Medical foods in Alzheimer’s disease
Klus W.Lng,Jinjun Guo,Shighiko Kny,Kthrin M.Lng,Yukiko Nkmur,Shiming Li
Keywords:
ABSTRACT
1. Introduction
Aging is commonly associated with a decline in cognitive functioning,which ranges from mild cognitive impairment to dementia.Up to 50% of individuals with mild cognitive impairment will develop dementia within 5 years [1]. Alzheimer’s disease (AD),the most prevalent cause of dementia, is a progressive neurodegenerative disorder characterized by global cognitive impairment affecting memory, language and other behavioral functions [2,3].Given its increasing prevalence and worldwide impact, AD is a challenge for both societies and healthcare systems. Neuropathological hallmarks of AD are the deposition of abnormal proteins in the form of extracellular amyloid plaques and intracellular neurofibrillary tangles[4,5].Chronic inflammation and oxidative stress in the brain are important in the pathophysiology of AD and lead to neuronal dysfunction and death [6]. The onset of neurodegeneration is thought to precede the clinical symptoms by many years.
Effective treatments capable of improving the cognitive impairment or retarding the progression of AD are not available[3].The drugs currently in use in AD, i.e. acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists(e.g.memantine),show no significant efficacy[3].A systematic review examined the efficacy and safety of acetylcholinesterase inhibitors (donepezil,rivastigmine,galantamine)and memantine in regard to mild cognitive impairment not related to AD [7]. It was concluded that these drugs did not improve cognition or function in individuals with mild cognitive impairment but were associated with a greater risk of headaches and gastrointestinal side-effects(e.g.nausea,diarrhea,vomiting)[7].Preventing or delaying the onset of AD is therefore a major public health challenge [8] and requires the development of novel interventions.
While old age is the main risk factor for dementia, other factors have been shown to be diet-related[9].These include obesity,hypertension, and unbalanced diets [10]. Dietary components are able to modulate cerebral structure and connectivity, to cause changes in brain and behavioral functions, and to modulate cognition and emotion [11]. Nutritional approaches to AD include“healthy”dietary patterns(e.g.Mediterranean diet)with individual components that may produce positive effects on pathophysiological processes of AD [12], ketogenic dietary approaches which target energetic deficits and reduced glucose utilization in AD[13],and medical foods meeting specific nutritional needs of individuals with AD[14].The present short review will provide an overview of nutritional interventions in AD, highlighting the available clinical trials assessing the effects of medical foods in people with AD.
2. Mediterranean diet
The dietary habits of populations living in regions bordering the Mediterranean Sea are characterized by a relatively high consumption of fresh fruits,vegetables,legumes,whole grains,seeds,and nuts, the use of olive oil as a main source of fat, a moderate intake of fish, poultry, and dairy products, and a low intake of red meat,refined grains,refined sugar,and convenience foods(see[15]).Adherence to the Mediterranean diet has been posited to contribute to better cognitive performance and to be among the most important modifiable protective factors against AD and cognitive decline[16].Both epidemiological studies(e.g[17–19].and clinical trials [20,21] have linked higher adherence to a Mediterraneanstyle diet to a reduced risk of cognitive decline and dementia.The potential of a Mediterranean diet to prevent neurodegeneration has been attributed primarily to its high content of vegetables, fruits,and olive oil,which contain a wide range of bioactive phytochemicals,such as polyphenols,carotenoids,and sulfur compounds(e.g.[17,18,22,23]).
Numerous studies assessing the impact of individual components of a Mediterranean diet on cognitive functions as the brain ages suggest that the intake of diet-derived or supplementary omega-3 fatty acids and plant polyphenols such as flavonols and resveratrol exerts positive effects on brain health and cognition in older people [24]. Neuroinflammation in the brain is a pathological feature of AD and is characterized by the activation of microglia, which is the first line of immune defense.Dietary polyphenolic compounds and their derivatives have been shown to mitigate microglia-mediated neuroinflammation in in vitro studies (for review see [25]). Large-scale randomized controlled trials, also controlling for the consumption of other fatty acids, are needed to investigate the potential benefits of regular omega-3 fatty acid intake in maintaining or improving cognitive performance. Neuroprotective effects of resveratrol on relevant features of AD, including a reduction in amyloid deposition and tau-hyperphosphorylation,an enhancement of hippocampal neurogenesis, and an improvement in memory deficits, have been shown in rodent models(for review see[26,27].However,evidence of a beneficial effect of resveratrol on cognitive functions in AD is lacking.
