Antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle against lead acetate-induced testicular toxicity in rat
Sri Agus Sudjarwo, Chairul Anwar, Giftania Wardani, Koerniasari Eraiko, Koerniasari
1Department of Pharmacology, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia
2Department of Histology, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia
3Department of Pharmacy Biology, Faculty of Pharmacy, Hang Tuah University, Surabaya, Indonesia
4Department of Conservative Dentistry, Faculty of Dentistry, Airlangga University, Surabaya, Indonesia
5Department of Microbiology, Study Program of Environmental Health, Polytechnic of Health, Surabaya, Indonesia
Keywords:Chitosan-Pinus merkusii nanoparticle Lead acetate Antioxidant Caspase 3
ABSTRACT Objective: To investigate the antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle on lead acetate-induced toxicity in rat testis. Methods: Chitosan-Pinus merkusii nanoparticles were identified by dynamic light scattering and scanning electron microscope. The male rats were divided into control group (rats were given with distilled water); lead acetate group [rats were injected with lead acetate 20 mg/kg body weight (BW)i.p.], and the treatment group (rats were given the chitosan-Pinus merkusii nanoparticle 150 mg; 300 mg; 600 mg/kg BW orally and were injected with lead acetate 20 mg/kg BW). The testis tissues were collected to evaluate the malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), histological evaluations of testis damage,and the caspase 3 mRNA expression was measured by reverse transcription-polymerase chain reaction. Results: The dynamic light scattering showed that the size of chitosan-Pinus merkusii nanoparticle was (530.2±38.2) nm. The scanning electron microscope images of the chitosan-Pinus merkusii nanoparticles showed an irregular shape, and the morphology surface showed the rough surface. The treatment with lead acetate resulted in significantly increasing MDA level and caspase 3 mRNA expression, and significantly decreasing level of SOD and GPx when compared with control group. The treatment with the chitosan-Pinus merkusii nanoparticle 600 mg/kg BW but not 150 and 300 mg/kg BW significantly decreased the MDA levels, caspase 3 mRNA expression, and also increased level of SOD and GPx when compared with lead acetate group. The lead acetate induced loss of the normal structure of testicular cells and necrosis, whereas treatment with chitosan-Pinus merkusii nanoparticle inhibited testicular cell necrosis. Conclusions: It can be concluded that chitosan-Pinus merkusii nanoparticle protects rat testis from oxidative damage and apoptosis caused by lead acetate, through increasing antioxidant and inhibiting caspase 3 expression.
1. Introduction
Lead is well known as one of most toxic heavy metals which can cause extensive environmental pollution and problems in human and animal health. The sources of lead toxicity mainly include industrial processes such as coal burning, lead-containing paint, lead smelting, lead-based pipes, grids, battery recycling,and leaded gasoline[1]. The lead has non-biodegradable nature, so it is prolongedly present in the environment. Lead acetate, one of toxic heavy metals, accumulates in almost all the body tissues and causes multiple systemic toxicities to several organs such as liver[2],reproductive organs[3,4], heart[5] and kidneys[6].
The mechanism of lead caused-testicular toxicity can induce oxidative stress through increasing reactive oxygen species (ROS)[3,7]. The focus of research attention is oxidative stress which causes damage effects on the body and also on the death of cells. Oxidative stress will occur if ROS increases and antioxidants decrease in the cells. It has been reported that lead-induced overproduction of ROS such as superoxide ion, nitric oxide and hydroxyl radical could consequently lead to increasing lipid peroxidation, impairment of antioxidant enzymes such as decreasing superoxide dismutase(SOD), and glutathione peroxidase (GPx)[7,8]. In addition, free radicals are very reactive to lipids of membrane, protein, DNA and to be the main factors to cause testicular cell damage[9]. Many researchers have demonstrated that lead induced apoptosis in rat tissues including brain, testis, fibroblasts, liver and lung[8,10,11]. Xuet al[9] have demonstrated that lead can increase Bax/Bcl-2 ratio and caspase 3 activity which caused DNA damage and apoptosis in a cell.Malondialdehyde (MDA) is a secondary product of lipid peroxidation, which can be used as a biomarker of lead acetateinduced testicular toxicity. The increase in MDA level showed decreased antioxidant activity to inhibit excessive free radicals formation[3,12].
