High uric acid model in Caenorhabditis elegans
Zhenjing Li,Yibin Xue,Nifei Wang,Jingli Cheng,Xiaoying Dong,Qingbin Guo,Changlu Wang,∗
Keywords:
ABSTRACT
1. Introduction
Characterized by high uric acid (UA) level in the blood, hyperuricemia with the symptom of deposition of urate crystals in the joints and kidneys, is the key risk factor for many diseases such as gouty arthritis, uric acid nephrolithiasis, cardiovascular, renal disease and hypertension [1,2]. Epidemiological studies from different countries suggested that the incidence of hyperuricemia has been dramatically increased in past few years[3–5].Medically, reducing production (e.g. allopurinol) and increasing excretion (e.g. probenecid, benzbromarone and so on) of UA are two main strategies for hyperuricemia treatment [6]. However,the medicine currently used for both methods impart severe side effects on human body such as allergic,hypersensitivity reactions and nephropathy [7]. Therefore, finding new strategy and developing novel safer drugs for hyperuricemia treatment has become increasingly important.To achieve this,a steady and efficient high uric acid model is indispensable.
C.elegans has been applied to model human diseases for many years [8–10]. About 60–80% of human genes homologues and counterparts for different human disease-causing genes have been identified in C. elegans [11]. Additionally, properties such as short life cycle and span all could make it a pivotal model for drug screening, e.g., a life cycle of 3.5 days and a lifespan of about 3 weeks at 20◦C.
However, to our best knowledge, no hyperuricemia model has been established previously based on C.elegans.The overall objective of this study was to construct a reasonable and efficient hyperuricemia model in C. elegans to better facilitate the development of novel and safe drug for hyperuricemia treatment.
2. Materials and methods
2.1. Materials
C. elegans wild-type strain N2 and E. coli strain OP50 were donated by professor Xiao-chen Wang from National Institute of Biological Sciences(Beijing);uric acid,hypoxanthine,adenine,xanthine, allopurinol and 5-fluorouracil were purchased from Sigma(St.Louis,MO,USA);chromatographic grade methanol and acetonitrile were obtained from Merch Drugs & Biotechnology; xanthine oxidase (XOD) kit was purchased from Nanjing Jiancheng Biology Engineering Institute (Jiangsu, Nanjing). All the other chemicals were of analytical grade unless specified.
C.elegans was cultivated at 20◦C on Nematode Growth Medium(NGM)agar plates or in a liquid S-Medium(SM),and fed ad libitum with E.coli OP50[12,13].Age-synchronized animals were prepared by hypochlorite treatment.
2.2. Drugs treatment of C.elegans
An experimental model induced by uric acid, hypoxanthine,adenine and xanthine was established.All groups consisted of 500 worms. The culture was carried out in six-well plates filled with 3.6 mL of SM and 1.2 mL of drugs at 20◦C.To obtain the optimal drug concentration,the experimental groups were treated for 24 h with four drugs at different final concentrations(0.05,0.15,0.25 mg/mL).Equal amount of M9 buffer was used for control group.And different intervention time (6 h, 10 h, 18 h, 24 h, 32 h) with drugs final concentrations of 0.25 mg/mL were used.
2.3. Preparation of urate samples
After drugs treatment, 500 worms of experimental or control groups were collected, washed with M9 buffer three times,homogenated with 3.0 mL M9 buffer, centrifuged (4000 rpm,20 min),then the supernatant was mixed with 6.0 mL 25%NH3·H2O and ultrasounded for 10 min.Protein was precipitated by remixed 4.0 mL acetonitrile and centrifuged (4000 rpm, 20 min), then the supernatant was analysed by HPLC.
2.4. Analysis of uric acid
Qualitative and quantitative analyses of uric acid were performed on an Intertsil ODS-SP column (5 μm, 4.6×250 mm)at 25◦C.Ammonium acetate solution(10 mmol/L)was used as eluent at a flow rate of 1 mL/min with a UV detector at 280 nm A standard curve of uric acid was constructed.
2.5. Survival assays of C.elegans
30 synchronized L4 worms were cultured in 6-well plates with 3.6 mL SM and 1.2 mL of xanthine solution with the final concentration of 0.25 mg/mL at 20◦C for different time interval(18 h,24 h,32 h), and then transferred to Petri dish containing NGM medium with OP50 and 5-fluorouracil. The worms were checked for survivability daily until all died. Equal amount of M9 buffer replaced xanthine solution was used for control groups.Each test was performed in triplicates.
2.6. Hyperuricemia model evaluation
To investigate the duration of the hyperuricemia model,xanthine-treated C.elegans were transferred to Petri dish containing NGM medium with OP50,and cultured for different times(0 h,6 h, 12 h, 18 h, 24 h) at 20◦C. Then worms were collected, and the uric acid content was determined.To study the hypouricemic effect of allopurinol on hyperuricemic C.elegans,xanthine-treated worms were treated to allopurinol with the concentrations of 0.25 mg/mL,and the uric acid content was then determined.
