Effect of butylated hydroxytoluene on quality of pre-frozen and frozen buffalo semen
Asmaa A. Mostafa, Mohamed S. El-Belely, Sayed T. Ismail, Reda I. El-Sheshtawy, Mohamed I. Shahba✉
1Abassia Frozen Semen Center, General Organization for Veterinary Services, Cairo, Egypt
2Department of Theriogenology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
3Department of Animal Reproduction and Artificial Insemination, National Research Centre, Dokki, Giza, Egypt
Keywords:Butylated hydroxytoulene Lecithin Egg yolk Buffalo semen Cryopreservation
ABSTRACT Objective: To clarify the antioxidant effect of butylated hydroxytoulene (BHT) at different concentrations on cooled and post frozen semen diluted in tris-citrate-fructose egg yolk glycerol and lecithin -based extenders. Methods: Forty ejaculates were harvested from four buffalo bulls by means of the artificial vagina. Ejaculated semen samples were diluted with each of the tris citrate-fructose egg yolk glycerol and lecithin-based extender diluents. The semen samples diluted with each of the two extenders were added to pre-warmed dried test tubes containing BHT (prepared in ethanol) to get concentrations at 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mM/mL BHT. These ingredients were put at 37 ℃ for 5 min to allow the proper BHT spermatozoal permeation. The diluted semen samples were cooled to 5 ℃ and then frozen to -196 ℃ in 0.25 mL ministraws before dipping in liquid nitrogen pending its evaluation.Sperm motility, viability, morphology, intact acrosome and membrane integrity were tested.Visual motility was tested using a high power ordinary microscope (at 400 ××) with closed circuit television, and sperm concentration was tested using Neubauer haemocytometer and abnormality % using eosin-nigrosin stain. Spermatozoal membrane integrity was tested using the hypo-osmotic swelling test. The sperm with swollen twisting tail was normally intact. Sperm acrosomal integrity % was tested as mentioned by Watson. Results: Addition of BHT improved (P<0.01) progressive motility, viability, morphology and acrosome as well as plasma membrane integrities at 0.5-2.0 mM/mL depending upon types of used extenders and stages of pre-and post-freezing process. Higher levels of 2.5 and 3.0 mM/mL BHT had a deteriorating (P<0.01) result if compared to the control and all extenders assayed.Conclusions: BHT addition at lower concentration can improve pre-frozen and post-thawed buffalo sperm quality.
1. Introduction
The value of pre-frozen and post-frozen spermatozoa is influenced by the oxygen free oocyte radicals[1]. These radicals decrease the sperm quality and the oocyte fertilizing capacity post-fertilization[2]. They have harmful properties on DNA of sperm and on male pronuclei development[3]. Moreover, these radicals inhibit motility, acrosome reaction and capacitation of sperm mainly due to lipid peroxidation[4]. Antioxidants improve frozen semen characteristics by lowering the oxidative stress[5].Semen freezing reduces semen antioxidants and spermatozoa are liable to oxidative damage. High levels of poly unsaturated fatty acid (PUFA) in sperm membrane of buffalo have increased lipid peroxidation percent as compared to cattle bulls[6]. Supplementation of antioxidants in semen diluents preserves spermatozoal status during freezing. Inclusion of butylated hydroxytoluene (BHT), a phenolic organic antioxidant that is a synthetic analogue of vitamin E, in different concentrations considerably lowers the sperm cryoinjury during freezing by protecting the sperm membrane.BHT can preserve liquid and frozen semen in buffalo bulls[7,8],bovine bulls[9-18], ram[19,20] and goats[21-25].
When antioxidants are used in higher levels, some vital metabolic functions may be deteriorated. Therefore, the current study aimed to assess the effects of BHT in tris-citrate-fructose egg yolk glycerol (TCFYG) and lecithin (TCFGL) based extenders on preand post-freezing semen parameters of buffalo bulls.
2. Materials and methods
2.1. Buffalo bulls
Four buffalo bulls (aged 3.5-5.0 years) kept at the Abassia Buffalo Semen Freezing Center, Central Organization for Veterinary Services, Ministry of Agriculture, Egypt, were selected for semen collection. The buffalo bulls were maintained under uniform standard nutrition and managerial practices. They were in a good general health condition (600-800 kg body weight).
