Antioxidant effects of quercetin in freeze-thawing process of mouse spermatogonial stem cells
Fardin Amidi, Zahra Rashidi, Zahra Khosravizadeh, Kajal Khodamoradi, Ali Talebi, Shadan Navid, Mehdi Abbasi
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Keywords:Spermatogonial stem cell Cryopreservation Oxidative stress Quercetin
ABSTRACT Objective: To evaluate the antioxidant effect of quercetin on cell viability, reactive oxygen species (ROS) contents and apoptosis of cryopreserved mouse spermatogonial stem cells(mSSCs). Methods: mSSCs were isolated from neonate mice and cultivated in culture medium containing 30 µM quercetin for 48 h and then frozen for 2 weeks. After thawing,MTT assay was carried out to analyze the cell viability. Moreover, intracellular ROS levels were measured by flow cytometery and apoptosis was evaluated by detection of phosphatidylserine externalization assay and also real-time polymerase chain reaction.Results: Pre-treatment of mSSCs by 30 µM quercetin significantly decreased intracellular ROS content and apoptotic cell numbers and improved viability of mSSCs. Moreover, the gene expression of Bcl-2 and Bax significantly increased and decreased respectively after the freeze-thawing process. Conclusions: Pre-treatment of mSSCs with quercetin can improve cell viability and reduce apoptosis during freeze-thawing process. It can be a promising way to improve the quality and efficiency of cryopreservation protocols used in fertility preservation strategies.
1. Introduction
With the increasing prevalence of cancer in human beings and the increasing use of methods such as chemotherapy and radiation therapy, the number of patients suffering from fertility complications is increasing nowadays[1,2]. One of the major challenges facing fertility is the toxic treatment on the reproductive function that enhances infertility development among cancer patients[3]. Although the first choice to preserve fertility among these patients is to store mature sperm, this method is only applied to adult males. In prepubescence, boy children may encounter loss or destruction of testicular stem cells.
In the recent studies, the experimental fertility reestablishment strategies for these children have been evaluated: autologous grafting of testicular tissue, spermatogonial stem cells (SSCs)transplantation, andin vitrospermatogenesis[4,5]. Duringin vitrospermatogenesis, isolated SSCs from testicular tissue will be used and sperm production can be induced[6]. Therefore, the proliferation of SSCs in the culture systems can provide a valuable source of germ cells for subsequent applications such as freezing,transplantation, genetic manipulation andin vitrodifferentiation[7,8].Since the SSCs in the harvested fragments of the testes are rare,in vitroculture of these cells can multiply them for use in the treatment and then increase the successful transplantations. To date, various types of SSCs culture systems have been evaluated in a variety of media and their results have been reported[7,9]. Freezing is one of the best main procedures for male fertility preservation[10].In spite of the usefulness of this method, it has been established that cryopreservation can induce the formation of reactive oxygen species (ROS) in the cells. Accordingly, ROS production promotes the damage in biological molecules such as proteins, lipids and DNA[11]. To hamper oxidative stress in cells, the presence of antioxidants is essential for the organism. Oxidation of antioxidants can eliminate free radicals and prevent oxidative damage[12,13]. It is reported that quercetin acts as an anti-inflammatory, anti-apoptotic,anti-oxidant and anti-cancer agent[14-16]. Considering the antioxidant properties of quercetin and the necessity of SSCs cryopreservation to fertility restoration approaches in pre-pubertal cancerous boys, here,we assessed the antioxidant effects of quercetin pre-treatment on survival, ROS production, apoptosis and apoptotic genes expression of mouse SSCs (mSSCs) during the freeze-thawing process.
2. Materials and methods
2.1. Animals
mSSCs were obtained from 3-6 day-old male mice (Naval Medical Research Institute). Animals were kept under controlled conditions(12:12 light-dark cycle and 22-25 ℃ temperature). All animal care and experimental procedures were performed according to the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1396.2507, Dated: 6 June 2017).
