Identification and Removal of Microbial Contaminants from Herbal Drugs for Traditional Chinese Medicine Injection
DUAN Jinlian,LI Yue,ZHANG Lumei,DONG Xianxiang,YANG Rong,YIN Hua,GUO Liang,DUAN Weigang△
(1.Key Laboratory of Molecular Biology for Sinomedicine,Yunnan University of Chinese Medicine,Kunming 650500,China;2.Institute of Pharmaceutical Research,Shiyao Yinhu Pharmaceutical Co.,Ltd.,Yuncheng 044000,China)
ABSTRACT:Objective The main aim of the present study was to limit contaminant microbes and their macromolecules in raw herbal drugs,then decrease TCMIs’safety problems.Methods A raw herbal drug from underground part(Danshen,root and rhizome of Salvia miltiorrhiza Bge.)and that from aboveground part(Honghua,flower of Carthamus tinctorius L.)were introduced.The contaminant microbes were assayed at steps of the mimic process of TCMI manufacturing.Microbes adhered to raw herbal drugs Honghua or Danshen were collected,and their DNA encoded 16S rRNA(for bacteria)and 18S rRNA(for fungi)were identified by high-throughput sequencing.The feasibility of removable lipopolysaccharide(LPS)adhered to the raw drugs was investigated with TAL(Tachypleus Amebocyte Lysate)kits by detecting flushing fluid and the mimic injections.Results The results showed that both raw drugs were contaminated with microbes,and the abundance of fungi was heavier than that of bacteria.As a raw herbal drug from aerial part,Honghua contained more bacteria and fungi than Danshen.The contaminant LPS was able to be largely removed by washing.Conclusion All the results suggested that the contaminant microbes,especially fungi,and their macromolecular impurities should be taken into account for TCMI manufacturing,and they were able to be removed largely by washing.
KEY WORDS:microbial contamination;bacteria;fungi;lipopolysaccharide;traditional Chinese medicine injection
Traditional Chinese Medicine injection(TCMI) is a unique preparation for clinical practice in China,which is made from the extracts of raw natural drugs,and most of which are herbal drugs.The drug preparation originated in 1940s[1],and widely used in clinic in the last decades of 20thcentury.There had been more than 1 000 TCMIs,but most of them were excluded or forbidden for clinical practice because of their safety problems,and there are only 132 TCMIs left approved by China Food and Drug Administration(cFDA)at present[2].According to chemicals and substances contained,TCMIs can be divided into four groups,namely,monomeric injections(containing only one active compound),active fraction injections(containing several active compounds shared similar structure),single herbal injections(a total extraction from a natural drug),and multiple herbal injections(an extraction from multiple natural drugs).In fact,most TCMIs contained two or more active compounds,which become a big challenge for pharmaceutical scientists to analyze and evaluate.
TCMIs contain impurities,and the component contributes little to drugs’efficacy,but a lot to adverse reactions,especially anaphylactic and anaphylactoid reactions[3-6].Based on their molecular size,the impurities can be grouped into small molecules and macromolecules,and the latter were usually neglected.According to their sources,macromolecular impurities can be grouped into endogenous impurities that come from the raw drug and can not be removed easily just by processing methods like washing,and exogenous ones are usually from microbes.Endogenous macromolecular impurities include nucleotides,proteins and polysaccharides which are essential components for herb to grow,and exogenous ones usually do little to the medicinal herb growing.According to the modern immunological theory,body’s macromolecules should be in place without disturbance of exogenous ones,and invasive macromolecules are able to result in innate immune response via pathogenassociated molecular pattern(PAMP),or damage associated molecular pattern(DAMP) if macromolecules are similar to the intracellular proteins of the host,or human[7-8].Moreover,microbes and their macromolecules,especially lipopolysaccharide(LPS) are PAMPs that are able to cause anaphylactic and ana phylactoid reactions[9],which became the top safety problem of TCMI.Therefore,the macromolecular impurities should be removed as thoroughly as possible during the process of TCMI preparation.However,most raw drugs for TCMI are herbal drugs contaminated with microbes,and the hazards were underemphasized.The microbes contained in herb can be classified as virus,bacterium and fungus,and the latter two are believed to be the main contaminants that harmful to human health.Therefore,it is needed to understand the microbes and their macromolecules left in the herbal drugs.Honghua injection,made from herbal drug Honghua(flower of Carthamus tinctorius L.,C.tinctorius),and Danshen injection,made from Danshen(root and rhizome of Salvia miltiorrhiza Bge.,S.miltiorrhiza),are representative injections that the present study concerned.The present study will investigate the microbes and LPS left in the two raw drugs for TCMI.