A suggestive but inconclusive protective effect of the Mediterranean diet on outcomes related to cognition in AD and mild cognitive impairment was demonstrated by several prospective cohort studies, the majority of which found significant positive relationships between adherence to the Mediterranean diet and cognitive health (for review see [28]). A meta-analysis including five prospective studies showed that in individuals adhering to the Mediterranean diet, the risk of AD or mild cognitive impairment was reduced by 33%in those in the highest tertile compared to those in the lowest tertile [29]. In addition, greater adherence to the Mediterranean diet was protective against the progression from mild cognitive impairment to AD [29]. In a brain imaging study of individuals with higher versus lower adherence to a Mediterranean-style diet, it was shown that lower adherence was associated with progressive abnormalities of AD biomarkers in middle-aged adults[30].
The external validity of most findings showing significant cognitive benefits in individuals on a Mediterranean diet remains problematic, since many interactions and overlaps exist between diet and other lifestyle factors, such as physical exercise (e.g.[8,31]). For example, a randomized controlled trial using a robust design and demonstrating cognitive benefits in participants on a Mediterranean diet was conducted in highly active people in a Mediterranean culture[21].Another major limitation and threat to external generalizability of studies on associations between cognitive functions and the Mediterranean diet is that there are no a priori determinations of cut-off points or recommendations regarding the exact composition of the Mediterranean diet. Research investigations usually compare participants within a given sample,who adhere to the Mediterranean diet to a greater or lesser extent.Interestingly, a recent cross-sectional study using a priori determined cut-off points for the adherence to the Mediterranean diet found that greater adherence was associated with improved cognition and a decreased risk of dementia[32].
In summary,the evidence for a protective effect of the Mediterranean diet on AD risk is suggestive but far from conclusive. The“prescription”of the Mediterranean diet as a preventive or therapeutic measure in AD is hampered by the lack of established levels of individual dietary components, especially of biologically active phytochemicals whose chemical structure,content and efficacy are well characterized[33].
3. Ketogenic diets
A region-specific decline in glucose uptake and metabolism can be observed in the brain of patients with AD; these changes may precede the onset of clinical signs and the diagnosis of AD by many years[34–38].The deterioration of glucose utilization is a potential target of treatment strategies,including interventions involving the supplementation of the normal glucose supply with ketone bodies, which are produced during glucose deprivation and can be metabolized by the brain. While glucose utilization in the brain declines in AD, ketone body utilization remains unaffected [39].Ketogenic diets increase the consumption of fat and reduce that of carbohydrates[40].The ensuing reduction in the release of insulin stimulates the hepatic oxidation of long-chain fatty acids to ketone bodies (β-hydroxybutyrate, acetoacetate, and acetone). Although glucose is the brain’s major fuel, β-hydroxybutyrate and acetoacetate can replace glucose during fasting,starvation,or demanding exercise [41,42]. The administration of ketone bodies or high-fat,low-carbohydrate ketogenic diets may help augment the supply of brain fuel in later life[43]and has been demonstrated to produce positive effects in AD animal models and in clinical trials with AD patients(for review see[13]).
A small number of human studies examining ketogenic medium-chain triglycerides in individuals with mild-to-moderate AD have been conducted[44–47].The main findings of these studies were as follows. An improvement in cognitive outcomes was associated with the level [44,45,47] and duration [47] of ketosis.In addition, improved cognitive outcome was more pronounced in ApoE4-negative patients [44–46,48]. (The epsilon-4 variant of apolipoprotein E is a major genetic risk factor for AD[49].)Cognitive improvement was also found in elderly,non-demented people[50].