2.2. Isolation and enrichment of mSSCs
An enzymatic method was applied to isolate mSSCs according to the previous study[17]. Briefly, testes of neonate mice were collected and washed in phosphate-buffered saline (Sigma, Steinheim,Germany). Then, the tunica albuginea was removed and isolated testes tissues were transferred to enzymatic medium containing typeⅣ collagenase (1 mg/mL; Sigma, Germany), deoxyribonucleaseⅠ(10 ug/mL; Sigma), hyaluronidase (0.5 mg/mL; Sigma, Germany) in minimum essential medium alpha (MEM-α) (Sigma, Germany) and incubated for 15 min at 37 ℃ and 5% CO2. Pipetting was done every 5 min. Later, the samples were centrifuged for 5 min at 1 500g. In the second stage of enzymatic digestion, the resulting cells were plated and incubated in the fresh enzyme solution mentioned above for 20 min at 37 ℃ and 5% CO2. Then, the digested cells were centrifuged and washed with phosphate-buffered saline again. mSSCs were enriched using differential plating method described in the previous study[17].
2.3. mSSCs culture
The isolated mSSCs were cultured for 2 weeks in MEM-α medium containing 10% fetal bovine serum (FBS), 1××nonessential amino acids (Invitrogen, USA), 0.1 mM 2-mercaptoethanol (Sigma Germany), 103U/mL human recombinant leukemia inhibitory factor(B&D, USA), 10 ng/mL glial cell line-derived neurotrophic factor(Sigma, Germany), 100 U/mL penicillin (Sigma, Germany), and 100 µg/mL streptomycin (Sigma, Germany).
2.4. Pre-treatment with quercetin before cryopreservation
In order to obtain the best concentration of quercetin, mSSCs were treated with culture medium containing different concentrations of quercetin (0, 10, 20, 30, 40 and 50 µM) for 48 h. Then, methyl thiazol tetrazolium (MTT) assay was carried out to assess the cell viability. After determination of optimum dose of quercetin,mSSCs were cultivated for 2 weeks, then the cells were cultured for additional 48 h as 2 groups: control group without quercetin and experimental group with an optimum dose of quercetin as pretreatment before freezing.
2.5. Cryopreservation and thawing
After cell cultivation for 48 h, mSSCs of experimental and control groups were harvested. An equal number of mSSCs in each group were suspended in MEM-αmedium containing dimethyl sulfoxide(1.4 M; Sigma, Germany) and 10% FBS as freezing medium[18].The cryovials containing cells were stored at -80 ℃ freezer for 1 day and then the cryovials were transferred into liquid nitrogen for 2 weeks.
After storage, the cryovials were thawed at room temperature for 30 s and then in a water bath at 37 ℃ for 2 min. The cryovials contents were transferred to a 15 mL falcon tube containing MEM-ααand 10% FBS. The cells were washed in medium and centrifuged for 5 min at 3 200g. After removal of the supernatant solution, the cell pellets were used for further experiments.
2.6. Cell viability
Cell viability was measured by MTT assay after thawing. MEM-α(400 µL) and MTT (40 µL) were added to the cells, and incubation at 37 ℃ was performed for 4 h. Then, MTT solution was removed and 400 µL of dimethyl sulfoxide was added to the cells with pipetting several times. At the end, optical density at 570 nm was evaluated using an enzyme-linked immunosorbent assay plate reader.
2.7. Evaluation of intracellular ROS
After thawing, intracellular hydrogen peroxide levels were determined using a 2’,7’-dichlorofluorescin diacetate assay (Sigma,Germany). First, 10 µL of 2’,7’-dichlorofluorescin diacetate was added to the cells followed by an incubation at 37 ℃ for 25 min. Next,the green fluorescence between the 500 and 530 nm wavelengths[in the fluorescence (FL)-1 channel] was assessed by FACScan flow cytometery (Becton Dickinson, USA).
2.8. Detection of phosphatidylserine (PS) externalization
In the primary phases of apoptosis, PS was relocated to the outer surface of the plasma membrane from its inner surface. This initial index of apoptotic cells can be identified by conjugated fluorescence color to the annexin-Ⅴprotein, with a high degree of PS binding.For detection of PS externalization, PS detection kit (IQ Products,Netherland) was used according to the manufacturer’s protocol.In summary, 106cells were washed with 500 µL of binding buffer.AnnexinⅤ-fluorescein isothiocyanate (5 µL) was added to the cells and a dark incubation on the ice was performed for 20 min. After adding 5 µL of propidium iodide (PI) to the cell sample, green fluorescence and red fluorescence were detected respectively by using FL1 channel for annexinⅤ-fluorescein isothiocyanate and FL3 channel for PI by FACScan flow cytometery (Becton Dickinson,USA).