1 MATERIALS AND METHODS
1.1 Materials
Honghua and Danshen for injection preparation were provided by Shiyao Yinhu Pharmaceutical Co.,Ltd and identified by the Department of Pharmaceutical Botany,Yunnan University of Chinese Medicine.TAL kits (0.125 EU/100 μL) were manufactured by Zhanjiang A&C Biological Ltd(Zhanjiang,China).E.Z.N.ATM Mag-Bind Soil DNA Kits were manufactured by Omega Biotec(Guangzhou,China).Ultrapure water free of lipopolysacchride was prepared by Milli Q water purification system manufactured by EMD Millipore Group(Darmstadt,Germany).Centrifugal filter devices(Amincon® Ultra-15)were manufactured by Millipore Corporation (Billerica,USA.).Other reagents of analytical purity were made in China.Multimicroplate reader of Infinite 200 pro was manufactured by Tecan Group(Berne,Switzerland).The freeze drier was manufactured by EYELA(Tokyo,Japan).NanoDrop ND-1000 spectrophotometer was manufactured by Thermo Scientific(Wilmington,USA.).
1.2 Microbe detection
Raw drugs of 50 g were washed with microbefree water for 30 min.The water extraction was made into powder by a freeze drier.Total DNA from 0.2 g powder was extracted and purified by the Soil DNA Kit and dissolved in 70 μL buffer provided by the Soil DNA Kit.DNA quality and quantity were measured by the NanoDrop ND-1000 spectrophotometer.
The DNA encoded by bacterial 16S rRNA was amplified by 25 cycles with primers Bakt_341F(CCTACGGGNGGCWGCAG) and Bakt_805R(GACTACHVGGGTATCTAATCC)[10].The DNA encoded by fungal 18S rRNA was amplified by 25 cycles with primersITS1-F(CTTGGTCATTTAGAGGAAGTAA)and ITS2-R(GCTGCGTTCTTCATCGATGC)[11].The 5’end of the primers was designed as adapters for purification,which were CCCTACACGACGCTCTTCCGATCTG and GACTGGAGTTCCTTGGCACCCGAGAATTCCA,respectively[10].The purified 16S rRNA and 18S rRNA were assessed by standard agarose gel electrophoresis.The DNA was sequenced by Sangon Biotech (Shanghai,China).The amplified sequences were BLASTed with gene bank.The OTUs(operational taxonomic units)and copies were recorded according to the results of BLAST.
1.3 LPS in pre-processing solution
Raw drug of 5 g (Honghua) or 10 g(Danshen)was put into a 500 mL beaker free of LPS.As soon as 200 mL water was added and mixed,1 mL water was sampled(0 min)and another 1 mL was replenished.Then,the raw drug and water were stirred by a magnetic stirrer.The stirred water of 1 mL was sampled and new water of the same volume was replenished at different time.
The LPS in the samples was detected by TAL kits.If the mixture of 100 μL sample and 100 μL TAL caused a positive reaction,the sample would be diluted two folds with LPS-free water until a negative reaction occurred.The time(s) of dilution just for negative reaction was recorded to evaluate the level of LPS.
In order to understand the dissolved substances in the samples from the raw drug,their absorption curve from 230 to 800 nm was scanned by the multimicroplate reader with an interval of 2 nm.The area under the absorption curve(AUC) was calculated based on trapezoidal method[2].
1.4 LPS in mimic injection solution
Honghua injection and Danshen injection were prepared according to the Quality Standard approved by cFDA,and the mimic injections were also prepared according to modified Quality Standards(Table 1).The sample of every step(Table 1)during preparation was also obtained for lipopolysaccharide(LPS)detection.