In summary,the mechanisms underlying the positive effects of ketogenic diets are unclear and may be related to the normalization of energy metabolism. There is currently limited evidence of the efficacy of these diets in AD. Best results may be expected if ketogenic diets could be administered early in the presymptomatic stages of AD[51].
4. Medical food trials
Foods for special medical purpose (“medical foods”) are produced according to a special processing formula in order to meet the specific dietary needs of people with metabolic disorders or other diseases.In the United States,a medical food is defined as,“a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation” [52].All ingredients of medical foods must be recognized as safe.In contrast to approved drugs,however,medical foods are not subject to the rigorous review of the US Food and Drug Administration.In the European Union,products considered medical foods in the United States are regulated as dietary foods for special medical purposes[53].
The identification of the specific nutritional requirements in AD, especially in the early stages, that cannot be met by modifications of the normal diet alone requires a better understanding of AD pathophysiology.Symptomatic benefits in AD have been claimed for three medical foods, i.e. Axona®, Souvenaid®and CerefolinNAC®[54]. Axona®provides ketone bodies as an alternative energy supply to neurons, Souvenaid®supplies precursors believed to improve synaptic function,and CerefolinNAC®is concerned with oxidative stress related to memory loss. An overview of findings regarding these medical foods is presented in Table 1.
4.1. Axona®
Axona®is consists of medium-chain caprylic triglyceride. The dietary intake of coconut or palm kernel oils is thought to be unable to provide caprylic triglyceride in sufficient quantities to meet the needs of people with AD [14]. Caprylic triglyceride is intended to be metabolized to ketones, which provide an alternative energy source for the brain. The rationale underlying the use of Axona®,as indicated above, is that the diminished glucose metabolism in the brain of people with AD should be compensated by ketone bodies serving as an alternative energy supply.Axona®is claimed to be helpful in the clinical dietary management of the metabolic processes related to mild-to-moderate AD[55].However,it is not approved as a medical food for the therapy of AD by the US Food and Drug Administration.
The efficacy and safety of Axona®were examined in a randomized,double-blind,placebo-controlled,parallel-group clinical trial with 152 participants with mild-to-moderate AD, who continued taking their usual AD medications [45]. Axona®was administered daily for 90 days. The primary outcomes were changes in the score of the AD Assessment Scale Cognitive subscale (ADASCog),and global scores in the AD Cooperative Study Clinical Global Impression of Change scale(ADCS-CGIC).In comparison to placebo,serum ketone bodies were significantly increased two hours after administration of Axona®[45].A significant difference between the Axona®and placebo groups was found in mean change from baseline in the ADAS-Cog score on days 45 and 90, with the Axona®group improving and the controls worsening.These changes were most notable in participants who did not carry the ApoE4 allele,while individuals with the ApoE4 genotype did not show any benefit.Differences between groups were not found in regard to changes from baseline in ADCS-CGIC scores [45]. Participants receiving Axona®presented more frequently with adverse events,primarily transient,mild-to-moderate gastrointestinal effects[45].
4.2. Souvenaid®
Synaptic dysfunction, which is a major factor underlying the cognitive impairment in AD [56,57], may be the consequence of deficient composition and function of neuronal membranes[58,59], which are mainly composed of phospholipids [60]. Interventions improving phospholipid metabolism in the brain may therefore be beneficial for cognitive functioning in AD.