Several antioxidants of natural product have protective effects against lead-induced oxidative damage and free radical generated formation in the body. Natural products are easily found as sources of antioxidants which contain a mixture of different chemical compounds to improve health and to treat diseases. The advantage of using natural products for the treatment of various diseases was low cost and little side effects compared with conventional drug[13].Many researchers tried herbal medicine likePanax ginseng[4],Fumaria parviflora[12],Oringa oleifera[14], andJuglans nigra[15]against lead-induced testicular toxicity.
This study has focused on natural products of chitosan-Pinus merkusiiwhich have antioxidant activities to inhibit free radicalcaused testicular cell injury. It has been demonstrated that these antioxidant activities ofPinusplants were due to the phytochemicals possessed, including alkaloids, polyphenols, flavonoids, lignans,triterpenes, sterols, glycosides, triterpenoids, and saponins[16,17].Some researchers have stated thatPinusplant showed stronger effects of antioxidant (free radical scavengers), antibacterial agents,anticancer, anti-inflammatory, immune-stimulating, antiviral, and estrogenic activities[17-20]. Recent research activities have shown thatPinusplant is an important source of pycnogenol that contains proanthocyanidins (procyanidins)[21]. Proanthocyanidin is potent antioxidant (free radical scavengers) and antibacterial agent, and it also exhibits vasodilatory, anticancer, anti-inflammatory, immunestimulating, antidiabetes, and anti-atherosclerosis properties[22,23].
In recent years, synthesis of natural product nanoparticles is an interesting issue of the nanoscience and nanobiotechnology. The natural product nanoparticle has the opportunities to prevent and treat diseases in both human and animal. Nanoparticles vary in size but are generally ranging from 100 to 800 nm. Nanoparticle-based natural product has a potential future for enhancing the activity, drug stability and treatment efficacy, and overcoming problems associated with a pure natural product[24,25]. Chitosan, one of natural products,is widely used in pharmaceutical and biomedical applications[26,27].Chitosan nanoparticles have been studied extensively by researchers for their controlled drug release properties and are used for bothinvitroandin vivoapplications[28,29]. Chitosan nanoparticles are also non-toxic and have many biological activities such as antibacterial,antioxidant, antihyperlipidemic, anti-diabetic, anti-HIV, antiinflammatory, drug delivery, and immunoenhancing activities, which makes chitosan nanoparticle as an ideal delivery agent for application in medicine[30,31]. The aim of the research is to investigate the antioxidant and anti-caspase 3 activity of chitosan-Pinus merkusiiextract nanoparticle against lead acetate-caused toxicity in rat testis.
2. Materials and methods
2.1. Making process of chitosan-Pinus merkusii extract nanoparticles
Chitosan-Pinus merkusiiextract nanoparticle was prepared using ionotropic gelation method[32]. The 0.2% (w/v) solution of chitosan was made in 0.1% (v/v) glacial acetic acid and then filtered.The 0.1% (w/v) solution of tripolyphosphate (TPP) was made in deionized water. Under constant stirring, 0.4% (w/v) extract ofPinus merkusiiin 70% ethanol was added to 0.2% (w/v) solution of chitosan, and then sonicated for 5 min. Furthermore under constant stirring, TPP solution was added dropwise. The ratio of chitosan : TPP solution was maintained at 2 : 1. The supernatant was centrifuged at 25 000 rpm for 20 min, and then sediment of chitosan-Pinus merkusiiwas characterized.
2.2. Characterization of nanoparticles by scanning electron microscopy and dynamic light scattering
The surface morphological features such as particle size, shape and topography of the chitosan-Pinus merkusiiextract nanoparticle were observed using scanning electron microscope.