2.2. Semen collection and initial evaluation
The four bulls were used weekly for semen early collection program using an artificial vagina. Semen samples were collected early in the morning. Two successive semen samples were collected by means of an artificial vagina attempts with 15 min interval. The ejaculates were pooled to eliminate variability among the collected samples. The collected ejaculates (10 per bull, total 40 in each experiment) were immediately put into a water bath at 35 ℃ for 10 min and evaluated for visual motility using a high power ordinary microscope (at 400 ××) with closed circuit television, sperm concentration using Neubauer haemocytometer and abnormality % using eosin-nigrosin stain. The heterogenic semen samples quantifying a minimum standard of 1 mL volume,70% motility, total sperm defects lower than 20% and with concentration of 600 × 106spermatozoa/mL of the ejaculate were selected for further processing. Additionally, percent of integrity status of the spermatozoal membrane using the hypoosmotic swelling test was done as recorded by Jeyendranet al[26].The sperm with swollen twisting tail was normally intact. Sperm acrosomal integrity % was undertaken as mentioned by Watson[27].Normal acrosome was differentiated by normal apical ridge.
2.3. Experimental design
The pooled semen was divided into two parts extended in two different extenders: TCFYG and TCFGL based extenders. The TCFYG diluent was set by dissolving 3.028 g tris, 1.678 g citric acid and 2.000 g fructose in 100 mL bi-distilled water, then adding 20% egg yolk and 7% glycerol with antibiotic (penicillinstreptomycin mixture) at 0.01 mL/mL of the diluent according to Ijazet al[7]. Since we had already documented the 1.0%soya lecithin enriched in egg yolk-free extender, as the optimal concentration providing cryopreservation of sperm quality variables in buffalo bulls, this concentration criterion was chosen during preparation of the TCFGL extender. The semen samples diluted with each of the two extenders were added to pre-warmed dried test tubes containing BHT (prepared in ethanol) to get concentrations at 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mM/mL BHT. These ingredients were put at 37 ℃ for 5 min to allow the proper BHT spermatozoal permeation[7] before cooling (cooled to 5 ℃) and freezing (frozen to -196 ℃). All diluents were adjusted to a pH of 6.7 using a pH meter (HANNA instrument, HI 8314). The concentration of sperm in the extended semen was 30×× 106cells in a 0.25 mL ministraws(IMV, France), then processed for freezing[28].
2.4. Statistical analysis
The data were tabulated as mean ± standard deviation (mean ± SD).All data were submitted to one way analysis of variance by using computerized statistical analysis. The analytical design was factorial design (general linear model) to clarify the effect of BHT concentrations and extenders on the pre- and post-freezing semen variables. Treated means were compared by the least significant difference test at 5% and 1% levels of probability. Five replicates were carried out for each treatment. For each replicate, four straws were used for assessing of sperm characteristics. All statistical methods were done according to Snedecor and Cochran[29].
3. Results
Table 1 clarified effects of BHT on buffalo semen characteristics after equilibration at 5 ℃ in TCFYG and TCFGL extenders.There was an interaction between treatment, extenders and semen variables as well as between treatment and extenders.
With semen cooled in TCFYG extender+1.0 and 1.5 mM/mL of BHT, progressive motility and sperm viability were enhanced(P<0.05) compared with the control group, with more better results reached with use of 1.5 mM/mL. As compared with the control, sperm morphology, intact acrosome and plasma membrane integrity were ameliorated (P<0.01) with 1.0 mM/mL BHT,respectively.
Semen cooled in TCFGL extender exhibited better semen quality with 0.5 mM/mL BHT compared with TCFYG extender.Progressive motility was improved (P<0.05) when compared to control group, at BHT concentrations of 1.0 and 0.5 and 1.0 mM/mL,respectivelywith superior in 1.0 mM/mL. In comparison with the control, sperm viability, sperm morphology, intact acrosome and membrane integrity were improved with 0.5 mM/mL of BHT.
