation, 1997, 96(10): 3602-3609.
[96] Vacaresse N, Vieira O, Robbesyn F, et al. Phenolic antioxidants trolox and caffeic acid modulate the oxidized LDL‐induced EGF‐receptor activation [J]. British Journal of Pharmacology, 2001, 132(8): 1777-1788.
[97] Devika P T, Prince P S M. Preventive effect of (-) epigallocatechin-gallate (EGCG) on lysosomal enzymes in heart and subcellular fractions in isoproterenol-induced myocardial infarcted wistar rats [J]. Chemico-Biological Interactions, 2008, 172(3): 245-252.
[98] Singh B N, Shankar S, Srivastava R K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications [J]. Biochemical Pharmacology, 2011, 82(12): 1807-1821.
[99] Choi J S, Choi Y J, Shin S Y, et al. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: role of MAPK-and JAK/STAT-signaling [J]. The Journal of Nutrition, 2008, 138(6): 983-990.
[100] Li H L, Huang Y, Zhang C N, et al. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and-independent signal pathways [J]. Free Radical Biology and Medicine, 2006, 40(10): 1756-1775.
[101] Arai H, Berlett B S, Chock P B, et al. Effect of bicarbonate on iron-mediated oxidation of low-density lipoprotein [J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(30): 10472-10477.
[102] Milde J, Elstner E F, Grassmann J. Synergistic inhibition of low-density lipoprotein oxidation by rutin, γ-terpinene, and ascorbic acid [J]. Phytomedicine, 2004, 11(2/3): 105-113.
[103] Gutteridge J M. Lipid peroxidation and antioxidants as biomarkers of tissue damage [J]. Clinical Chemistry, 1995, 41(12): 1819-1828.
[104] Potapovich A I, Kostyuk V A. Comparative study of antioxidant properties and cytoprotective activity of flavonoids [J]. Biochemistry (Moscow), 2003, 68(5): 514-519.
[105] 赵秀兰, 宫爱华, 李建华, 等. 茶多酚抗动脉粥样硬化机制研究[J]. 中国公共卫生, 2003, 19(8): 930-931.
[106] Jayashree G V, Krupashree K, Rachitha P, et al. Patulin Induced Oxidative Stress Mediated Apoptotic Damage in Mice, and its Modulation by Green Tea Leaves [J]. Journal of Clinical and Experimental Hepatology, 2017, 7(2): 127-134.
[107] Zhu Q Y, Huang Y, Tsang D, et al. Regeneration of α-tocopherol in human low-density lipoprotein by green tea catechin [J]. Journal of Agricultural and Food Chemistry, 1999, 47(5): 2020-2025.
[108] Liu Z Q, Ma L P, Zhou B, et al. Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein [J]. Chemistry and Physics of Lipids, 2000, 106(1): 53-63.
[109] Dimmeler S, Haendeler J, Zeiher A M. Regulation of endothelial cell apoptosis in atherothrombosis [J]. Current Opinion in Lipidology, 2002, 13(5): 531-536.
[110] Wintergerst E S, Jelk J, Rahner C, et al. Apoptosis induced by oxidized low density lipoprotein in human monocyte‐derived macrophages involves CD36 and activation of caspase-3 [J]. The FEBS Journal, 2000, 267(19): 6050-6059.
[111] Salvayre R, Auge N, Benoist H, et al. Oxidized low-density lipoprotein-induced apoptosis [J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2002, 1585(2): 213-221.
[112] Jeong Y J, Choi Y J, Kwon H M, et al. Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids [J]. British Journal of Nutrition, 2005, 93(5): 581-591.
[113] Liu M L, Yu L C. Potential protection of green tea polyphenols against ultraviolet irradiation-induced injury on rat cortical neurons [J]. Neuroscience letters, 2008, 444(3): 236-239.
[114] Yan X, Li J. GW28-e0321 Epigallocatechin-3-gallate inhibits H2O2-induced apoptosis in Mouse Vascular Smooth Muscle Cells via 67 kD Laminin Receptor [J]. Journal of the American College of Cardiology, 2017, 70(Sup 16): C57-C58. DOI: 10.1016/j.jacc.2017.07.196.
[115] Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2004, 24(6): 998-1005.
[116] Vanhaesebroeck B, Leevers S J, Panayotou G, et al. Phosphoinositide 3-kinases: a conserved family of signal transducers [J]. Trends in Biochemical Sciences, 1997, 22(7): 267-272.
[117] Dimmeler S, Zeiher A M. Nitric oxide-an endothelial cell survival factor [J]. Cell death and differentiation, 1999, 6(10): 964-968.
[118] Jacob R A, Gretz D M, Taylor P C, et al. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women [J]. The Journal of nutrition, 1998, 128(7): 1204-1212.
[119] Liu S, Sun Z, Chu P, et al. EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways [J]. Apoptosis, 2017, 22(5): 672-680.
[120] 蔡宏文, 毛威. 动脉粥样硬化斑块内新生血管中西医治疗进展[J]. 中华中医药学刊, 2015, 33(5): 1168-1170.
[121] Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia [J]. Journal of Neuroscience, 2001, 21(19): 7724-7732.
