Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
CHEN Tun-Wei ZHEN Wen-Bo YAN Zhi-Ming LIN He-Tong CHEN Guo-Ying CHEN Sho-Jun
Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
CHEN Tuan-WeiaZHEN Wen-BoaYAN Zhi-MingaLIN He-TongaCHEN Guo-YingbCHEN Shao-Juna
a(350002)b(19038)
A novel 1-methylimidazole ionic liquid modified SBA15 mesoporous silica (1-MIIL@SBA15) was synthesized and applied to selective separation of inorganic arsenic (iAs) in rice by dispersive solid phase extraction (DSPE), followed by hydride generation-atomicfluorescence spectrometric(HG-AFS) quantification. The prepared sorbent was characterized by FTIR, FESEM, BET and Zeta potential. Key parameters of adsorption and desorption in DSPE wereoptimized using standard reference material 1568b rice flour. Under optimal conditions, the limit of detection was 8.776 ng/kg, relative standard deviation was ≤2.0%, and recoveries of iAs were in the 92.3~94.4% range.This method was successfully applied to the determination of iAs in rice. Under acidic condition, the electrostatic interaction between the positively charged 1-MIIL@SBA15 and anionic iAs played an important role in selective iAs separation, rendering this method suitable for iAs analysis.
SBA15, ionic liquid, inorganic arsenic, dispersive solid phase extraction, hydride generation-atomic fluorescence spectrometric (HG-AFS);
1 INTRODUCTION
Arsenic (As), recognized as a global pollutant and non-threshold carcinogen, isassociated with both cancer (lungs, liver, bladder, skin,etc.) and non-cancer (keratosis,cardiovascular disease, diabetes, etc) health impacts[1-4]. Toxicity of inorganic arsenic (iAs, including arsenite (AsIII) and arsenate (AsV)) is much higher than its organic counterparts[5,6]; so, the focus of regulatory monitoring is shifting from total arsenic to iAs, making speciation critical in food safety monitoring.
Rice is the dietary staple for half of human population, especially in Asia. Typically grown in anaerobic flooded soil, unfortunately, rice is more vulnerable to arsenic in sub-soil and irrigation water than other terrestrial crops, leading to much higher iAs accumulation[7,8].To protect public health from chronic iAs exposure via rice consumption, the Codex Alimentarius Committee (CAC) on contami- nants in food set iAs maximum level (ML) in polished rice at 200 ng/g in 2014[9]. China and South Korea have also set iAs ML in polished rice at 200 and 150 ng/g, respectively[10]. European Union has established iAs MLs at 200 ng/g for polished rice, 250 ng/g for parboiled rice, and 100 ng/g for the rice destined for foods for young children and infants[11].In 2016, US FDA proposed an actionlevel of iAs in rice for the infant foods at 100 ng/g[12]. However, sensitive, accurate and reliable determination of iAs in rice samples is still difficult due to low-level presence and complexmatrix.
Conventional separation by high performance liquid chromatography (HPLC) using strong anion exchange sorbent suffers from high cost and low sample throughout[13,14].Thus, non-chromatographic approaches such as liquid-liquid extraction, cloud point extraction, and solid phase extraction (SPE) were developed for iAs separation to gain simplicity, cost, and time advantages[15,16]. Dispersive SPE (DSPE) is an attractive technique with rapid opera- tion, high recovery, and large enrichment factor[17]. Up to now, a variety of adsorbents[18-26]such as ion exchange resin, metal organic frameworks (MOFs), carbon-based materials, metal oxides, and meso- porous materials have been used for the adsorption of arsenic from water and biological samples. Amongst these,ordered mesoporous silicas, espe- cially SBA-15, have gainedsignificant attention due to their large specific surface area,uniform internal pore structure, controllable pore size, andframework stability[27].However, low selectivity disqualifies these adsorbents for iAs separation; modification with selective functional groups is recommended. Fortunately, imidazolateionic liquids (IILs) with surface cationic imidazolegroups have been demonstrated to enhance selectivity via electrostatic interaction[28-30]. Based on the fact that iAs existed as H2AsO4−, HAsO42−, and H2As2O32−, we propose imidazole modified SBA15 as an effective DSPE adsorbent for selective iAs separation.