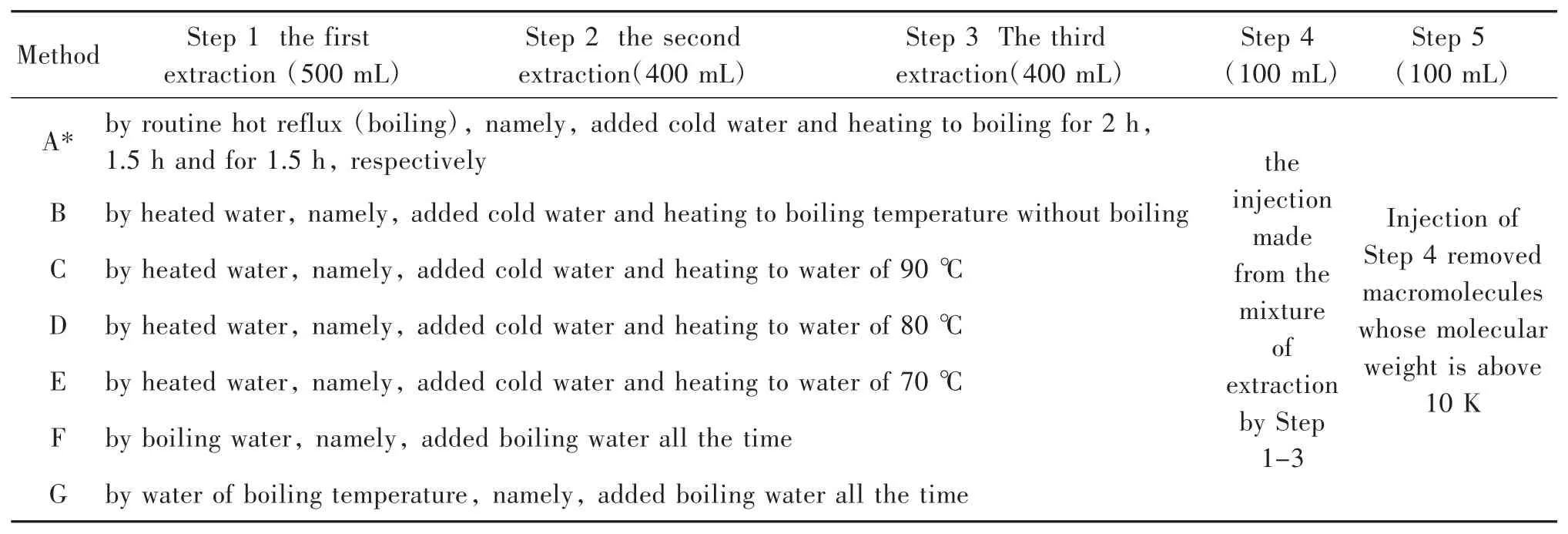
Table 1 Steps and methods for mimic injection preparation
Briefly,Honghua of 60 g and Danshen of 150 g were used.Step 1-4 of Method A was agreed with the Quality Standard approved by cFDA.The colour of the extract of Step 1-3 of Method B and others was controlled to be very similar to that of Method A of same volume by adjusting the processing time.
In order to prove the effect of water-washing pre-processing in removing LPS,the raw drugs(Honghua of 60 g and Danshen of 150 g)were preprocessed with distilled water(500 mL)for 6 min in three times.Then,the mimic injection solutions of Honghua and Danshen were prepared according to Method A(Table 1).
2 RESULTS
2.1 Abundance of microbes
Extract powder of 7.6 g from raw drug(50 g)of Honghua and 0.6 g powder from raw drug(50 g)of Danshen were obtained.The 16S rRNA and 18S rRNA in the powder were amplified and sequenced.The sequencing yielded 1 722 16S rRNA and 1 090 18 rRNA from the extract powder.OTUs of 16S rRNA in Honghua(1 325)were more than those in Danshen(636),and OTUs of 18S rRNA in Honghua(623)were close to those in Danshen(699).According to the total copies showed in Figure 1A,the flower(Honghua)was contaminated with more bacteria and fungi than the root and rhizome(Danshen,Figure 1A and Figure 2A).Even further,the copies of 18S rRNA(Figure 2A)were more than those of 16S rRNA(Figure 1A)whether in the flower or in the root and rhizome,suggesting that the contaminant microbes mainly belonged to fungi rather than bacteria whether in herb from aerial part or underground part.