There was a great deterioration (P<0.01) in pre-freezing semen quality extended in both TCFYG and TCFGL extenders with use of 2.0 mM/mL then 2.5 mM/mL concentrations and there was a significant increase in deterioration when the 3.0 mM/mL BHT was used.
Table 2 clarified the effects of BHT on post freezing semen variables after one week storage in TCFYG and TCFGL diluents.In general, there was an enhancement in semen characters with an increase in BHT concentrations until there was an optimal level depending on extender used and consequently the quality decreased with a further increase in BHT concentrations.
With TCFYG TCFGL, post-thawed sperm quality improved at higher levels of BHT if compared with samples frozen in TCFGL TCFYG. When compared with the control group, sperm progressive motility improved at 1.5 mM/mL BHT. Vviability,morphology, intact acrosome and membrane integrity were all significantly (P<0.01) improved at 2.0 mM/mL BHT. With use of 2.5 mM/mL BHT, sperm progressive motility, viability,morphology decreased when compared with the control. And there was a deterioration (P<0.01) when 3.0 mM/mL BHT concentration was used.
In comparison with post-thawed control samples extended in TCFGL, sperm motility, viability and morphology were greater(P<0.01) with use of 1.5 mM/mL BHT followed by 1.0 mM/mL.Intact acrosome and membrane integrity were all significantly(P<0.01) improved at 0.5 mM/mL These three semen variables substantially (P<0.01) decreased with the increase in BHT concentrations above 1.5 mM/mL. The use of 0.5 mM/mL concentration significantly (P<0.01) increased both intact acrosome and plasma membrane integrity as compared to the control, and then decreased as concentrations of BHT increased.
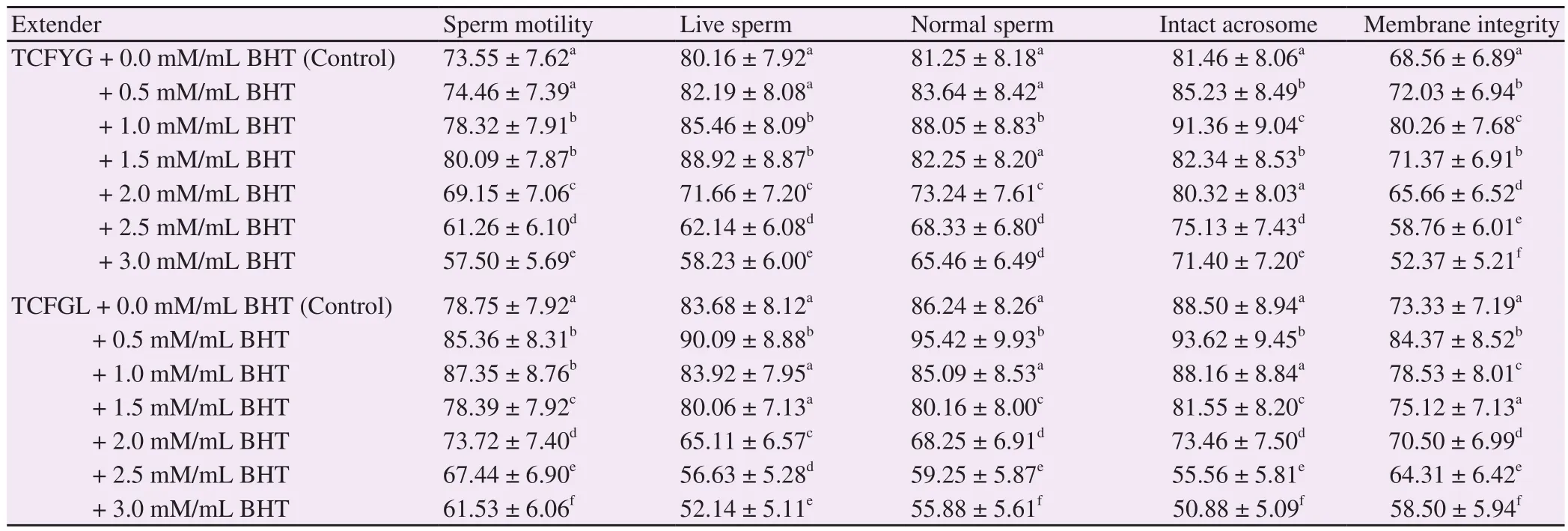
Table 1 Effects of BHT concentrations on pre-freezing semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls (%) (n=4) (mean ±SD).
