Rapid and easy determination of morphine in chafing dish condiments with colloidal gold labeling based lateral flow strips
Wei Chen,Xin-ni Li,Qian Wu,Li Yao,Jianguo Xu
School of Food and Biological Engineering,Engineering Research Center of Bio-process,MOE,Hefei University of Technology,Hefei,230009,China
Keywords:
ABSTRACT
1. Introduction
Over past decades, the reported public food safety incidents have shown that abusing non-food additives brought serious food safety risks to consumers. Poppy is the raw material of opium,and its shell contains more than 30 types of alkaloid [1,2] with some of them,such as morphine(MOP),convincingly demonstrated with the pharmacological and toxicological activities[3].In recent years, a few illegal food producers and operators seek to attract customers and make outsized gains via adding poppy shells and other ingredients to the chafing dish condiments to improve the sense of taste and smelling.Although the flavor was better-changed to whet the consumers’ appetite, these poisonous additives are strictly forbidden by Chinese governments and laws. As a consequence, it is imperative to establish effective detection methods towards MOP, the metabolite of heroin, identification form chafing dish condiments to guarantee the individual health and social stability.
In general,there are several representative methods existed to detect MOP with certain degree of success such as gas chromatography, high performance liquid chromatography [4,5]. Raman spectroscopy,infrared spectroscopy,chemiluminescence[6],capillary electrophoresis[7,8],and immune-assay[9,10].However,the major drawbacks of them are the requirements of time-consuming operation processes,expensive instruments and professional technicians,making them cannot be usefully employed as point-of-care and on-site analytical tools for rapid,simple,convenient,and costeffective food safety tests.From this point of view,the development of new methodologies to face the abovementioned issues is of great importance[11].
Fortunately, with the appealing features of low detection cost,easy operation, and user-friendly model, the rapid detection method of lateral flow strip(LFS)has been emerged and regarded as one of the most promising alternatives for analyzing the target of interest.According to literature reports,various types of LFS via the use of colored-nanomaterials as observed reporters have been wellconstructed and widely applied in the field of daily life monitoring[12]. Particularly, the proven advantages of easy synthesis, good stability,and large surface area of gold nanoparticles(GNPs)make the corresponding GNP-labeling LFS[13]overall explored and popularly commercialized by using GNP-labeled antigen or antibody as the tracer. To date, a variety of target analytes including small molecules[14,15],protein[16,17],and pathogen[18,19]were qualitatively or semi-quantitatively screened by direct observation of the red-colored GNPs on test line (T line). Much endeavor is thus encouraged to continually devote to the development of valuable GNP-labeling LFS platforms.

Scheme 1. Schematic illustration of the GNP-labeling LFS for MOP determination.
Taken above into consideration, in this work, we for the first time presented a simple,rapid,convenient,and cost-effective LFS platform by using GNP as the easily-observed reporter.As the MOP is categorized as small molecule,the competitive mechanism was adopted to execute the recognition event. Experimental results have shown that this facile LFS was able to highly sensitive and selective determination the target MOP for both the standard MOP or the MOP-added real chafing dish condiments less than 10 min.The favorable data (see below) implies the GNP-labeling LFS was afforded with a great potential for point-of-care and on-site detection of MOP.
2. Materials and methods
2.1. Chemicals and reagents
HAuCl4and bovine serum albumin(BSA)were purchased from Sigma-Aldrich.The goat-anti-mouse antibody,the antibody against MOP and MOP-BAS conjugates, and the semi-rigid polyethylene sheets were supplied by Shanghai Joey Biotechnology Company(Shanghai,China).All standard substances such as MOP and cocaine were obtained from the National Institutes for Food and Drug Control (China). Double-distilled water from a RephiLe PURIST UV Ultrapure water system (China) was used throughout the experiment to prepare the aqueous solutions. The nitrocellulose membranes were ordered from Whatman GmbH (Dassel,Germany). The glass fiber membranes and absorbent pads were bought from Millipore Crop (USA). All solvents and other chemicals were of analytical reagent grade and used without further purification.
2.2. Preparation of the GNPs
The GNPs were prepared via the classical citrate reduction method. In brief, 850 μL of chloroauric acid solution (5 g/L) was firstly added into 60 mL of double-distilled water and thoroughly mixed.After heating the solution to 310◦C,the 1%trisodium citrate solution at certain volume was rapidly added and keep stirring at 1500 r/min. With the solution color gradually changed to red, the obtain GNPs were stabilized by stirring the mixture for another 5 min.Of note,the utilized glassware should be overall washed and cleaned by Aqua regia solution before usage.