Dynamic light scattering was done using Malvern instruments version 2.2. Average particle size of the chitosan-Pinus merkusiiextract nanoparticle was determined.
2.3. Experimental animal
The male Wistar rats were used in this research with weighing about 200-250 g which were purchased from Gadjah Mada University, Yogyakarta, Indonesia. The rats were housed in plastic cages with a temperature at (24 ± 2) ℃ in an air-conditioned room and given feed and waterad libitum. This study was reviewed by the Ethical Clearance Committee for preclinical research, Institute of Tropical Disease, Airlangga University and obtained ethical clearance under No.178/ITD/1/2018. Date: January 14, 2018.
2.4. Experimental design
The 50 male rats were divided into: control group (rats were given with distilled water); lead acetate group [rats were injectedi.p.with lead acetate 20 mg/kg body weight (BW)], and the treatment group(rats were given orally the chitosan-Pinus merkusiinanoparticle 150 mg/kg, 300 mg/kg, 600 mg/kg BW once a day for 11 days, and on 4th day rats were injectedi.p.with lead acetate solution at a dose of 20 mg/kg BW one hour after the chitosan-Pinus merkusiiextract nanoparticle). On day 11, the rats were sacrificed, and testis tissues were homogenized with 50 mM sodium phosphate buffer (pH 7.4)containing 0.1 mM ethylenediamine tetraacetic acid in ice-cold. The supernatant was centrifuged at 1 000 ××gfor 20 min at 4 ℃. The supernatant was used for analyzing MDA, SOD and GPx and the expression of caspase 3. The testis was also fixed in a 10% solution of neutral buffered formalin for histopathological evaluation of the testis damage.
2.5. Measurement of MDA
The supernatant of homogenate testis tissue was measured to determine MDA level by the thiobarbituric acid method, on the absorbance at 532 nm, which was expressed in nanomoles MDA/g tissue[5,33].
2.6. Measurement of antioxidant enzymes
The SOD activity was measured with a kit of SOD detection according to the manufacturer’s instructions. The level of SOD was measured at 505 nm, which was expressed in U/mg protein[34].
The GPx activity was measured with a kit of GPx detection according to the manufacturer’s instructions. The GPx was evaluated spectrophotometrically at 340 nm, which was expressed in U/mg protein[34].
2.7. RNA extraction and reverse transcription-polymerase chain reaction analysis
Total RNA was isolated from rat testis by using a solution of Trizol, re-suspended in 50 µL diethyl pyrocarbonate-treated water,and stored at 80 ℃. Synthesis of cDNAs for caspase 3 detection was made by using reverse transcription of Promega[11]. The used specific primers were 5-AGAGAACAATGGCGGATA-3 for forward and 5-CCAGTTGAGGGATGAAAG-3 for reverse, as control was carried out amplification of ratβ-actinmRNA in each sample using the primer: 5-GAGGCTCAGAGCAAGAGAGG-3 for forward, and 5-TGACATCTCGCACAATCTCC-3 for reverse. All polymerase chain reactions were conducted usingTaqDNA polymerase (Life Technologies, Inc., Monza, Italy) with 200 ng cDNA for each of 30 cycles of amplification, which consisted of a denaturing phase of 1 min at 94 ℃, an annealing phase of 30 s at 65 ℃, and an extension of 1 min at 72 ℃. The products of amplified polymerase chain reaction were separated on a 2% agarose gel, and ethidium bromide was used for the visualization of the band. Band densities of caspase-3 expression was quantified by densitometry using the Scion Image software (Scion Corporation Frederick, MD, USA).
2.8. Histopathological examination of testis damage
The tissue of testis was fixed in a 10% solution of neutral buffered formalin, embedded in paraffin and stained with hematoxylin and eosin[3].
2.9. Statistical analysis
Data were shown as mean±standard deviation (mean±SD) and analyzed with one-way ANOVA. The statistical comparisons among the groups were carried out with an Fisher’s least significant difference test (SPSS V. 17.0).