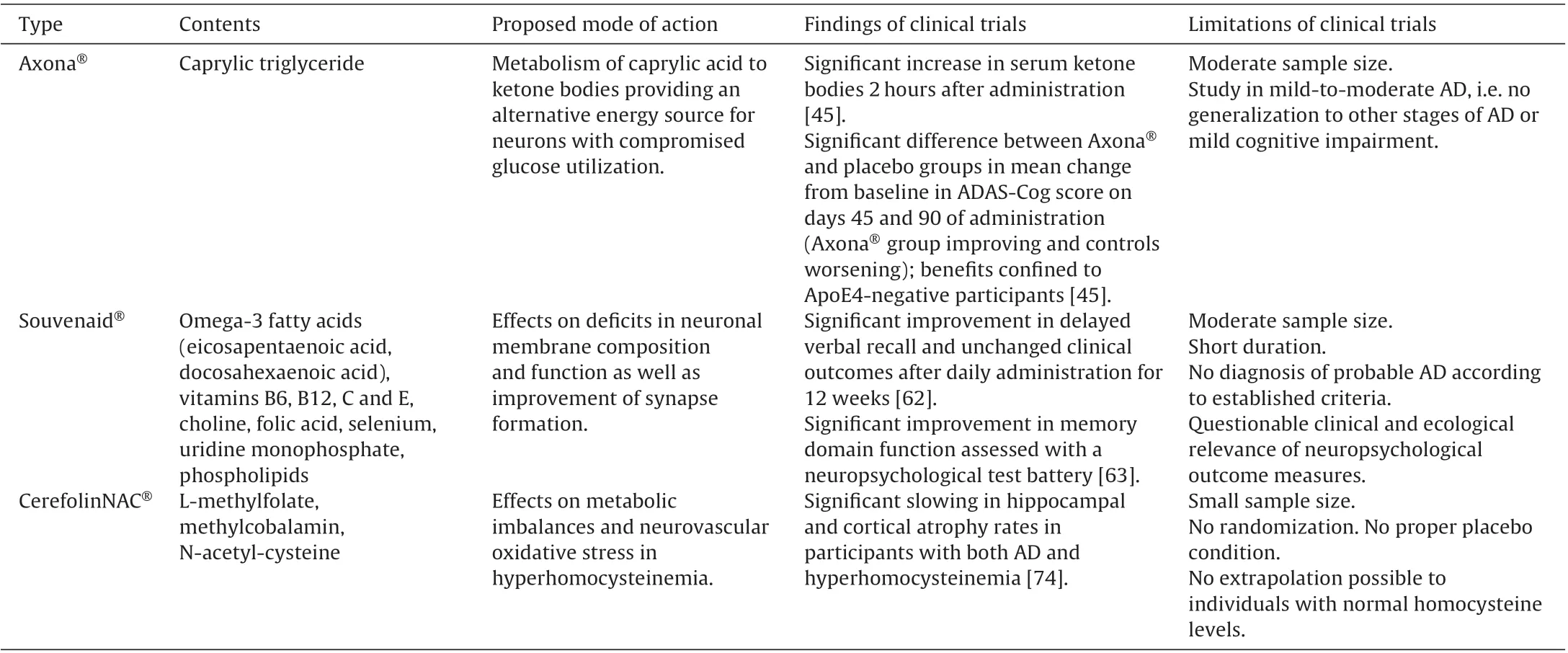
Table 1 Summary of medical foods in AD.
The nutritional formulation,Souvenaid®,is designed to improve synapse formation[61].This medical food is a multinutrient beverage,which combines 11 vitamins and supplements and is approved for early AD in some European countries and Australia.The formulation consists of phosphatide precursors and supporting nutrients that are intended to act synergistically to enhance the formation of membranes and to improve the function of synapses in individuals with AD [62]. The mixture contains the omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid,vitamins B6,B12,C and E,choline,folic acid,selenium,uridine monophosphate,and phospholipids at levels above those contained in the normal diet.
In a randomized,controlled,double blind,multicenter trial,the effect of Souvenaid®on cognitive function was compared with a control formulation in 225 drug-naïve subjects with mild AD[62]. Souvenaid®or the control drink were administered once daily for 12 weeks. A statistically significantly improved performance in the delayed verbal recall task of the Wechsler Memory Scale-Revised was found in the active group, compared to controls,after the 12-week supplementation[62].However,modified AD assessment scale–cognitive subscale and other outcome scores(e.g., clinician interview, neuropsychiatric inventory, activities of daily living,quality of life in AD)were unchanged.Gastrointestinal side-effects were the most commonly reported adverse events in both groups[62].Major limitations of this study are the short duration and the lack of improvement in clinically important outcomes.The ecological and clinical validity of test results in a verbal recall task is not proven.Longer-term studies and a more comprehensive neuropsychological assessment using suitable tests are therefore needed.