2.3. Procedure of GNP coupling with anti-MOP antibody
Prior to coat the GNPs with the anti-MOP antibody,the pH value of GNPs solution was adjusted to 8 using K2CO3(0.1 M).Then,the monoclonal antibody of MOP (1 mg/mL) was added to the preprocessed GNPs solution with gentle stirring. After incubated at room temperature for 1 h,a certain volume of blocking agent of BSA was injected to the above mixture and stirred for 30 min to seal the un-occupied active sites for eliminating the nonspecific adsorption effect. The removal of excess antibody was finally performed by centrifuging the mixture at 9800 rpm for 10 min.The obtained precipitates of GNP-antibody conjugates were re-dissolved to a volume of 100 μL to prepare the conjugation pad.
2.4. LFS fabrication
Before the LFS fabrication,the solutions of MOP-BSA conjugate and goat-anti-mouse antibody were sprayed as T line and C line on the NC membranes,respectively.The conjugation pad and sample pad were treated with 10 mM phosphate buffer (5% sucrose,1% trehalose, and 0.3% Tween 20) and 50 mM Tris-HCl (pH=8.0,0.15 mM NaCl,and 0.25%Triton X-100),respectively,and placed in 28◦C drying oven.Subsequently,the sample pad,conjugation pad,NC membrane,and adsorbent pad were laminated and pasted onto the plastic sheet orderly. It should be noted that each pad should be overlapped with adjacent pads with length of 2 mm to ensure the successful migration of the detection samples.
3. Results and discussion
3.1. Principle of GNP-labeling LFS and feasibility demonstration
As shown in Scheme 1,the LFS was typically composed by one sample pad, one conjugation pad, one NC membrane, and one adsorbent pad from left to right.The T line was sprayed with MOPBSA,while the C line was sprayed with goat-anti-mouse antibody.When the target MOP is absent from the added sample (panel a),the antibody-coated GNPs would be fixed on both the T line and C line through the specific recognition pairs of MOP antibody/MOPBSA and MOP antibody/goat-anti-mouse antibody, respectively.The red-colored GNPs could be aggregated and observed on the test zone and control zone. In contrast, upon addition of MOPpresented sample onto the sample pad, the capillary force firstly driven the sample solution entered into the conjugation pad and reacted with the GNP-antibody conjugate to form GNP-antibody-MOP. Due to the competition effect, this part GNPs could not be trapped in T line any more,resulting in a weakened or disappeared T line.Meanwhile,the excess GNPs without target analytes binding would continually interact with the MOP-BSA immobilized T line and goat-anti-mouse antibody immobilized C line.As the intensity of T line was inversely proportional to the target MOP concentration,the easily constructed GNP-labeling LFS platform is expected to achieve the goal of MOP analysis.
After construction of the LFS, the feasibility was directly verified in Fig.1. It can be found that the negative sample containing only loading buffer (10 mM PB) displayed with strong red T line and C line within 10 min. However, only obvious C line could be observed by the positive sample containing 100 ppb MOP in PB buffer,while the T line was completely disappeared.The shape contrast powerfully confirms that the GNP-labeling LFS is available for MOP detection.
3.2. Optimization of experimental conditions
In order to achieve best assay performance,several parameters that were essential to the LFS detection behavior were systematically studied in Fig.2. As depicted in panel a, with the increase of MOP antibody dosage,the T line was intensified from left to right.However,the sensitivity was compromised when the antibody was overused at 3.0, 4.0, 5.0 μL/mL considering the T line could not be totally disappeared at low MOP concentration (1 ppb). This is attributed to the fact that increasing the amount of antibody leads to the unbound GNP-antibody increased and then trapped in the T line.In other words,the T line was not decreased obviously.So,the coupled antibody was chosen as 2.0 μL/mL in view of the balance between T line intensity and detection sensitivity.Panel b provided the experimental results of blocking regents selection.One can see that the GNPs were significantly aggregated at the conjugation pad and could not be easily driven by the capillary force when using 0.5% casein, 0.5% casein sodium, or 0.5% ovalbumin as the blocking substances. Although the GNPs were migrated by using 10%HAS or 10%BSA to block the excess active sites,a darker red color was obtained at the T line of BSA group. Therefore, 10% BSA solution was selected as the most suitable blocking agent.In addition,the dropped dosage of GNP-antibody(panel c)has a similar influence as that of single antibody, in where the T line intensity was gradually increased and the sensitivity was gradually decreased.Accordingly, 2 μL of GNP-antibody was adopted on each pad for subsequent experiments.