In this work, 1-methylimidazole ionic liquid func-tionalized SBA15 mesoporous silica (1-MIIL@SBA15) was synthesized for iAs separa- tion from rice, followed by hydride-generation atomic fluorescence spectrometric(HG-AFS) quan- tification. To the best of our knowledge, this is the first report on the use of 1-MIIL@SBA15 sorbent in arsenic speciation. Characterizations of the adsor- bent and related technical issues were discussed in detail.
2 EXPERIMENTAL
2. 1 Chemicals and reagents
AsV, AsIII, monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) standard stock solutions (1000 mg/Lin 1 mol/L HNO3) were purchased from National Standard Materials Research Center (Beijing, China) and diluted prior to use. SBA15 ordered mesoporous silica was obtained from Xianfeng Nanomaterial Technology (Nanjing, China). All the other analytical grade chemicals were supplied by Sinopharm Chemical Regent (Shanghai, China). All the solutions were prepared using deionized water (DIW) with a resistivity of 18.2 MΩ·cm from a TM-D24UV water system (Millipore, Bedford, MA, USA). The standard reference material (SRM) 1568b rice flour (certified value of inorganic arsenic 92±10g/kg) was provided by National Institute of Standard and Technologies (NIST, Boulder, CO, USA).
2. 2 Synthesis of 1-methylimidazolium ionic liquid modified SBA15 (1-MIIL@SBA15)
The synthesis was conducted according to the report by Yuan[31]with some modifications. A typical procedure is: 2.0 g SBA15 was dispersed in 50 mL of dried toluene to which 3.0 mL of 3-chloro- propyltriethoxysilane (CPTES) was added, followed by reflux at 120 °C for 24 h under N2atmosphere. After cooling to room temperature, the reaction solution was extracted to dichloromethane by Soxhlet extraction for 24 h and dried at 70 °C over- night in a vacuum oven to obtain the precursor (CPTES/SBA15). Next, 2.0 g CPTES/SBA15 was suspended in 50 mL of dried toluene to which 3.0 mL of 1-methylimidazole (1-MI) was added, followed by reflux at 120 °C for 24 h under N2atmosphere. After cooling to room temperature and centrifugation, the resulting sorbent was washed sequentially with toluene, absolute ethanol, and methanol, and finally desiccated at 70 °C overnight in a vacuum oven to obtain solid sorbent, designated as 1-MIIL@SBA15, to be kept in a desiccator. Fig. 1 illustrates the schematic diagram of the synthesis procedure of 1-MIIL@SBA15.

Fig. 1. Schematic diagram of the synthesis procedure of 1-MIIL@SBA15
2. 3 Selective adsorption experiment
Abatchselective adsorption experiment was carried out with 5 mg 1-MIIL@SBA15 and 5 mL of AsV, MMA, DMA aqueous solutions at desired pH and concentrations. The aqueous solutions were adjusted to desired pH using 1 mol/L HCl or NaOH. The adsorption process was aided with ultrasonic agitation at room temperature to achieve adsorption equilibrium (in the case of adsorption isotherms). After centrifugation at 10000 rpm for 15 min, 1.6 mL of supernatants was transferred to 10 mL volumetric flask, to which 0.4 mL of 50 g/L thiourea-50g/L ascorbic acid and 2 mL of 10%HCl were added, and allowed to stand at room temperature for30 min. Finally, the content was analyzed by HG-AFS. The adsorption efficiency (%) and adsorptioncapacity (q,g/g) were calculated with the following equations:
%= (1–C/C)×100 (1)
q= (C–C)/, (2)
whereCandCarethe initial and equilibrium concentrations of arsenic solution (g/L),is thevolume (L), andis the amount of adsorbent (g).