3. Results
3.1. Characterization of chitosan-Pinus merkusii extract nanoparticles by scanning electron microscope
Scanning electron microscopy images of the chitosan-Pinus merkusiinanoparticles prepared using ionic gelation revealed that the nanoparticle surface showed the rough surface morphology and an irregular shape (Figure 1).

Figure 1. Scanning electron microscope images of chitosan-Pinus merkusii extract nanoparticles showed irregular shape, and the morphology surface showed the rough surface.
3.2. Characterization of chitosan-Pinus merkusii nanoparticles by dynamic light scattering
Dynamic light scattering showed that the average particle size of the chitosan-Pinus merkusiiextract nanoparticles was (530.2±30.2) nm(Figure 2).
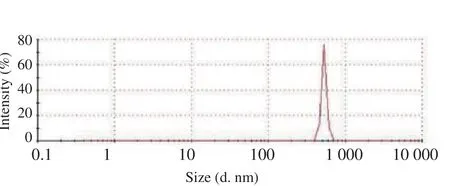
Figure 2. Size distribution of chitosan-Pinus merkusii extract nanoparticles by dynamic light scattering.
3.3. Effects of chitosan-Pinus merkusii extract nanoparticle in MDA, SOD, and GPx of lead acetate-caused testis toxicity
Table 1 showed the level of MDA of testis tissue in the lead acetate treatment group significantly increased when compared with the control group (P<0.05). Treatment with dose 600 mg/kg BW chitosan-Pinus merkusiiextract nanoparticle significantly decreased testis tissue MDA when compared with the lead acetate treatment group (P<0.05). Table 1 also showed the levels of SOD and GPx of the lead acetate treatment group on testis tissue were significantly decreased when compared with the control group (P<0.05).Treatment with dose 600 mg/kg BW chitosan-Pinus merkusiiextract nanoparticle on lead acetate toxicity significantly increased testis SOD and GPx when compared with the lead acetate treatment group(P<0.05).
3.4. Effects of chitosan-Pinus merkusii extract nanoparticle on expression of caspase 3 mRNA of lead acetate-induced testicular toxicity
The increasing of the expression ofcaspase 3mRNA indicated cell apoptosis. Figure 3 showed the expression ofcaspase 3mRNA on testis cell apoptosis. In the lead acetate treatment group, thecaspase 3mRNA expression of testis tissue (1.58±0.09) was significantly increased when compared with the control group (0.43±0.04)(P<0.05). Band densities of caspase-3 expression was quantified by densitometry using the Scion Image software (Scion Corporation Frederick, MD, USA). Treatment with dose 600 mg/kg BW chitosan-Pinus merkusiiextract nanoparticle (0.80±0.06), significantly reducedcaspase 3mRNA expression in testis tissue when compared with lead acetate treatment (1.58±0.09), but not dose 150 mg/kg BW(1.63±0.14) and dose 300 mg/kg BW (1.32±0.11).
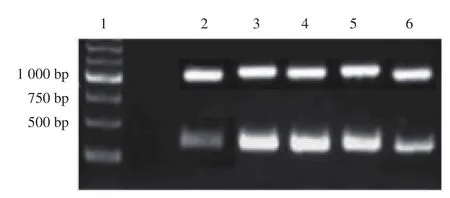
Figure 3. Caspase 3 mRNA expression by the reverse transcriptionpolymerase chain reaction.