In another randomized,controlled,parallel-group,double-blind trial, 259 drug-naïve participants with mild AD were randomly assigned to two groups receiving either Souvenaid®(n=130)or an isocaloric control product(n=129)once daily for 24 weeks[63].The primary outcome was memory domain function,assessed using a neuropsychological test battery;secondary outcomes consisted of electroencephalography measures as a marker for synaptic connectivity. Assessments were performed at baseline, at 12, and at 24 weeks. The memory domain score was significantly higher in the Souvenaid®group compared to the control group over the 24-week intervention period [63]. A significant difference in EEG measures of functional connectivity in the delta band was observed between the study groups during the study period, with superior measures in the group receiving the active compound. High levels of compliance were present in both groups. No difference between the two groups in the occurrence of serious adverse events was found [63]. The findings of this study suggest that, in drug-naïve patients with mild AD, Souvenaid®improves memory performance,as assessed using neuropsychological tests,and has a positive effect on brain functional connectivity, as assessed with EEG measures. These findings support the hypothesis of altered synaptic activity following the administration of Souvenaid®.However, several limitations of this study should be considered. The sample was of moderate size and the duration of 24 weeks was short,when considering that AD is a chronic disease.A longer-term study should therefore be conducted.Furthermore,the participants were in an early stage of AD and, while the diagnosis of probable AD was made according to established criteria, biomarker evidence was not available.In addition,a placebo effect was observed for several neuropsychological test parameters after 12 weeks.This effect could also be due to learning or familiarity with the tests and should be controlled for by the use of parallel test versions. Finally, the respective effects of the sole administration of the multi-nutrient drink Souvenaid®and of the add-on therapy together with approved anti-AD medication should be investigated.
The ability of Souvenaid®to affect brain phospholipid metabolism in AD was investigated in an exploratory,double-blind,randomized controlled, 4-week intervention study; participants were drug-naive individuals aged 60–86 years with mild AD [64].Phosphorus magnetic resonance spectroscopy was performed prior to and following the 4-week administration of Souvenaid®(n=16)or an isocaloric control product(n=17).These measurements allow the assessment of surrogate measures of phospholipid synthesis and breakdown, i.e. phosphomonoesters and phosphodiesters,respectively,as well as several other parameters.Following 4 weeks of treatment, total choline and the ratio of phosphomonoesters and phosphodiesters were increased in the Souvenaid®group compared to controls[64].It was not possible to attribute the increase in this ratio to elevated phosphomonoester or reduced phosphodiester levels.Moreover,the sample size of this study was relatively small. The findings of this study suggest that Souvenaid®affects phospholipid metabolism in multiple brain regions in mild AD after 4 weeks.This may lead to an increase in neuronal membrane formation,which would support the hypothesized mode of action of this medical food.The long-term effects of Souvenaid®on phospholipid formation,synaptic function,and cognitive abilities in people with and at risk for AD needs to be examined in randomized controlled trials in larger samples and over substantially longer periods of time.
4.3. CerefolinNAC®
Hyperhomocysteinemia is a common condition globally in individuals aged over 65 years, with a prevalence from 5.1% to 29%[65–67].In comparison to people with normal homocysteine levels,those with hyperhomocysteinemia show poorer cognitive performance [68] as well as greater regional brain atrophy [69] and ischemic lesions [70]. The risk of brain atrophy in people with hyperhomocysteinemia is up to 10 times higher than in the general population [71]. High baseline homocysteine levels predict moderately-severe whole brain atrophy 5 years subsequently[72].Treatment effects of supplementation of B vitamins on cognition and brain volume in patients with hyperhomocysteinemia have been inconsistent [70–73]. The prescription medical food CerefolinNAC®, containing L-methylfolate, methylcobalamin, and N-acetyl-cysteine,has also been used in the treatment of hyperhomocysteinemia. The intended action of the special formulation of CerefolinNAC®is to address metabolic imbalances of hyperhomocysteinemia and neurovascular oxidative stress, which have been shown to be related to cognitive impairment.