At the same time, the adsorption of AsVby native SBA15 served as control.
2. 4 Sample preparation
Eighteen rice samples of different varieties (polished rice, brown rice, glutinous rice and rice flour) were collected from local markets in Fuzhou, China. After being washed three times with DIW, rice grains were dried at 60 °C in an oven to constant weight and then milled using a small mill into fine powders. Microwave assisted acid digestion was conducted following our previous report[16]with some minor modifications. Briefly, aliquots of 0.25 g (±0.005 g) rice flour were weighed into 100 mL microwave vessels, 10 mL of 0.06 mol/L HNO3-3%H2O2was added, then the vessels were sealed and digestion was performed using a MarsX Express (Anton Paar, Austria) microwave reaction system at 95℃for 30 min. The contents were allowed to cool to room temperature, and then filtered through 0.22m polytetrafluoroethylene (PEFE) membrane filter (Jinteng, Tianjin, China).
2. 5 DSPE procedure
Fifty mg 1-MIIL@SBA15 was added to adjust the pH value of the rice extract. The suspension was mixed under ultrasonic agitation at room tempera- ture for 20 min, and then centrifuged at 10000 rpm for 5 min. After centrifugation, the solid phase was mixed with 5 mL of 1.0 mol/L HCl and subjected to ultrasonic agitation for 5 min. After centrifuge at 10000×for 10 min, 1.6 mL of supernatant was transferred to 10 mL volumetric flask, then 0.4 mL of 50 g/L thiourea-50g/L ascorbic acid and 2 mL of 10%HCl were added, and finally the mixture was allowed to stand at room temperature for30 min to reduce AsVto AsIII. The recoveries (%R) were calculated with the following equation:
%R=C/C×100 (3)
whereCandCare the detection value and the certified value of inorganic arsenic in the SRM1568b rice flour, respectively.
2. 6 Instrumentation
The microstructural images of 1-MIIL@SBA15 were observed by field emission scanning electron microscopy (FESEM) using an S4800 microscope (Hitachi, Tokyo, Japan) with accelerating voltage set at 10 KV. Fourier transform infrared (FTIR) spec- trum was collected on a Nicolet 6700 spectrometer (Waltham, Massachusetts, USA) in the 4000~400 cm-1frequency range with 4 cm-1resolution. The N2adsorption-desorption isotherms of SBA15 and 1-MIIL@SBA15 were measured using an ASAP2020micromeritics analyzer (Micromeritics,Georgia, USA) at 77 K with samples previouslydegassed at 300 °C for 48 h to determine the Brunauer-Emmett-Teller (BET) specific surface area(m2/g) and total pore volume (m3/g). Zeta potential was measured using a Zeta potential analyzer (Malvern, UK). Triplicate measurements were taken for each sample; quantification was based on peak height using a 3100 atomic fluorescence spectro- meter (Haiguang, Beijing, China). The major AFS parameters were set as follows according to the manufacturer’srecommendations: total primary current at 50.0 mA, detection wavelength at 193.7 nm, Argon flow rate at 800 mL/min, air flow rate at 300 mL/min. A standard curve was constructed daily.
3 RESULTS AND DISCUSSION
3. 1 Characterization of 1-MIIL@SBA15
FTIR was employed to confirm the successful introduction of methylimidazole groups onto the surface of SBA15 mesoporous silica, where native SBA15 was used as reference material. The FTIR spectra of the 1-MIIL@SBA15 and SBA15 are depicted in Fig. 2A. The adsorption bands at 960 cm-1are attributed to the O–H stretching vibration of the surface silanol groups; vibration of 1-MIIL@SBA15 was weakerSBA15 because some O–H groups were coupled toimidazolyl groups. The adsorption band at 2928 cm-1isascribed to C–H vibration of imidazole, and the band at 1573 cm-1corresponds to the stretching of C–C and C–N groups[31,32]. Overall,the FTIR spectra proved successful coupling of 1-MIIL onto the surface of SBA15. The morphologies of SBA15 and 1-MIIL@SBA15 were studied by FESEM. The FESEM images revealed that the pore size was slightly decreased due to 1-MIIL filling, but the uniform mesoporous structure of SBA15 was still intact (Fig. 2B).