Fig.1. Comparative images of the GNP-labeling LFS in the absence and presence of MOP(100 ppb).
3.3. Sensitivity investigation
To evaluate the detection sensitivity, a serious of target MOP at different concentrations(0,0.01,0.1,0.25,0.5,1,5,and 10 ppb)were examined by the GNP-Labeling LFS under optimal conditions.As gathered in Fig.3, we can notice that even the MOP at minute amount (0.1 ppb), the corresponding T line was clearly weakened than the negative sample without target analyte involved. This value was thus defined as the visual limit of detection(LOD).Moreover, the more the MOP was added, the more decrease of the T line was observed. When the sample pad was loaded with 1 ppb MOP,there was no GNP reporter dragged on the T line,indicating the coated antibodies on GNP surface were fully bound with MOP molecules. Further increase of the MOP amount, such as 10 ppb,could no longer has effect on the T line and be differentiated by visual observation. The change tendency of the T line was consistent with the theoretical prediction, and compared with reported methods for small molecules detection, the LFS is outperformed with a greatly improved LOD[20,21].
3.4. Selectivity study

Fig.2. Effects of the MOP-antibody amount(a),blocking agent species(b),and GNP-antibody amount(c)on the LFS assay ability.

Fig.3. Typical results of GNP-labeling LFS loaded with different concentrations of MOP.From left to right:0,0.01,0.1,0.25,0.5,1,5,and 10 ppb.
Given the reliability for MOP determination, the selectivity of the LFS was affirmed by analyzing other small molecules including cocaine (COC), methamphetamine (MET), clenbuterol (CL), salbutamol (SAL), and ractopamine (RAC) against the target MOP. As expected in Fig.4, no matter which target species was interrogated, the C line was evidently presented, ensuring the better quality of the prosed LFS. Interestingly, as can be seen from the T lines,even the non-target analytes at the concentration of 1000 ppb(100-fold higher than MOP), they cannot cause any distinguishable decreased intensity, while the T line was invisible in the presence of target MOP. The comparative results confirmed the excellent selectivity of GNP-Labeling LFS for potential practical application.
3.5. Real samples analysis

Fig.4. Selectivity assay of the LFS corresponding to MOP,COC,MET,CL,SAL,and RAC,respectively.Experimental conditions:MOP,10 ppb;Non-target,1000 ppb.
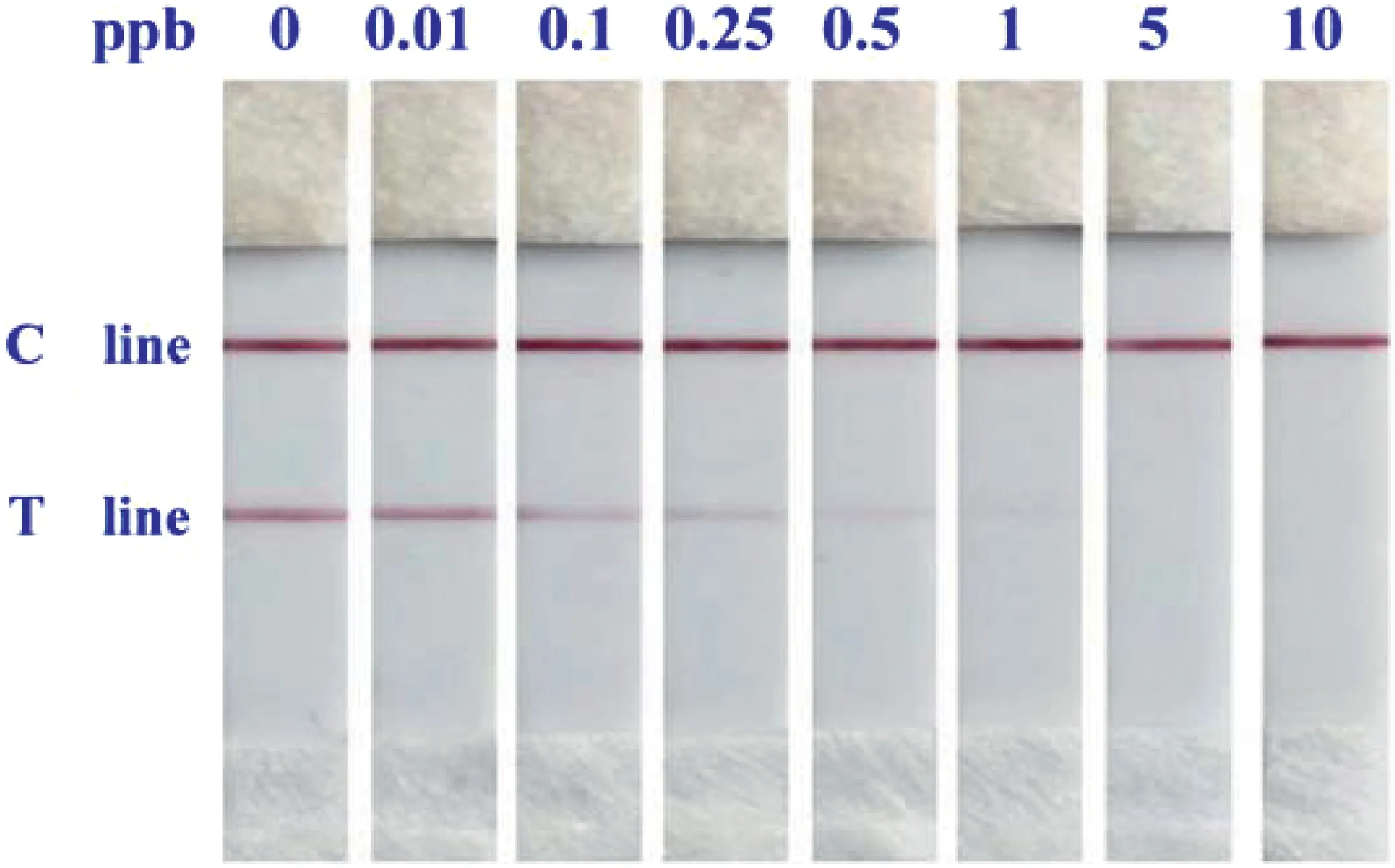
Fig.5. MOP-spiked chafing dish condiments examination with the proposed LFS.