2.7. Determination of XOD activity
The samples of XOD solutions were prepared as described elsewhere [13]. The xanthine-treated 500 worms were collected,washed with M9 buffer, homogenated with 3.0 mL M9 buffer at ice bath,and centrifuged(4000 rpm,20 min)at 4◦C.Then the XOD activity of the supernatant obtained was determined by XOD kit.Typically,protein concentrations were determined using Albumin Bovine Serum(BSA)as the standard[14].
2.8. Statistical analysis
All statistical analyses of data were carried out using SPSS.The data were expressed as the mean±standard deviation (S.D.) and both ANOVA and t-test were used to determine the level of significance.A value of p< 0.05 was considered statistically significant.
3. Results
3.1. Effects of hypoxanthine,uric acid,adenine and xanthine on uric acid levels of C.elegans
The effects of different concentrations of hypoxanthine, uric acid,adenine,xanthine on uric acid levels of C.elegans were shown in Fig.1. As shown in Fig.1, when treating worms with hypoxanthine, uric acid and xanthine at the dose of 0.25 mg/mL for 24 h,the content of uric acid were significantly increased by 12.02 μg(p<0.05), 14.42 μg (p<0.001) and 19.20 μg (p<0.001), respectively. However, there was no significant difference (p> 0.05) in uric acid levels of C.elegans between adenine and controls.
For the experimental groups,worms were treated with hypoxanthine, uric acid, adenine and xanthine at the dosage of 0.25 mg/mL for 6, 10, 18, 24 and 32 h were compared (Fig.2). For xanthine treatment,the levels of uric acid gradually increased with the treating time,especially after 10 h.A significant increase of uric acid content(p<0.001)at 18,24 and 32 h were all detected.
Therefore,the highest uric acid level could be obtained after C.elegans were treated with xanthine at 0.25 mg/mL for 32 h.
3.2. Lifespan test of C.elegans
Taking account of the effect of xanthine on C.elegans,the lifespan test was carried out, and the results were shown in Fig.3.The mean lifespan of control group was 19.61 days, whereas those of experimental groups were 17.93, 14.76 and 10.92 days,respectively.These findings indicated that a significant decrease of lifespan (p<0.01) was detected for both 24 h and 32 h treatment,but treatment by xanthine for 18 h showed no significant impact on the lifespan of worms. Therefore, 18 h treatment of xanthine was selected for the further experiment.