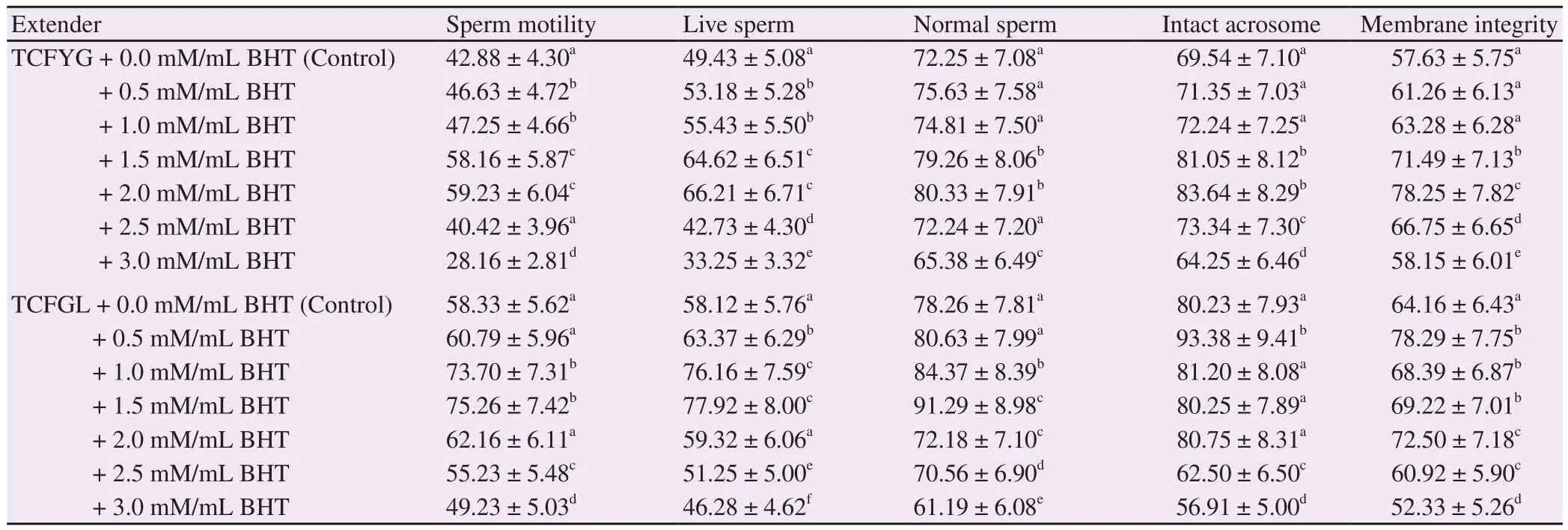
Table 2 Effects of BHT concentrations on post-thawed semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls (%) (n=4) (mean ±SD).
4. Discussion
Higher concentration of PUFA in the plasma membrane of buffalo sperm renders it liable to lipid peroxidation in the presence of reactive oxygen species (ROS). Seminal antioxidants such as superoxide dismutase and glutathione peroxidase prevent oxidation by lowering the ROS production[7]. Consequently, the quantity of such antioxidants are limited and insufficient to counteract the oxygen free radical metabolite over accumulation[28]. This result revealed that proper BHT levels were required to obtain the best results with the various semen diluents. Better findings were attained in pre-frozen and post-thawed buffalo semen with a lower BHT concentration with the use of TCFGL than the TCFYG.Better semen characteristics were noticed on using of TCFGL if compared to TCFYG extenders. High density lipoprotein in egg yolk interferes with sperm function, causing reduced protective effect of egg yolk during cryopreservation[30]. So, higher concentrations of BHT in TCFYG is required .