Bacteria distributed in raw drugs of Honghua and Danshen were different whether at phylum(Figure 1B),class(Figure 1C),order(Figure 1D),family(Figure 1E)or genus(Figure 1F)levels.In Honghua,the bacteria most frequently detected were belonged to Proteobacteria,Alphaproteobacteria,Rhizobiales,Rhizobiaceae and Anderseniella at phylum,class,order,family,and genus level,respectively.However,in Danshen,the bacteria most frequently detected were belonged to Proteobacteria(similar to Honghua),Gammaproteobacteria(different from Honghua),Enterobacteriales(different from Honghua),Enterobacteriacae(different from Honghua)and Pseudomonas(different from Honghua).At the species level,the top 10 bacterias most frequently identified in Honghua and Danshen were shown in Table 2.Obviously,the pattern of bacterial distribution in Danshen was differ-ent from that in Honghua.
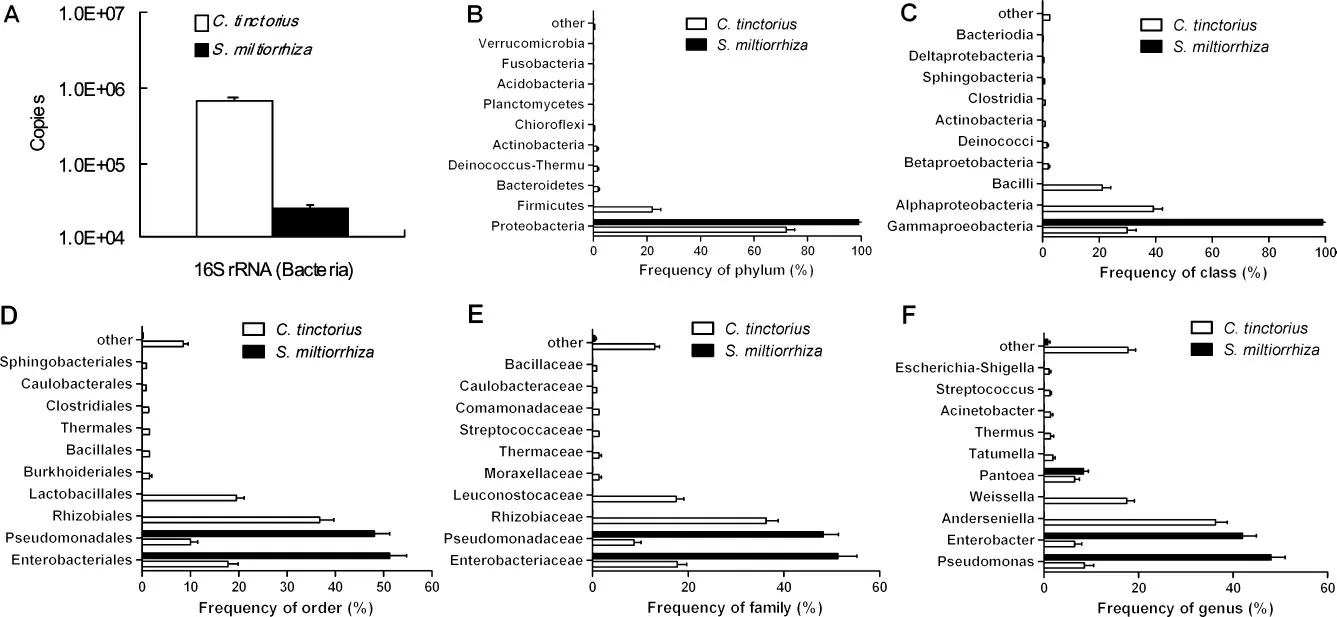
Figure 1 Bacteria in raw drugs of Honghua(C.tinctorius)and Danshen(S.miltiorrhiza)(mean±SD,n=3).The total abundance of bacteria in the raw drug of 50 g was evaluated based on copies of DNA encoding 16S rRNA(A).The main bacteria were evaluated at phylum(B),class(C),order(D),family(E)or genus(F)level.