The intake of CerefolinNAC®has been claimed to delay regional brain atrophy in individuals with AD or cognitive impairment due to cerebrovascular disease. Thirty individuals with both hyperhomocysteinemia and AD (63% females; mean age and standard deviation at baseline, 76.0±10.6 years), received CerefolinNAC®for a mean duration of 18.6 (± 16.1) months [74]. A subgroup of this sample did not receive CerefolinNAC for varying periods of time (12.6±5.6 months). A group of 37 individuals with AD and related disorders but without hyperhomocysteinemia (65% females; mean age, 71.6±8.7 years) did not receive CerefolinNAC®for 13.3 (± 17.7) months. All participants were receiving a cholinesterase inhibitor and memantine throughout the study period.None of the participants received B-vitamin supplementation [74]. Regional brain volumes were measured using quantitative MRI at baseline and end of study, and covariateadjusted rates of cortical,hippocampal,and forebrain parenchymal(including white matter)atrophy were predicted.Participants with both hyperhomocysteinemia and AD/related disorders receiving CerefolinNAC®showed an adjusted hippocampal atrophy rate 4.25 times slower than those with AD and related disorders but no hyperhomocysteinemia who did not receive CerefolinNAC®[74].The rate of cortical atrophy was 11.2 times slower in the treatment group. Furthermore, the rate of forebrain parenchymal atrophy was significantly reduced only in participants with hyperhomocysteinemia and cerebrovascular disease. In summary, the intake of CerefolinNAC®was associated with significantly slowed hippocampal and cortical atrophy rates in patients with AD/related disorders and hyperhomocysteinemia;it further slowed forebrain parenchymal atrophy rates in subjects with cerebrovascular disease and hyperhomocysteinemia.Future studies will need a larger sample size with a longer follow-up period and a randomized assignment to the CerefolinNAC®treatment and a proper placebo condition. It needs to be emphasized that the findings in individuals with hyperhomocysteinemia cannot be extrapolated to those with normal homocysteine levels.
5. Future directions
5.1. Epigenetic diet
The main epigenetic mechanisms are DNA methylation,histone post-translational modification, and microRNAs [75]. Emerging evidence suggests that various modifications of these epigenetic mechanisms are associated with the pathogenesis of neurodegenerative diseases,and epigenetic regulator mechanisms may be important targets in the treatment of AD[76,77].Epigenetic alterations caused by external factors can be reversed, with nutrition and dietary factors being some of the principal epigenetic regulators [78]. It has also been proposed that epigenetic regulation at DNA methylation near stress gene loci may link stress responses to AD and that nutrient-based epigenetic changes play a role as protective factors in stress-related disorders,which are recognized as risk factors of AD [79]. Many food bioactives, including folic acid, choline, vitamin B12, selenium, zinc, omega-3 fatty acids,and polyphenols, have been suggested to play an important role in maintaining methylation patterns and genomic stability and to affect neuronal dysfunction and the progression of AD by interfering with deregulated epigenetic processes and gene expression[78]. Since the pathological mechanisms related to AD are established early in life,epigenetic diet may provide a novel preventive approach in preclinical phases of AD or a therapeutic strategy in later stages of the disease.The efficacy and dose-effect relationships of the epigenetic diet need to be examined in the future. A focus on nutrition and epigenetics may lead to new avenues of research[80].
5.2. Outcome measures
The dietary and nutritional approaches to the treatment of AD discussed above have in common that they require a valid and reliable assessment of various outcomes in early phases of the disease process. A consensus concerning the optimal approach to outcome assessment in dementia research does not exist.In order to represent comprehensively the impact of interventions,outcome measures should reflect not only cognitive functioning,but also the personal experiences and patient-centered aspects of functional performance and quality of life [81]. A standardized approach to these problems is needed.In view of the heterogeneity of AD phenotypes,the development of valid and reliable as well as clinically and ecologically meaningful outcome measures is a major challenge[82].