To carry out the real sample analysis experiment,the simulate real food samples were made by dissolving purchased solid chafing dish condiments with boiling double-distilled water at a mass ratio of 1:20 for 30 min.Next,the solution was crudely filtered with gauze and filter paper in sequence until no precipitates existed.The obtained mixture was then spiked with MOP to final concentrations of 0 ppb,0.01 ppb,0.1 ppb,0.25 ppb,0.5 ppb,1 ppb,5 ppb,and 10 ppb,respectively.After the pretreatment,the prepared samples were identically detected as that of standard MOP.As described in Fig.5,the T line was definitely weakened or disappeared by certain amounts of MOP-doped real samples,and the variety trend of T line was not interfered by the chafing dish condiment brought complex substances. The desirable data was in great accordance with the recorded results in Fig.3, suggesting the LFS holds great practical applicability and can acts as very useful analytical platform for illegal additives detection.
4. Conclusions
In summary, by using GNP as the reporter, a GNP-labeling LFS was rational constructed for rapid detection (within 10 min) of MOP from chafing dish condiments without any complicated pretreatments.Compared with reported representative methods,the LFS can be easily fabricated and simply operated,achieving a LOD for MOP detection as low as 0.1 ppb by visual observation.In addition,the high specificity of the MOP antibody confers the LFS with an excellent selectivity to only respond to the MOP involved samples.We therefore envision that the LFS is prospective for daily food safety assurance and clinical diagnosis.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grants No. 21475030, 21804028),the Fundamental Research Fund for central university(Grants No.2017HGPA0162, JZ2018HGTA0205, PA2017GDQT0018), the grant of 2017YFF0208600, the China Agriculture Research System-48(CARS-48),Anhui Provincial Modern Argo-industry Tech.Research System(NYCYTX-2016-84),and the S&T Research Project of Anhui Province(Grant No.15czz03109).
- 食品科学与人类健康(英文)的其它文章
- Microalgae:A potential alternative to health supplementation for humans
- Autophagy-associated signal pathways of functional foods for chronic diseases
- Screening of potential GCMS derived antimigraine compound from the leaves of Abrus precatorius Linn to target“calcitonin gene related peptide”receptor using in silico analysis
- Optimization of process conditions for drying of catfish(Clarias gariepinus)using Response Surface Methodology(RSM)
- QSAR modeling of benzoquinone derivatives as 5-lipoxygenase inhibitors
- High uric acid model in Caenorhabditis elegans