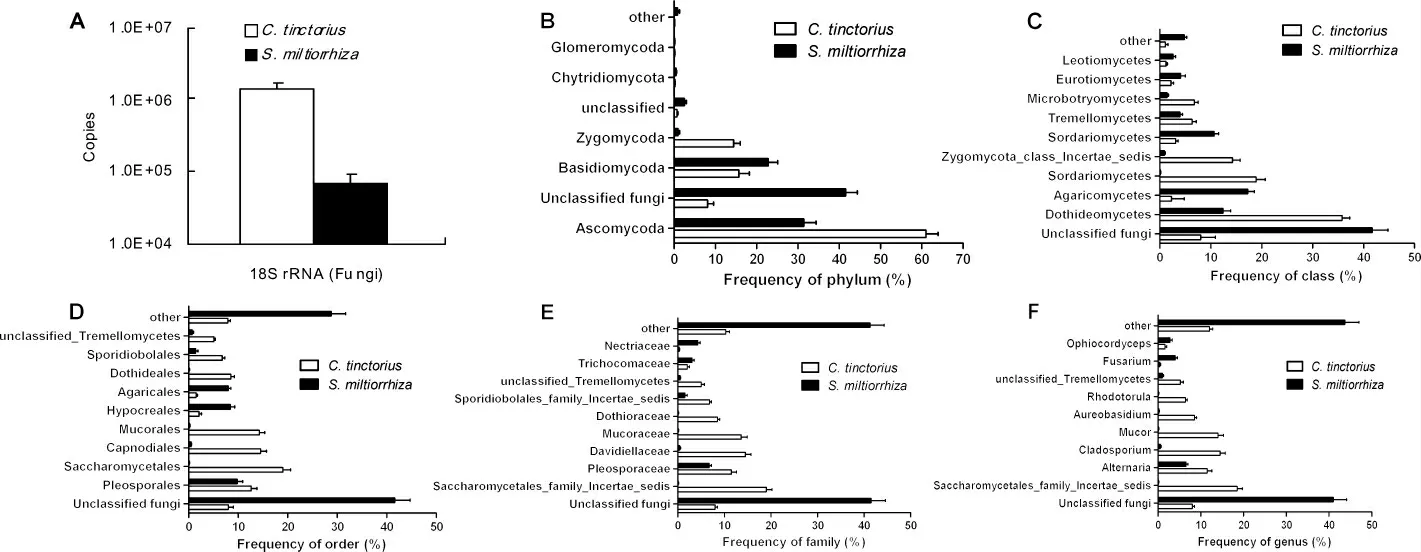
Figure 2 Fungi in raw drugs of Honghua(C.tinctorius)and Danshen(S.miltiorrhiza)(mean±SD,n=3).The total abundance of fungi in the raw drugs of 50 g was evaluated based on copies of DNA encoding 18S rRNA(A).The main fungi were evaluated at phylum(B),class(C),order(D),family(E)or genus(F)level.
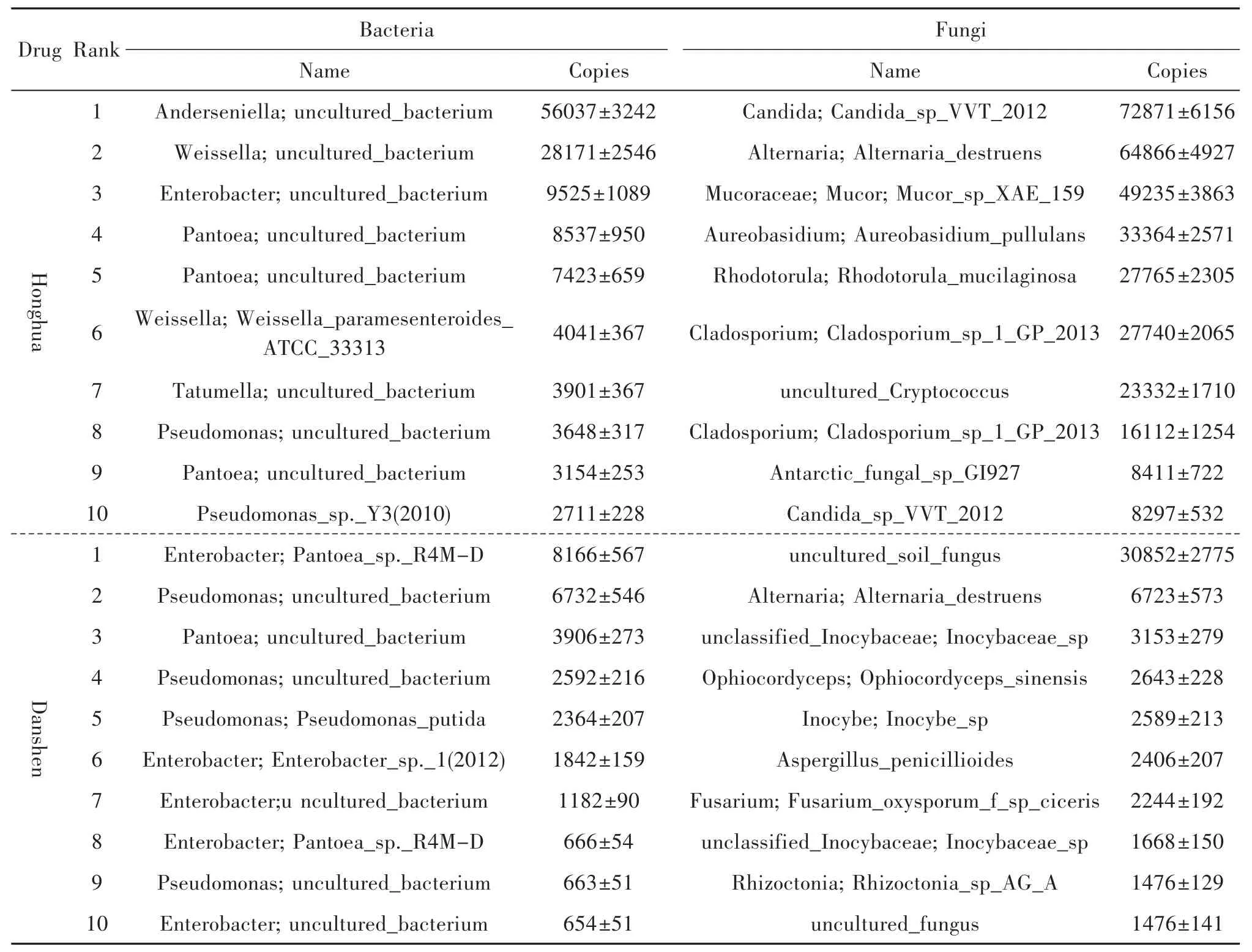
Table 2 Top 10 microbes mostly frequently identified at species level identified in Honghua(flower of C.tinctorius)and Danshen(root and rhizome of S.Miltiorrhizae)(Mean±SD,n=3)*
Fungi distributed in raw drugs of Honghua and Danshen were also different whether at phylum(Figure 2B),class(Figure 2C),order(Figure 2D),family(Figure 2E)or genus(Figure 2F)levels.In Honghua,the fungi most frequently detected were belonged to Ascomycoda,Dothideomycetes,Saccharomycetales,Saccharomycetales family,Incertae sedis and Saccharomycetales family,Incertae sedis at phylum,class,order,family and genus level,respectively.However,in Danshen,the fungi most frequently detected were all belonged to Unclassified fungi(different from Honghua)whether at phylum,class,order,family or genus level.At the species level,the top 10 fungi most frequently identified in Honghua and Danshen were shown in Table 2.Obviously,the pattern of bacterial distribution in Danshen was also different from that in Honghua.
2.2 LPS in pre-processing solutions
LPS in the pre-processing solutions was detected by TAL(Tachypleus Amebocyte Lysate)kits.LPS in the pre-processing solution of Honghua was at a higher level than that of Danshen(Figure 3A and 3B).LPS in pre-processing solution at 0 min(just mixed with LPS free water)was detectable,and the LPS level increased as washing time increases.When raw drugs were pre-processed for 6 min,LPS in Honghua or Danshen solution arrived at the first plateau.Then,LPS in Honghua solution arrived at the second plateau at 18 min,and that in Danshen solution did so at 35 min.The two plateaus suggested that there were two phases for LPS digested from the raw drugs.LPS digested in the first stage should be mainly from the surface of raw drugs that adhered to loosely,and that in the second stage mainly from the surface adhered to relatively tightly.As for Danshen,strangely,there was an unaccountable,but really existed peak at 30 min.