An important problem is the insufficiency of psychometric properties of functional and quality of life outcome measures used to demonstrate changes in AD drug trials[83].Most of the scales are impaired by serious limitations,such as incomplete assessment of validity and reliability for the intended purposes. A further major problem is the lack of data on responsiveness to change[83].New assessment tools are therefore needed in regard to functional ability and quality of life.In addition to studies attempting to enhance cognition and function in individuals with AD,the focus of research has shifted towards prevention trials in early disease stages,when treatment responses may be subtle and difficult to detect. This requires new cognitive and functional outcome measures that will be of value in clinical trials assessing the gradual progression to cognitive and functional impairment[84].An evidence-based consensus has recently been provided on core outcome measures for trials of disease modifying interventions in mild-to-moderate dementia[85].
6. Conclusions
Several dietary approaches have been claimed to have symptomatic benefits in AD and, by providing nutritional components meeting the specific dietary needs of people with AD,to fulfill the criteria for approval as medical foods It is important to consider that the approval of medical foods by the U.S. Food and Drug Administration does not require the same high standards for testing as those for medications.The meaningful interpretation of the available findings of medical food clinical trials in AD is hindered by the relatively small number of participants,short follow-up periods for interventions that may need to be administered over many years,and positive findings for only some of the primary outcomes. In addition,the issue of the questionable capability of outcome measures commonly used to detect subtle changes in both cognition and function in early AD needs to be addressed.
Whether or not cognitive deterioration can be delayed by enhancement of cerebral hypometabolism preclinically or early in the course of AD remains unclear.Evidence of the efficacy of ketogenic dietary approaches in AD, including Axona®, in overcoming the impaired cerebral glucose utilization is limited. The potential neuroprotective properties of ketone bodies in regard to the prevention or improvement of cognition and function require further research. The suggestive evidence that Souvenaid®can improve synaptic function and provide long-term benefits in early AD needs to be confirmed.
It should be emphasized that the findings in individuals with hyperhomocysteinemia cannot be extrapolated to those with normal homocysteine levels.Studies based on methodologically sound trial designs need to establish whether the reduced hippocampal and cortical atrophy rates following the administration of CerefolinNAC®in AD patients with hyperhomocysteinemia can be confirmed in individuals with normal homocysteine levels.
Other open questions concern the sole versus combined administration of medical foods. In addition to the examination of the effects of individual medical foods,it may be interesting to investigate combinations of these foods with different hypothesized modes of action. Furthermore, the combination of medical foods with approved drugs or non-pharmacological approaches,such as physical exercise [86,87], should be examined. The conducting of comparative effectiveness studies regarding these interventions should be encouraged.
The most beneficial results of dietary approaches in AD,including the use of medical foods,may be expected when applied early in presymptomatic stages of the disease.However,this would require the reliable identification of people with minimal neuropathological AD-related changes. Most studies of medical foods have been conducted in patients with mild AD; thus, their findings cannot be generalized to other stages of AD or mild cognitive impairment.The efficacy shown for medical foods is,at best,comparable to available symptomatic medications.Medical foods appear generally to be safe and to have fewer and less severe side-effects than drugs. Large-scale clinical studies using valid, sensitive, and reliable assessment tools are needed to establish the efficacy of medical foods. In addition, specific dietary patterns, such as the Mediterranean diet,appear to show promise in reducing the risk of developing AD and should be investigated further.
Conflict of interest
All authors declare no conflict of interest in regard to this paper.
- 食品科学与人类健康(英文)的其它文章
- Comparative analysis of antioxidant activities of essential oils and extracts of fennel(Foeniculum vulgare Mill.)seeds from Egypt and China
- Oral microbiota:A new view of body health
- Phenolics,tannins,flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats,India
- High uric acid model in Caenorhabditis elegans
- QSAR modeling of benzoquinone derivatives as 5-lipoxygenase inhibitors
- Optimization of process conditions for drying of catfish(Clarias gariepinus)using Response Surface Methodology(RSM)