[122] Park J W, Hong J S, Lee K S, et al. Green tea polyphenol (-)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemia [J]. The Journal of Nutritional Biochemistry, 2010, 21(11): 1038-1044.
[123] Cheng X W, Kuzuya M, Nakamura K, et al. Mechanisms of the inhibitory effect of epigallocatechin-3-gallate on cultured human vascular smooth muscle cell invasion [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2005, 25(9): 1864-1870.
[124] Bolduc V, Baraghis E, Duquette N, et al. Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr−/−: hApoB+/+ mice and improves cerebral blood flow [J]. American Journal of Physiology-Heart and Circulatory Physiology, 2012, 302(6): H1330-H1339.
[125] Cheng X W, Kuzuya M, Sasaki T, et al. Green tea catechins inhibit neointimal hyperplasia in a rat carotid arterial injury model by TIMP-2 overexpression [J]. Cardiovascular Research, 2004, 62(3): 594-602.
[126] 王贤波, 成浩, 赵芸, 等. 茶叶中EGCG对小鼠抗凝血作用实验研究[J]. 茶叶科学, 2011, 31(6): 532-536.
[127] 房磊, 王捷熙, 刘敏霞, 等. 负载表没食子儿茶素没食子酸酯对血小板功能与凋亡等影响[J]. 中国实验血液学杂志, 2011, 19(3): 764-768.
[128] Kang W S, Lim I H, Yuk D Y, et al. Antithrombotic activities of green tea catechins and (-)-epigallocatechin gallate [J]. Thrombosis research, 1999, 96(3): 229-237.
[129] Lill G, Voit S, Schrör K, et al. Complex effects of different green tea catechins on human platelets [J]. Febs Letters, 2003, 546(2): 265-270.
[130] Ok W J, Cho H J, Kim H H, et al. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner [J]. Journal of Atherosclerosis and Thrombosis, 2012, 19(4): 337-348.
[131] Chen X Q, Wang X B, Guan R F, et al. Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice [J]. Food & function, 2013, 4(10): 1521-1525.
[132] Choi J H, Chang H W, Rhee S J. Effect of green tea catechin on arachidonic acid cascade in chronic cadmium‐poisoned rats [J]. Asia Pacific journal of clinical nutrition, 2002, 11(4): 292-297.
[133] Steptoe A, Gibson E L, Vuononvirta R, et al. The effects of chronic tea intake on platelet activation and inflammation: a double-blind placebo controlled trial [J]. Atherosclerosis, 2007, 193(2): 277-282.
[134] Duffy S J, Vita J A, Holbrook M, et al. Effect of acute and chronic tea consumption on platelet aggregation in patients with coronary artery disease [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2001, 21(6): 1084-1089.
[135] Rashidi B, Malekzadeh M, Goodarzi M, et al. Green tea and its anti-angiogenesis effects [J]. Biomedicine & Pharmacotherapy, 2017, 89: 949-956.
[136] Jain R K, Finn A V, Kolodgie F D, et al. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization [J]. Nature Reviews Cardiology, 2007, 4(9): 491-502.
[137] Ahn H Y, Hadizadeh K R, Seul C, et al. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB–induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation ofsis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172) [J]. Molecular Biology of the Cell, 1999, 10(4): 1093-1104.
[138] Lin C M, Hou S W, Wang B W, et al. Molecular mechanism of (-)-epigallocatechin-3-gallate on balloon injury-induced neointimal formation and leptin expression [J]. Journal of Agricultural and Food Chemistry, 2014, 62(6): 1213-1220.
[139] Hofmann C S, Sonenshein G E. Green tea polyphenol epigallocatechin-3 gallate induces apoptosis of proliferating vascular smooth muscle cells via activation of p53 [J]. The FASEB Journal, 2003, 17(6): 702-704.
[140] Kavantzas N, Chatziioannou A, Yanni A E, et al. Effect of green tea on angiogenesis and severity of atherosclerosis in cholesterol-fed rabbit [J]. Vascular Pharmacology, 2006, 44(6): 461-463.
Prevention of Tea Polyphenols on Atherosclerosis and Relative Mechanisms
ZHANG Shuping, WANG Yuefei, XU Ping*
Department of Tea Science, Zhejiang University, Hangzhou 310058, China
Atherosclerosis is the pathological basis of many cardiovascular diseases. Its injury to the cardiovascular could cause damage to other organs. Numerous data had indicated that tea polyphenols have a good preventive effect on atherosclerosis, such as anti-inflammatory, regulating blood lipid levels, inhibiting LDL oxidation, improving endothelial function and maintaining the stability of atherosclerotic plaque. The aim of this article is to review the health benefits of tea polyphenols against atherosclerosis and to outline the molecular mechanisms of tea polyphenols in atherosclerosis prevention.
atherosclerosis, tea polyphenols, cardiovascular, anti-inflammatory, vascular smooth muscle cell proliferation
S571.1
A
1000-369X(2019)03-231-16
2018-07-04
2018-09-07
国家广东省产学研合作项目(2016B090918118)
张姝萍,女,硕士研究生,主要从事天然产物与人体健康及其机理研究。*通信作者:zdxp@zju.edu.cn