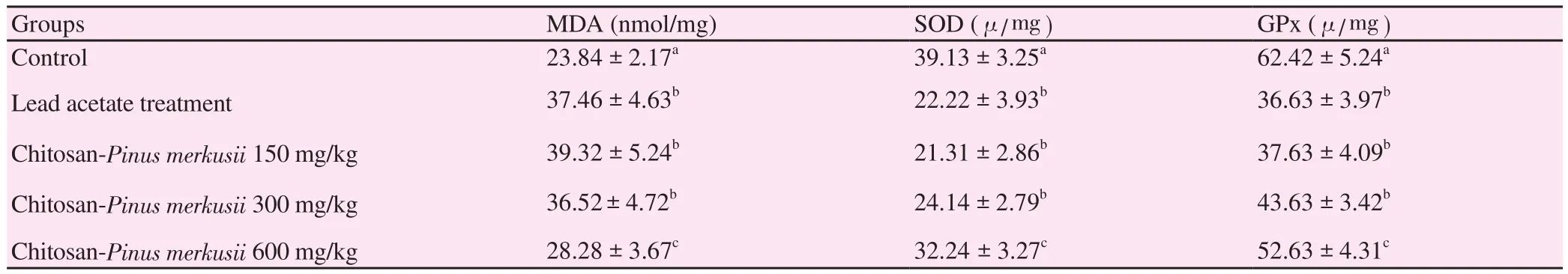
Table 1 Effects of Chitosan-Pinus merkusii extract nanoparticle on lead acetate induced changes in MDA, SOD and GPx (Mean ± SD).
3.5. Effects of chitosan-Pinus merkusii extract nanoparticle on lead acetate-caused testis cell damage
Histological observations on the control group showed testicular cells were observable and they appeared with normal architecture of the testicular cells. The positive lead acetate group showed testicular cell damage (necrosis). In the rats, treated with chitosan-Pinus merkusiiextract nanoparticle, the number and morphological integrity of testicular cells were preserved. Observations indicated that the testicular toxic effect of lead acetate was reduced by chitosan-Pinus merkusiiextract nanoparticle (Figure 4).
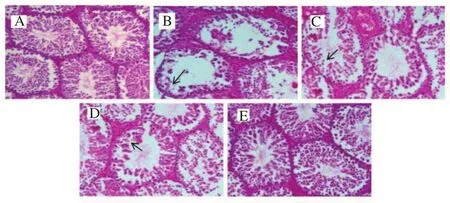
Figure 4. Histological results of chitosan-Pinus merkusii extract nanoparticle on lead acetate-caused testis cell damage.
4. Discussion
In recent years, the new advance of nanotechnology, synthesis of natural product nanoparticles is an interesting issue of the nanoscience and nanobiotechnology[24,28]. The natural product nanoparticle has the opportunities to prevent and treat diseases in both human and animal.
The chitosan has gained great attention in both the pharmaceutical and medical fields, which is used for herbal extract-loaded nanoparticles. Chitosan is a natural polysaccharide which is biodegradable and biocompatible[28,29]. In this study, we madePinus merkusiiextract encapsulated into chitosan nanoparticle using a cross-linking agent (sodium tripolyphosphate) by ionotropic gelation method, which has more advantages overPinus merkusiiextract.The dynamic light scattering showed that the size of chitosan-Pinus merkusiiextract nanoparticle was (530.2±38.2) nm. Nanoparticles vary in size but are generally ranging from 100 to 800 nm. The scanning electron microscope images of the chitosan-Pinus merkusiiextract nanoparticles showed an irregular shape, and the morphology surface showed the rough surface. The researchers were interested in using natural product antioxidant for protect lead-induced toxicity. In the experiment, we investigated the chitosan-Pinus merkusiiextract nanoparticle as a protector against lead acetate-caused damage to rat testis tissue. In our study, administration of lead acetate can increase MDA and induce oxidative damage in testis. MDA can be used for identifying cell membrane damage and it was the product of lipid peroxidation after free radicals were increased. Increase of MDA level in testis showed that antioxidant failed to inhibit the formation of free radicals and the enhancement of lipid peroxidation which caused tissue injury[6,7].