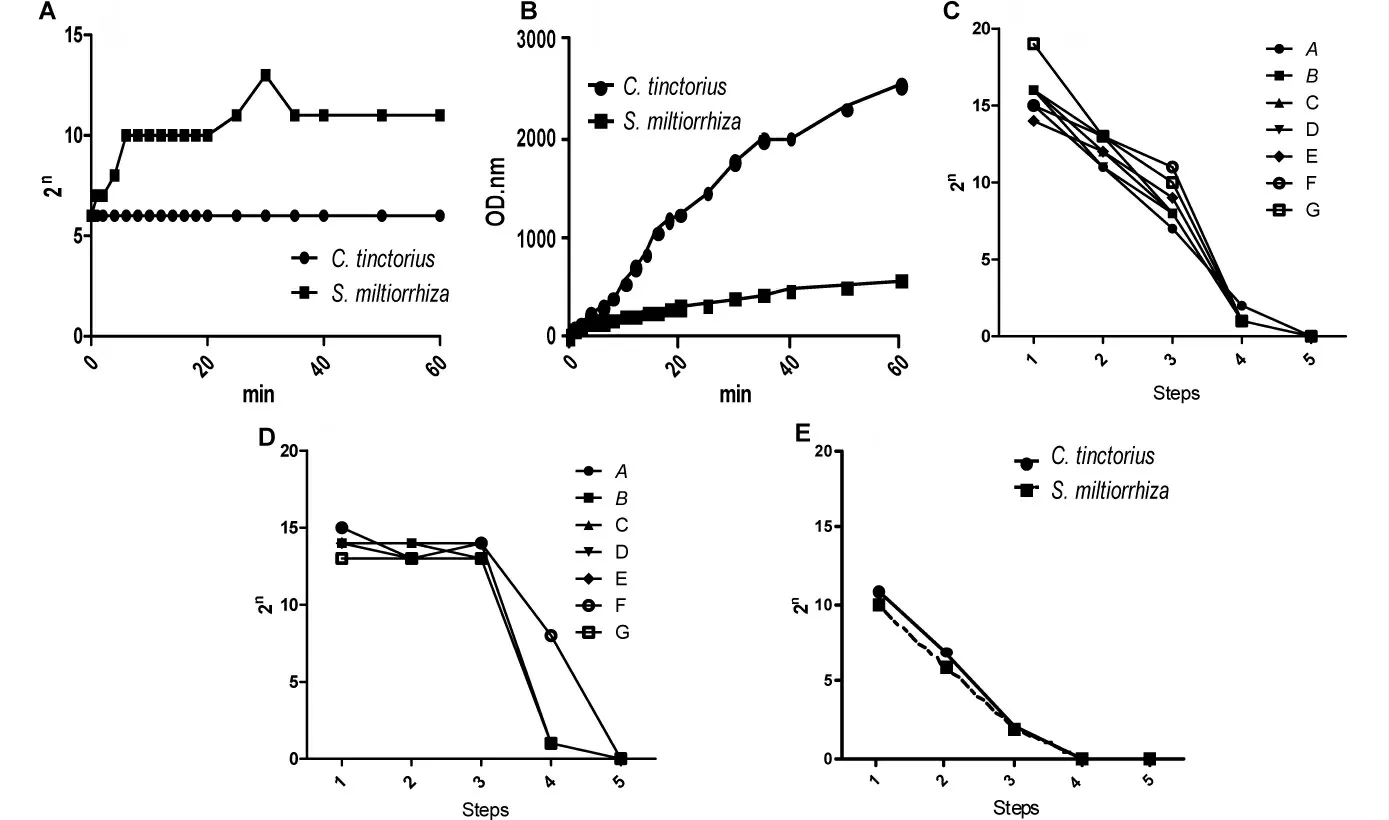
Figure 3 LPS was able to be largely removed at a small cost of active components losing.LPS(A)and total components(B)based on the area under the absorbance curve in the washing solution were detected.LPS in the mimic TCMI solution of Honghua injection(C)and Danshen injection(D)with modified methods was detected.LPS in mimic injection solution started with three times(3 min× 3)of washing followed with Method A was detected(E).All the data repeated three times and similar results can be obtained.2n,the diluted times for just the negative TAL(Tachypleus Amebocyte Lysate)results by double dilution;Step 1-5 and Method A referred to Table 1;OD nm,the area under absorption curve
Raw herbal drugs contained small molecular components accounted for their pharmacological effects,some of which were substances with ultravioletvisible absorbance.According to previous study[2],AUC before the isobestic point of the spectrum can be used to evaluate the active small molecules.Isosbestic point of Honghua injection was 480 nm,and that of Danshen injection was 360 nm[2].Therefore,the AUC before 480 nm(from 230 nm to 480 nm of the specturm)and AUC before 360 nm were calculated.When washed for a long time,the amount of the micro molecules with ultraviolet-visible absorbance would be digested from raw drugs.Since the small molecules existed in the inner of the raw drug rather than at the surface,there were no obvious saturation phrases during the washing period within 60 min(Figure 3C),suggesting that microbes and their biomacromolecules adhered to the raw drugs can be largely removed at a small cost of their active components losing.