Fig.1. Effects of different concentrations(0.05,0.15,0.25 mg/mL)of hypoxanthine,uric acid, adenine and xanthine on uric acid levels of C. elegans for 24 h. 0: control group;0.05/0.15/0.25:experimental groups.Data were expressed as the mean±S.D.for 500 C.elegans.(*p<0.05,**p<0.01,***p<0.001 vs.control group).
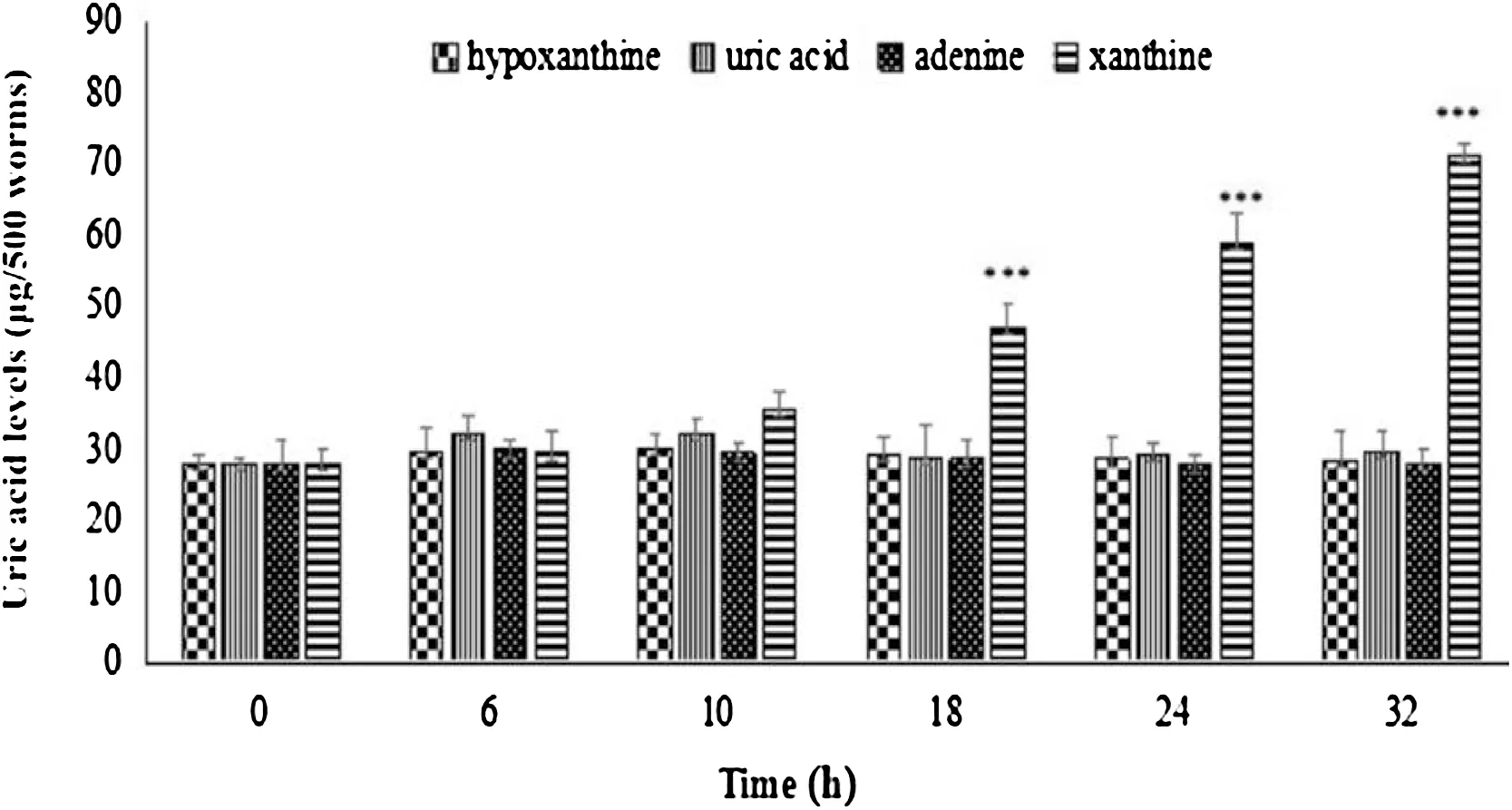
Fig.2. Effects of 0.25 mg/mL hypoxanthine,uric acid,adenine and xanthine on uric acid levels of C.elegans for the different times(6 h,10 h,18 h,24 h,32 h).0:control group;6/10/18/24/32:experimental groups.Data were expressed as the mean±S.D.for 500 C.elegans.(*p<0.05,**p<0.01,***p<0.001 vs.control group).

Fig.3. Effects of 0.25 mg/mL xanthine on mean lifespan of C.elegans for the different times(18 h,24 h,32 h).0:control group;18/24/32:experimental groups.Data were expressed as the mean±S.D. for 30 C. elegans. (*p<0.05, **p<0.01, ***p<0.001 vs.control group).

Fig.4. Time course of uric acid levels in C. elegans after treatment with xanthine(0.25 mg/mL,18 h).control:C.elegans without xanthine treatment.Data were expressed as the mean±S.D.for 500 C.elegans..(*p<0.05,**p<0.01,***p<0.001 vs.control group).
3.3. Hyperuricemia model evaluation
The uric acid content-time profile of C. elegans with and without treatment by xanthine (0.25 mg/mL, 18 h) was compared in Fig.4. A relative higher uric acid level could be maintained for 12 h in hyperuricemic C. elegans (p<0.01 vs. control group) and started to shrink after 18 h.Fig.5 showed the hypouricemic effect of allopurinol on hyperuricemic C. elegans. The treatment of xanthine (0.25 mg/mL, 18 h) caused a significant increase of uric acid level (p< 0.01) in hyperuricemic C. elegans, which was also presented above(Fig.3).After given allopurinol(0.25 mg/mL)for 12 h in hyperuricemic worms, the level of uric acid was decreased by 15% (p<0.01) as compared to hyperuricemic C. elegans. This suggested that C. elegans can be used as a stable and accurate model within 12 h of xanthine treatment(0.25 mg/mL,18 h).

Fig.5. Effects of allopurinol on uric acid level in hyperuricemic C.elegans.Normal:C. elegans without xanthine treatment; Model: C. elegans with xanthine treatmen(0.25 mg/mL,18 h);Allopurinol:C.elegans given allopurinol(0.25 mg/mL,12 h)after treatment with xanthine(0.25 mg/mL,18 h).Data were expressed as mean±S.D.for 500 C.elegans.(a,p<0.01 vs.normal control;b,p<0.01 vs.model control;c,p<0.05 vs.normal control).