The present study for the first time documented that the beneficial effect of BHT supplementation on sperm quality of buffalo bulls depended primarily on cryopreservation stage, in addition to the effect of used extender. During equilibration (cooling) process, use of concentration of 0.5 mM/mL in TCFGL and 0.5-1.0 mM/mL in TCFYG extenders increased sperm progressive motility, viability,morphology and acrosome/membrane integrities when compared with the control group. On the other hand, greater concentrations of BHT were required during the freezing stage to improve the aforementioned semen variables. There was an amelioration in post-freezing semen quality parameters extended in TCFYG with use of 1.5-2.0 mM/mL BHT. Concerning semen extended in TCFGL, the three variables motility, viability and morphology were increased with the addition of 0.5-1.5 mM/mL BHT, whereas acrosome and membrane integrities were greater with 0.5 mM/mL BHT. Alternatively, use of the greater BHT concentrations above 2.0 mM/mL decreased sperm characteristics when added to all extenders. This reduction may be attributed to hazardous property of BHT as more antioxidants decrease physiological level of oxidants which is concerned with normal sperm metabolism[31].Furthermore, superior levels of BHT make spermatozoa liable to cryodamage[12] with failure in permeation of nutrients into the spermatozoa[8]. Furthermore, higher oxidative damage during cryopreservation of buffalo sperm due to its higher PUFA contents results in DNA damage[32,33]. Similar to our results, Ijazet al[7] and Wadoodet al[8] recorded that addition of 2.5-3.0 mM/mL BHT to buffalo semen extended in TCFYG revealed hazardous effects in the spermatozoa opted for the freezing process.
In the present study, different concentrations of BHT(0.5-3.0 mM/mL) were tested and optimal results were obtained by addition of 0.5-2.0 mM/mL BHT depending on extender used and stage of cryopreservation. Also, it seems that optimal concentrations of BHT depend upon the species of animals. For instance, the optimal concentration of BHT was 0.2-1.6 mM/mL for boar[31,34]and 0.5-1.5 mM/mL for cattle bull[12,13,16-18]. However, greater concentrations of BHT (2.0-5.0 mM/mL) were recommended for achieving the desired post-thawed sperm quality in the ram[19,20]and goat bucks[21-25]. The sperm membrane disruption which occurs in the ram and buck semen during cold shock is extensive owing to its higher concentrations of PUFA, and is difficult to account for by concepts of lipid-phase changes alone[20,24]. That is why higher levels of BHT enriched in the semen diluent are optimal for achieving greater post-freezing semen quality of in such animals.
Positive effect of BHT on pre-freezing and post-thawed semen characteristics may be attributed to the information that BHT protected sperm membrane fluidity due to its lipid solubility[21].Secondly, BHT is a synthetic analogue of vitamin E and is a breaker for the ROS chain and not a scavenging antioxidant, thus it preserves the integrity of sperm membrane without influencing oxygen free radicals production. Another BHT improving effect is due to its anti-viral activity and thereby it can inactivate lipidcontaining viruses in semen[12].
In conclusion, BHT concentration ranging from 0.5 and 2.0 mM/mL are optimal for buffalo semen freezing depending on type of used extender and stage of freezing. Higher supplementation of BHT results in hazardous effects on semen characteristics. The TCFGL exhibited better upon sperm quality evaluation than TCFYG when BHT was used in the semen diluents.More research is needed to be sure if these beneficial effects could be applied to improve buffalo fertilizing capacity.
Conflict of interest statement
All the authors declare that there is no conflict of interest.
 Asian Pacific Journal of Reproduction2019年1期
Asian Pacific Journal of Reproduction2019年1期
- Asian Pacific Journal of Reproduction的其它文章
- Infertility in China: Culture, society and a need for fertility counselling
- Total segmental aplasia of uterus body in bitch
- Chronic atrophic endometritis and pyometra in a ferret: A case report
- Comparison of transvaginal cervical length and modified Bishop’s score as predictors for labor induction in nulliparous women
- Value of α-fetoprotein,β-HCG, inhibin A, and UE3 at second trimester for early screening of preeclampsia
- Blood indicators of dry cows before and after administration of a drug STEMB