2.3 LPS in mimic injection solutions
LPS in injection is toxic for body,and the substance could be removed as thoroughly as possible.The first extract from the raw drugs(Step 1) contained the most of LPS,and then,the amount of LPS decreased step by step(Figure 3C and 3D).However,the mimic injection at Step 4 still contained small amount of LPS that positively tested by TAL kits.LSP was macromolecule that could be removed thoroughly by an ultra-filter(10K)(Figure 3C and 3D,Step 5).
If the raw drugs pre-processed with water of ambient temperature(20 ℃)washing for 3 min three times,the amount of LPS in the mimic injection solution of every step dramatically decreased(Figure 3E).Not surprisingly,LPS in the mimic injection solutions at Step 4 and the latter was undetectable(Figure 3E).
3 DISCUSSION
TCMIs are important members of Chinese drug system for clinical practice and their efficacy are well-accepted in Chinese mainland.However,events of TCMI’s adverse reaction occurred in recent decades made TCMIs face suspicion and blame.Obviously,impurities,especially macromolecular impurities were the main reason for the adverse reactions,because oral TCMs were much safer than TCMIs with similar formulation.Macromolecules are non-absorbable substances and can be kept out by gastrointestinal barriers[12].Macromolecules in TCMIs could be derived from the tissue of raw drugs,the contaminant microbes,and pharmaceutical excipients.Among them,macromolecules from microbes were the most dangerous for they can damage body via PAMP pathway[13].LPS is a wall component of microbes,and can result in serious innate immune reactions.Therefore,microbes and their macromolecules remained should be excluded in TCMIs.
However,the present study found that the main contaminant microbes in raw herbal drugs were fungi rather than bacteria,though bacteria were also heavily contaminated.It can be deduced that the microbes in flower should be from the air exposed to and those in root and rhizome should be from the soil they grew.Honghua,with a huge surface area exposed to the air,was easy to adhere to floating microbes.Therefore,the copies of 16S and 18S rRNA in Honghua were much higher than those in Danshen.Danshen,with a small surface area exposed to soil,was able to adhere to soil microbes and quickly arrived at a balance.Most contaminant bacteria in Honghua were Anderseniella (belonged to Rhizobiales)and Saccharomycetales family,Incertae_sedis(belonged to Saccharomycetales).Comparing with Honghua,most contaminant bacteria in Danshen were soil bacteria(belonged to Enterobacteriales) and soil fungi(Unclassified fungi).
The high level of microbes doomed that the raw flower drug should contain more LPS than raw drugs from root and rhizome.Without pre-processing,the mimic injection solutions of Honghua contained higher level of LPS than those of Danshen.Fortunately,the contaminant substance was able to be dramatically removed simply by water washing.With water washing pre-processing,LPS in the injection solution obviously decreased and even was able to obtain LPS free injection solutions without ultrafiltration.
Therefore,all the results suggested that the contaminant microbes and their macromolecular impurities should be taken into account for TCMIs manufacturing,and pre-processing is an important treatment in removing them,which would make TCMIs much safer.