Furthermore, the porous structures of SBA15 and 1-MIIL@SBA15 were confirmed by N2adsorp- tion/desorption isotherms. As shown in Fig. 2C, the isothermal hysteresisof SBA15 and 1-MIIL@SBA15 appeared in the 0.6~0.8 relative pressure (/0) range in the IV type adsorp- tion/desorptionisotherm, which was characteristic of highly ordered mesoporous materials[33].The BET specific surface areas and total pore volume of 1-MIIL@SBA15 were calculated to be 391.4 and 0.68 cm3/g, respectively, based on the BET equation for adsorption isotherm. Compared to native SBA15 (BET specific surface area 781.7 m2/g and pore volume 1.18 cm3/g), the reduction of BET specific surface area and pore volume of 1-MIIL@SBA15 was due to 1-MIIL filling in the pore channel of SBA15, in good agreement with the results of FTIR and FESEM.Moreover, the zeta potential of 1-MIIL@SBA15pH (Fig. 2D) indicated that the material carried positive charges (28.6~36.8 mV) at pH up to 11.5, the isoelectric point (IP) of the 1-MIIL@SBA15.

Fig.2. Characterizations of SBA15 and 1-MIIL@SBA15. (A) FTIR spectra of SBA15(a) and 1-MIIL@SBA15(b); (B) FESEM images ofSBA15 (a)~(b) and1-MIIL@SBA15 (c)~(d); (C) N2adsorption-desorption isotherms of the SBA15 and 1-MIIL@SBA15; (D) Zeta potential of 1-MIIL@SBA15
3. 2 Selective adsorption behaviors
To understand the adsorption capacity of iAs on the adsorbent, the adsorption efficiency of 20g/L AsVon 5 mg native SBA15 and 1-MIIL@SBA15 was firstly compared at pH 6 after adsorption for 15 min (Fig.3). As could be seen, no adsorption occurred on native SBA15(3%) but high adsorption on 1-MIIL@SBA15 (95%). These contrasting adsorption behaviors of AsVcould be attributed to adsorption of anionic AsVspecies (H2AsO4−and HAsO42−) on cationic imidazole groups on 1-MIIL@SBA15 surface, rendering 1-MIIL@SBA15 an effective iAs adsorbent.
Solution pH affects both the surface charge of the adsorbent and iAs adsorption[28]. To better unders- tand the interaction of iAs adsorption at different pH, adsorption efficiencies of 20g/L AsV, MMA, and DMA on 1-MIIL@SBA15 were studied in the pH 2~13 range (Fig. 4). In Fig. 4A, adsorption efficiency of AsV, MMA and DMA increases in the pH 2~11range then decreases drastically beyond pH 11. The adsorption efficiency of AsVincreased rapidly from 10% at pH 2 to 85% at pH 3. With pKa=2.3, AsVexisted in neutral form below pH 2.3, resulting in no electrostatic interaction[16]. In the pH 4~11 range, AsVcarries negative charge (H2AsO4−and HAsO42−), while 1-MIIL@SBA15 carries positive charge (IP =11.5). The strong electrostatic interaction was therefore responsible for the high adsorption efficiencies (>95%). Beyond pH 11.5, 1-MIIL@SBA15 carries negative charge; electro- static repulsion caused adsorption efficiency to decrease drastically (<7%).