2.9. Real-time polymerase chain reaction (PCR)
After cryopreservation, the expression levels ofBaxandBcl-2genes were evaluated by real-time PCR. Total RNA was extracted using Trizol reagent according to the manufacturer’s protocol(Ready Mini Kit, Qiagen, USA). Then complementary DNA was synthesizedviareverse transcription, using synthesis kit (Transcript First Strand cDNA Synt, Roche, USA) according to manufacturer’s instructions. Real-time PCR was carried out using gene specific primers and the SYBR Green PCR Master Mix (Qiagen) in fortyreaction amplification cycles with Applied Bioscience 7500HT Fast. The primer sequences were shown in Table 1. The melting curve analysis was done to detect the presence of non-specific amplification products. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control to normalize samples. The expression level of each gene was calculated as 2-△CT.
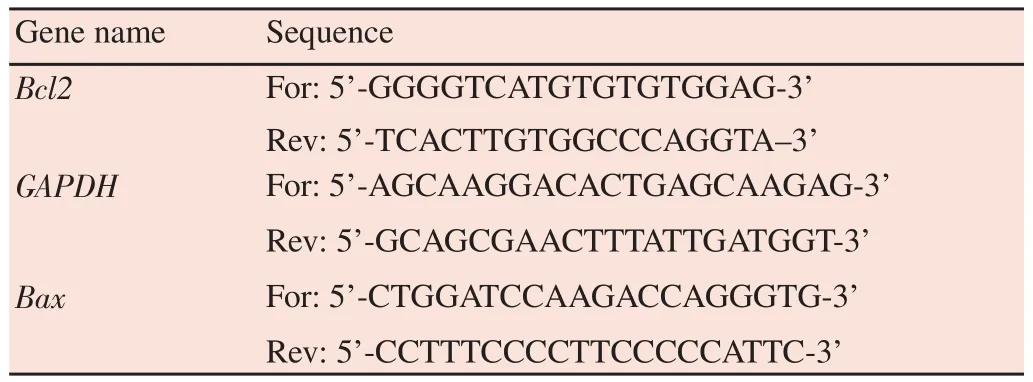
Table 1 Primers sequence used for real-time PCR analysis.
2.10. Statistical analysis
The data were representative of at least triplicate experiments.The data were analyzed by one-way analysis of variance, expressed as mean ± standard error of mean (mean ± SEM) and statistical significance was considered whenP<0.05.
3. Results
3.1. Evaluating cytotoxic effects of quercetin on mSSCs
The potential cytotoxicity of quercetin on mSSCs was evaluated by MTT assay. The results indicated that after treatment of cells with different concentrations of quercetin (10, 20, 30, 40 and 50 µM)for 48 h, cell viability did not significantly decrease compared to the control group (optical density, 0 µM: 0.743±0.033, 10 µM:0.706±0.049, 20 µM: 0.635±0.040, 30 µM: 0.898±0.039, 40 µM:0.689±0.049 and 50 µM: 0.720±0.037,P<0.05). The concentration of 30 µM of quercetin significantly (P<0.05) increased cell viability of cells in quercetin-treated group in comparison to the control group. Therefore, this concentration was selected as an appropriate dose to investigate the effect of quercetin on the freeze-thawing process of mSSCs.
3.2. Effect of quercetin on viability of frozen-thawed mSSCs
Quercetin decreased the mortality of mSSCs induced by cryodamage which was indicated by MTT assay. The results showed that the survival rate of the frozen-thawed mSSCs in quercetinpretreated group was significantly higher compared to the control group (optical density: 0.540±0.007vs.0.486±0.011,P<0.001).