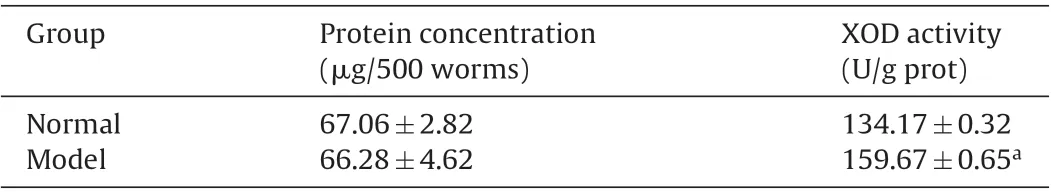
Table 1 Xanthine oxidase(XOD)activity and protein concentrations of normal and model C.elegans.
3.4. XOD activity assay
XOD, which catalyzes the oxidation of hypoxanthine and xanthine to uric acid,is a key enzyme in the formation of uric acid,and is the major target for the treatment of hyperuricemia.At present,the research on the effects of certain substances on XOD activity has become one of the main strategies for developing the novle drugs for hyperuricemia treatment and exploring pharmacological mechanism of uric acid-lowering drugs [15,16]. The effects of xanthine on XOD activity of C. elegans and protein concentrations were shown in Table 1. The results suggested that XOD activity of hyperuricemic C.elegans significantly increased(p<0.001)compared with normal control group,which indicated that the high uric acid level in C.elegans might be attributed to the high XOD activity induced by xanthine.It is consistent with the hyperuricemic mice or rats in vivo[17,18].
4. Discussion
Most mammals can produce uricase enzyme to breaks down uric acid,but humans lack the ability due to the lack of the correspondence gene [19]. Uric acid, therefore, become the final metabolic product of purine metabolism and can only be metabolized by kidney in human [20]. In other mammals including rodents, uric acid is degraded into allantoin through uricase in the liver. Hyperuricemic mice or rats induced by injecting or feeding potassium oxonate (uricase inhibitor) has been used for screening uric acidlowering drugs-like compounds inhibit XO or uric acid transporter[21,22]. Nevertheless, it is difficult to elucidate the mechanism of hypouricemic effects, because the uric acid metabolisms in both rodents are quite different from human. Construction of uricase genes null mice, which based on gene knockout technique commendably simulates urate metabolisms of human It’s difficult to popularize genetically engineered mice at present because it has a lot of disadvantages,such as high costs,technical bottlenecks,high mortality rates for mice,and so on[23,24].Birds,such as chickens and quail, that are consistent with human urate metabolism and can also be used as hyperuricaemia induced by enriching the diet with purine or urate,although which showed many disadvantages including poor reproducibility and administration difficult[25,26].
C.elegans,has been a powerful tool in studying various biological processes,including development,aging and a range of pathological conditions[27–29].For this work,there are several reasons why C.elegans was chosen to set up high uric ucid model.On one hand,there is no uricase in C. elegans, which does not exist in the uric acid metabolism of human either.On the other hand,feeding and management of C. elegans in liquid culture or agar plates culture,are simple and fast due to plenty of time and cost saved. Furthermore,no research has been involved in C.elegans as hyperuricemia model. In this report, a high uric acid model with low drug damage,high efficiency and stability was established in C.elegans after just simply xanthine treatment in liquid culture(0.25 mg/mL,18 h).It also foreseeable that the hyperuricemic C.elegans model can be established through genetic engineering to clarify the molecular mechanism of urate transport in C.elegans.This however,still has a long way to go.
In conclusion,high uric acid model was established when C.elegans was treated by xanthine(0.25 mg/mL,18 h)in this study.The high uric acid level in model C. elegans could keep for 12 h, and the content of uric acid would decline by 15% after giving allopurinol(0.25 mg/mL,12 h).The model was of relatively stability and curability. Furthermore, the XOD activity and protein concentrations were determined and the result indicated that the high uric acid level in model C.elegans may be because of high XOD activity induced by xanthine. In summary, a new high uric acid model in C.elegans with improved XOD,has been constructed to screen perfect uric-acid-lowering drugs for the treatment of hyperuricemia and gout.
Conflict of interest
No conflict of interest associated with this work.
Acknowledgments
The study was funded by a grant from the Scientific Research Fund of Tianjin University of Science and Technology (No.20120105)and National Natural Science Foundation of China(No.31701551).
- 食品科学与人类健康(英文)的其它文章
- Medical foods in Alzheimer’s disease
- Comparative analysis of antioxidant activities of essential oils and extracts of fennel(Foeniculum vulgare Mill.)seeds from Egypt and China
- Oral microbiota:A new view of body health
- Phenolics,tannins,flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats,India
- QSAR modeling of benzoquinone derivatives as 5-lipoxygenase inhibitors
- Optimization of process conditions for drying of catfish(Clarias gariepinus)using Response Surface Methodology(RSM)