To further investigate the AsVadsorption effi- ciency and the interference of organic arsenic species, the pH value was fine tuned to 3.0, 3.2, 3.4, 3.6, 3.8 and 4.0(Fig. 4B). The adsorption effi- ciencies of AsV, MMA and DMA were 95%, 14% and 5.3%, respectivelyat pH 3.4; on the other hand, higher than 30% adsorption efficiency of DMA was obtained at pH >3.4. So, pH3.4 was selected as the optimal for iAs adsorption.
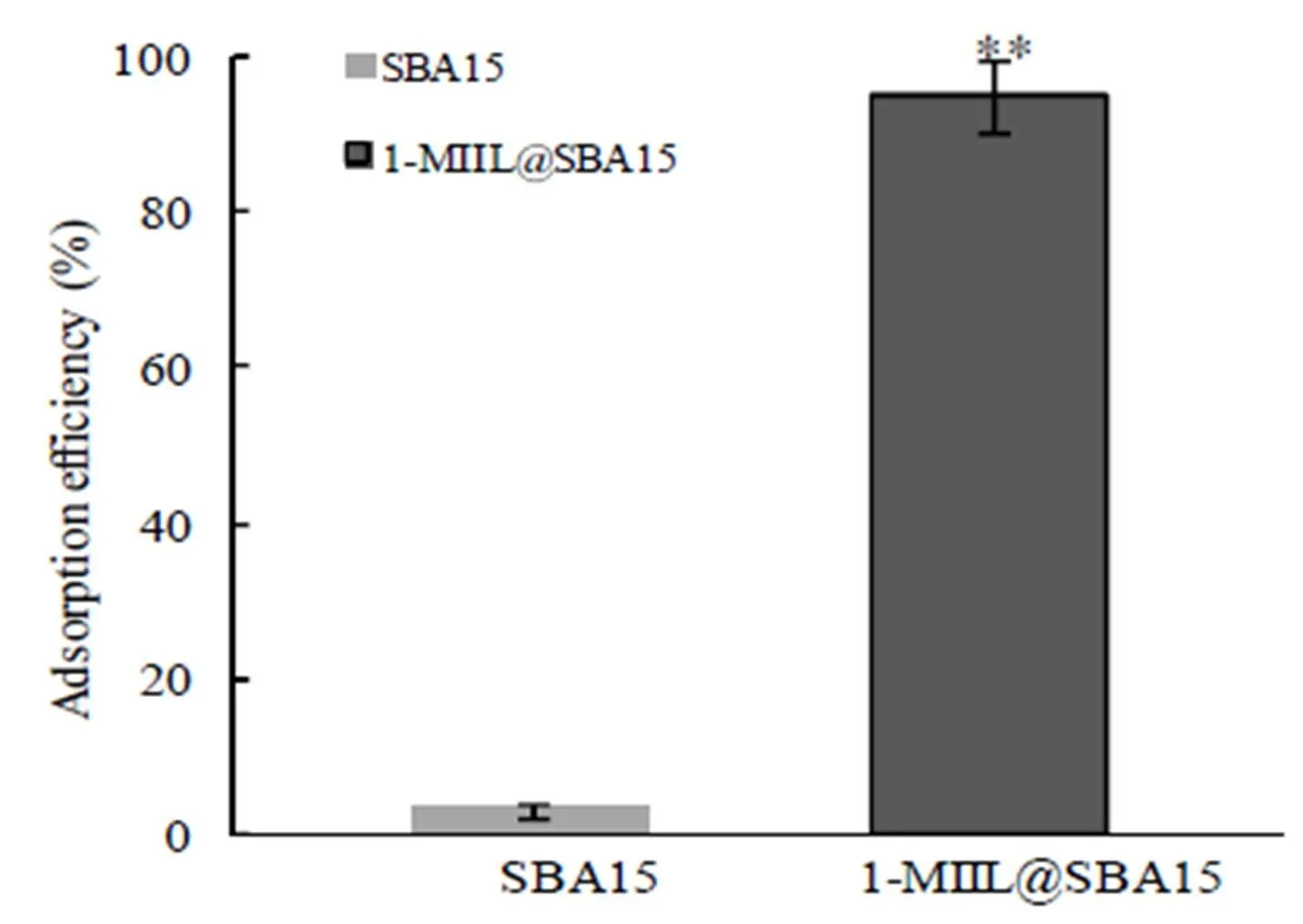
Fig. 3. Comparison of the adsorption efficiency of AsV(20 μg/L) between SBA15 and 1-MIIL@SBA15 after adsorption at pH6 for 15 min at room temperature
Fig. 4. Effect of solution pH on the adsorption efficiencies of AsV, MMA, and DMA on 1-MIIL@SBA15. (A) pH range from 2 to 13; (B) pH at 3.0, 3.2, 3.4, 3.6, 3.8 and 4.0
3. 3 Adsorption kinetic