3.3. Effect of quercetin on levels of ROS in frozen-thawed mSSCs
This experiment showed that the mean percentage of mSSCs with dichlorofluorescin (DCF) positive as an indicator of intracellular hydrogen peroxide levels significantly decreased in quercetin-pretreated group in comparison with the control group(35.790±2.236vs.39.420±2.058,P<0.05) (Figure 1).
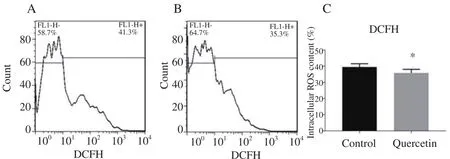
Figure 1. Effect of quercetin on levels of ROS in frozen-thawed mSSCs.
3.4. Flow cytometry analysis of PS externalization
AnnexinⅤwas used to detect the PS externalization. A significant difference between the mean percentage of vital mSSCs (annexinⅤ-/PI-)in quercetin-pretreated group and the mean percentage of the same cells in the control group was observed (61.9±1.0vs.28.6±5.4,P<0.01). The mean percentage of early apoptotic mSSCs (annexinⅤ+/PI-) significantly decreased in quercetin-pretreated group(9.345±0.750vs.29.650±1.650,P<0.001). Also, the mean percentage of late-apoptotic mSSCs (annexinⅤ+/PI+) in quercetin-pretreated group was decreased compared to the control group (19.29±1.11vs.35.30±4.30,P<0.001) (Figure 2).

Figure 2. Flow cytometry study of PS externalization.
3.5. Gene expression
After cryopreservation, the results ofBcl-2gene expression indicated higher significant expression in quercetin-pretreated group compared to the control group (0.489±0.051vs.0.021±0.005,P<0.05). Significantly, pretreated cells with quercetin showed lower expression ofBaxgene relative to the control group (0.006±0.003vs.0.012±0.003,P<0.01).
4. Discussion
In this study, we developed a new approach against oxidative stress which was produced during mSSCs cryopreservation procedure.We observed that viability of mSSCs after freeze-thawing process can be increased by pre-treatment of the cells with quercetin as an antioxidant. Quercetin could also decrease apoptosis and ROS content in post-thawing mSSCs and affect pro- and anti-apoptotic gene expression patterns. Our results demonstrated that pre-treatment of mSSCs by quercetin can increase the gene expression ofBcl-2and decrease the gene expression ofBaxduring cryopreservation.
Cryopreservation of SSCs from the testicular tissue before the start of chemotherapy and radiotherapy has provided significant hope for the fertility preservation of children confronting cancer[2]. Despite the advantage of cryopreservation, it leads to a disturbance in cellular redox through an increasing of ROS content and subsequently oxidative stress. Excess cellular oxidant agents result in the impairment of membranes, organelles and biological molecules such as proteins, lipids and DNA which can cause the activation of cell death. ROS plays an imperative role in apoptosis signaling pathways happening in cell organelles such as mitochondria controlled by Bcl-2 family proteins[19,20]. Our results showed that quercetinpretreatment of mSSCs improved cell viability after cryopreservation and thawing procedures. In supporting our data, Aliakbariet aldemonstrated that adding antioxidants catalase orα-tocopherol to the freezing medium can be an appropriate way to improve mSSCs viability after cryopreservation[18]. Our results indicated that the mean percentage of mSSCs with DCF positive as an indicator of intracellular hydrogen peroxide content significantly decreased in quercetin-pretreated group compared to the control group after freeze-thawing process. The protective effects of quercetin in our study are similar to the observed findings in another study, in which catalase orα-tocopherol protected mSSCs against oxidative stress due to cryopreservation[21]. Habaset alshowed that quercetin protected spermatogonial cells against oxidative damage and apoptosis that induced by diethylstilbestrol[22]. Moreover, Zhanget alconfirmed that quercetin can inhibit the oxidative damage resulted from aroclor 1254 by increasing the intracellular antioxidant levels like glutathione and superoxide dismutase in cultured embryonic chicken spermatogonial cells[23]. Also, some of the studies showed that ROS neutralization by quercetin may prevent ROS-induced spermatozoa damages and then preserve the function of male reproductive cells[24-26]. So, it seems likely that antioxidant activity of quercetin ameliorates oxidative stress by scavenging free radicals during freeze-thawing in mSSCs, which follows an increase in cell viability after cryopreservation procedures.