The administration of chitosan-Pinus merkusiiextract nanoparticle at a dose of 600 mg/kg BW can prevent the increased levels of MDA in the rats testis due to lead acetate. These might be due to the ability of chitosan-Pinus merkusiiextract nanoparticle to reduce the accumulation of free radicals. It has been reported that chitosan-Pinus merkusiiextract nanoparticles have antioxidant activity and free radical scavenger potential, which can decrease the MDA level.This suggests that chitosan-Pinus merkusiiextract nanoparticles inhibit the toxic effect of lead acetate through its antioxidant activity.The results of this study show that chitosan-Pinus merkusiiextract nanoparticle could inhibit oxidative stress by decreasing the lipid peroxidation (MDA level) in the lead acetate-induced testis damage.SOD and GPx are antioxidant enzymes which have protective capacity on biological systems against free radical attack[1,7]. SOD is a powerful endogenous antioxidant enzyme in the cell that has detoxification activity as a component of a first-line defense system against ROS. While GPx is an important antioxidant enzyme that can break hydrogen peroxides and lipid peroxides in the mitochondria and sometimes in the cytosol, and then protect cells from oxidative damage[3,14]. In the current study, lead acetate can decrease SOD and GPx activities in rat testis, whereas the administration of chitosan-Pinus merkusiiextract nanoparticle increases the levels of SOD and GPx. The result shows that chitosan-Pinus merkusiiextract nanoparticle acts as a scavenger for the oxygen-derived free radicals which can inhibit the formation of free radicals, thus protect lead acetate induced rat testis damage.
The decrease in lipid peroxidation due to chitosan-Pinus merkusiiextract nanoparticle can increase the antioxidant defense system such as SOD and GPx which protect against free radical exposure.The primary mechanism of action of chitosan-Pinus merkusiiextract nanoparticle may involve the scavenging of free radicals which can inhibit free radical formation.
The lead toxicity can cause increase of oxidants product and decrease of the defense systems of antioxidant which may induce overproduction of ROS and damage of proteins, lipoproteins, lipids,mitochondria, and DNA, leading to testicular cell death through apoptosis or necrosis[9,10]. This study showed that in the lead acetate treatment group, thecaspase 3mRNA expression of testicular tissue was significantly increased compared with the control group. The caspase is an executioner caspase and as a hallmark of apoptosis[8].Considering all these findings, it would be logical to conclude that lead acetate results in caspase-3 overexpression by triggering the apoptotic pathway. Dose dependent-manner of chitosan-Pinus merkusiiextract nanoparticle decreasedcaspase 3mRNA expression of testicular tissue in lead acetate treatment. The mitochondrial injury and changes in levels of apoptogenic proteins including Bcl-2, Bax,and caspase-3 may cause tissue cell apoptosis[9]. In lead toxicity, the expression levels of caspase-3 and Bax were significantly increased,while the level of Bcl-2 was significantly decreased. Thecaspase-3mRNA expression plays an important role in the lead acetateinduced testis apoptotic cell.
In the present study, histopathological image of the lead acetate group showed destroyed seminiferous tubules, loss of spermatid and necrosis in rat testis compared with the control group. The testis damage (necrosis) was mild in the groups treated with chitosan-Pinus merkusiiextract nanoparticle.
In conclusion, the present study indicates that the administration of chitosan-Pinus merkusiiextract nanoparticle inhibits the effects of lead acetate-induced testicular toxicity, through increasing antioxidant and inhibitingcaspase 3mRNA expression. Furthermore,protective effects of chitosan-Pinus merkusiiextract nanoparticle can be developed to treat patients with lead acetate-induced testicular toxicity.
Conflict of interest statement
The authors have no conflict of interest.
Acknowledgements
The authors would like to acknowledge the support of Airlangga University, Surabaya, Indonesia in conducting this research work.
 Asian Pacific Journal of Reproduction2019年1期
Asian Pacific Journal of Reproduction2019年1期
- Asian Pacific Journal of Reproduction的其它文章
- Antioxidant effects of quercetin in freeze-thawing process of mouse spermatogonial stem cells
- Effect of butylated hydroxytoluene on quality of pre-frozen and frozen buffalo semen
- Blood indicators of dry cows before and after administration of a drug STEMB
- Value of α-fetoprotein,β-HCG, inhibin A, and UE3 at second trimester for early screening of preeclampsia
- Comparison of transvaginal cervical length and modified Bishop’s score as predictors for labor induction in nulliparous women
- Chronic atrophic endometritis and pyometra in a ferret: A case report