The time-dependent adsorption of AsVon 1-MIIL@SBA15 in Fig. 5 demonstrated that the adsorption equilibriums of AsVwas achieved within 25 min. And two known kinetic models including pseudo-first-order rate and pseudo-second-order rate fittings were applied to the behavior of the adsorptiontime. The relevant kinetic equations and parameters are represented in Table 1. It could be seen that the pseudo-second-order model (2=0.9997) fits better than pseudo-first-order model (2=0.9780); meanwhile, the experimental equilibrium adsorption (e=28.6g/g) is much closer to that of the pseudo-second order model (e=29.0g/g).
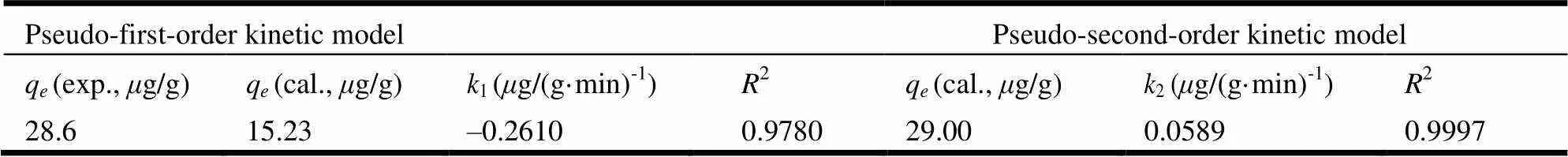
Table1. Kinetic Model Parameters for the Adsorption of iAs on 1-MIIL@SBA15
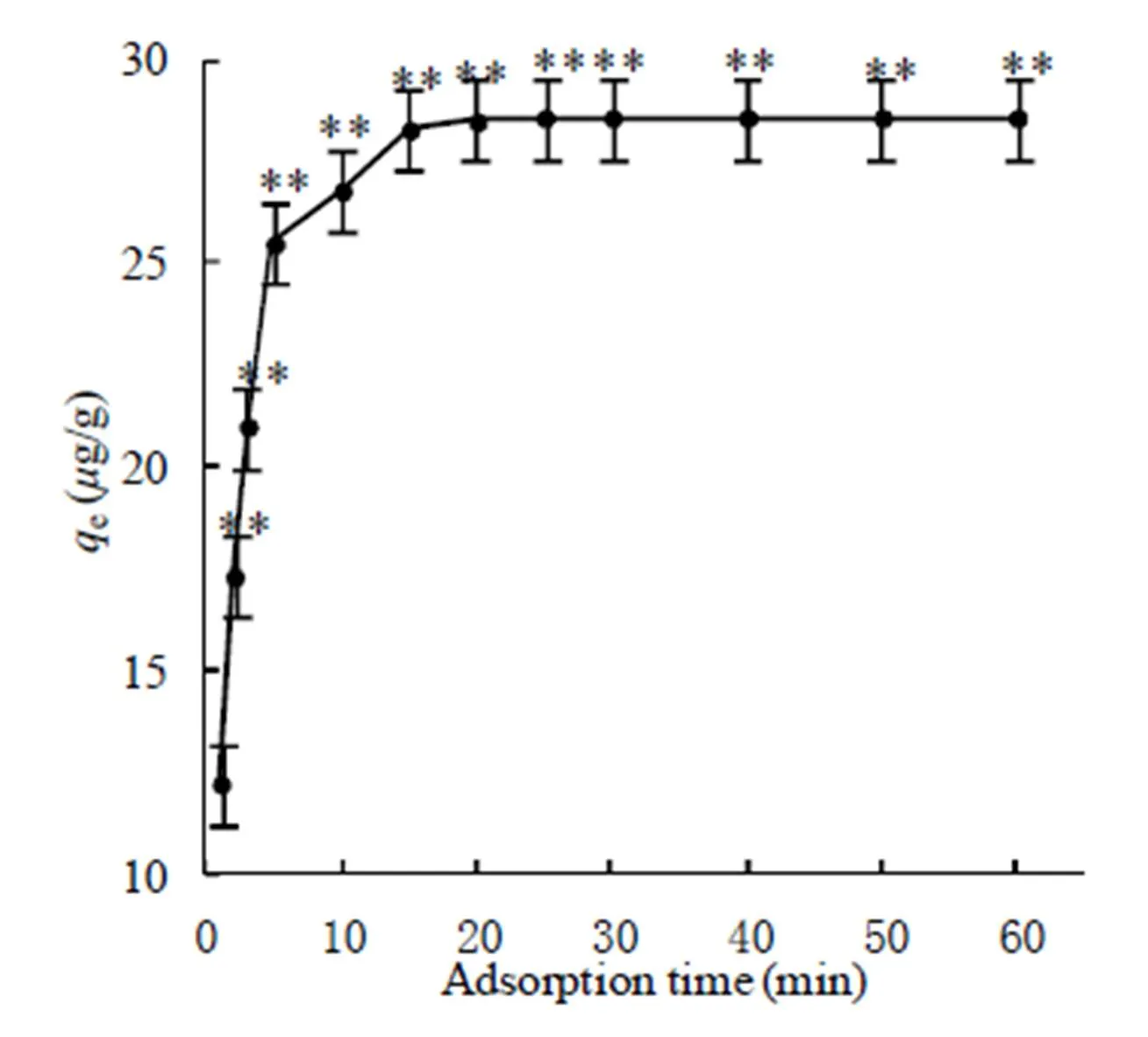
Fig. 5. Time-dependent adsorption of AsVon 5 mg 1-MIIL@SBA15 at different initial concentrations
3. 4 Adsorption isotherms
Two different models of adsorption isotherm, Langmuir and Freundilich models were used to interpret the relationship between the adsorbed amount of AsVand its equilibrium concentration in aqueous solution (Table 2). The Langmuir model is based on a monolayer sorption with homogeneous sorption energies, whereas the Freundilich model is based on multilayer sorptionand heterogeneous sorption energies[34]. In term of2values and experimental data, the sorption of AsVfollowed the Langmuir model (2=0.9965), indicating a mono- layer adsorption process. The maximum adsorption capacity (q) of 1-MIIL@SBA15 to AsVwas 231.48g/g. On the other hand, the 1/n <1 (0.6972) indicated high adsorption intensity[34].