The positive effects of antioxidant supplementation on cell apoptosis have been evaluated in several studies. One study demonstrated that the addition of catalase andα-tocopherol to the basic freezing medium of mSSCs can decrease the level ofBaxexpression and increase the level ofBcl-2expression in mSSCs[27].In agreement with this study, our results demonstrated that the quercetin can decrease the apoptotic rate of mSSCs and the mean percentage of pre-apoptotic mSSCs (annexinⅤ+/PI-). Remarkably,Bcl-2 family proteins contribute to the modulation of cell death through their anti-apoptotic activity[28]. Production of ROS in the cells can activate apoptotic pathways of Bax/Bcl-2 by an increase in the expression of apoptotic proteins such as P38, c-JunN-terminal kinase and P53[29]. The findings reported by Shabaniet alindicated that the combination of vitamin C and E in a basal freezing medium promoted the expression of anti-apoptotic genes (Bcl-2andBcl-2l1)in the transfected ovine SSCs after cryopreservation[30].
Different antioxidants have been used for managing and improving the quality and quantity of freezed SSCs. Hemadiet alindicated that use of melatonin supplementation can improve cellular activity,restoration, and transplantation and also decrease apoptosis in vitrified neonate testes of mice[31]. Researchers supported the possible mediatory role of melatonin on the expression of several factors involved in cell apoptosis such as Bcl2 and Caspase 3[32,33].It is assumed that the potential adverse effects of ROS production during cryopreservation can be reduced by antioxidants capacity to remove ROS and convert hydrogen peroxide into water and oxygen[34]. It has been indicated that quercetin has an inhibitory influence on cell death in rat glioma C6 cells[35].
Previous investigations have shown that the effect of quercetin on apoptosis may vary between different cell types. Quercetin’s anticancer activities can generally facilitate apoptosis of tumor cells due to inhibition of heat shock protein 70, which has a vital role in cell thermotolerance[36]. However, quercetin has an inhibitory effect on hydrogen peroxide-induced apoptosis of some non-tumorigenic cells such as neuronal cells[37]. In addition, the mitogen-activated protein kinases are imperative molecules in controlling cell death. The antiapoptotic effects of quercetin can inhibit mitogen-activated protein kinases including c-JunN-terminal kinase and p38 pathways and result in a decrease of theBaxgene expression[38]. The present study showed the anti-apoptotic activities and antioxidants capacity of quercetin in removing ROS which decreased the mSSCs death.
Finally, we should note that although semen cryopreservation is a routine strategy in adult men, yet this is not applicable in prepubertal boys that are undergoing chemotherapy and radiotherapy treatments. So SSCs preservation either in form of a cell suspension or tissue biopsies is a technique for fertility preservation in these patients. It is well established that cryopreservation protocols can result in oxidative stress and subsequently damages to different cell macromolecules such as proteins, lipids and DNA. Thus, antioxidant supplementation is a strategy against increased levels of ROS and other oxidative agents which can be used for improving the efficiency of cryopreservation procedures. Our results suggest that quercetin can increase the antioxidant potential of mSSCs for facing to high production of oxidative species during cryopreservation and so our approach can be a promising strategy to improve fertility preservation techniques.
Conflict of interest statement
The authors declare no conflict of interest in any form.
Foundation project
This work was supported by Tehran University of Medical Sciences(Grant No. 34398).
 Asian Pacific Journal of Reproduction2019年1期
Asian Pacific Journal of Reproduction2019年1期
- Asian Pacific Journal of Reproduction的其它文章
- Antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle against lead acetate-induced testicular toxicity in rat
- Effect of butylated hydroxytoluene on quality of pre-frozen and frozen buffalo semen
- Blood indicators of dry cows before and after administration of a drug STEMB
- Value of α-fetoprotein,β-HCG, inhibin A, and UE3 at second trimester for early screening of preeclampsia
- Comparison of transvaginal cervical length and modified Bishop’s score as predictors for labor induction in nulliparous women
- Chronic atrophic endometritis and pyometra in a ferret: A case report