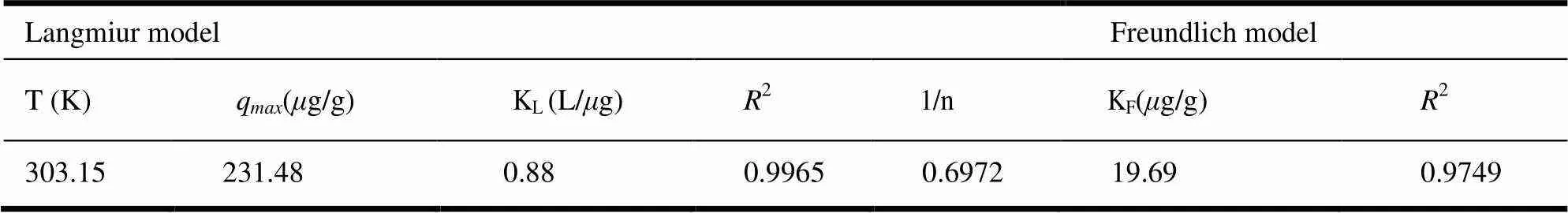
Table 2. Langmuir and Freundlich Parameters for the Adsorption of iAs on 1-MIIL@SBA15
3. 5 Optimization of DSPE procedure
In order to obtain quantitative recoveries of iAs with good sensitivity and precision in rice sample, key analytical parameters for DSPE procedure such as amount of adsorbent, adsorption time, con- centration and volume of elution solvent were optimized.
Adsorbent amount and adsorption time were cru- cial factors that affect extraction efficiency[35].Firstly, amounts of 1-MIIL@SBA15 in the range of 3.0~60 mg were studied based on iAs recovery (Fig.6A). It was found that >82% recovery was obtained using 50 mg 1-MIIL@SBA15; higher amount of adsorbent had no significant effect, likely due to higher degree of difficulty to elute iAsfrom a larger amount of adsorbent. In the final protocol, 50 mg of 1-MIIL@SBA15 was used. Secondly, the influence of adsorption time on iAsrecovery was investigated at 10, 15, 20, 25, 30, 35 and 40 min. As shown in Fig.6B, quantitative iAs recoveries (>85%) were achieved at 20 min, then reached a plateau beyond 20 min. Therefore, 20 min was set in the final protocol.

Fig. 6. Effect of adsorption conditions on the recovery of inorganic arsenic content in SRM1568b rice flour(A) adsorbent amount; (B) adsorption time
A suitable eluent is required for quantitativeelution[36],which was possible only when AsVcarried zero net charge at low pH; thus, strong acid HCl was selected as an eluent[16], and elution conditions were investigated (Fig. 7). The recovery of iAs increases with increase in the concentration in 0.5~1 mol/L, then reaches a plateau (93%) in the 1~1.5 mol/L range (Fig. 7A). So, 1.0 mol/L HCl was chosen as the optimal eluent concentration. The effect of HCl volume on iAs recovery was further investigated in the 4~8 mL range (Fig.7B), no significant effect on iAs recovery was observed; beyond 6 mL, decreased iAs recovery was likely caused by dilution. Therefore, 5 mL of HCl was used in the subsequent experiments. Finally, the effect of elution time on the iAs recovery was studied in the 1~15 min range. The results showthat iAs recovery reaches 93.1% when the elution time increased to 5 min, beyond which elution time had hardly any effect on recovery(Fig.7C). Thus, 5 min elution time was set in the final protocol.
In order to evaluate the reusability of 1- MIIL@SBA15 adsorbent, the used adsorbent was dipped into 1 mol/L HCl for 30 min, and then rinsed with DIWseveral times before the next cycle. As shown in Fig. 8, no obvious change was observed in iAs recovery after 5 cycles.
3. 6 Analytical performance
The method accuracy was verified by spike-reco- very studies. SRM 1568b rice flour was spiked with 70, 100, 130g/L AsV, and the calibration curve was obtained with a linear equation=137.5991+17.7836, and2=0.9991. The analytical merits are summarized in Table3; the recoveries were acceptable in the 92.3~94.4% range; the relative standard deviation (RSD) was 1.5~2.0%, demons- trating reliability and freedom from matrix effects.The limit of detection (LOD) and limit of quanti- fication (LOQ) were 8.776and 26.3 ng/kg, respec- tively, calculated from 3based on 11 blank measurements[16].In short, the developed method using 1-MIIL@SBA15 as adsorbent showed high sensitivity was proved by the much lower LODs and LOQs compared with traditional and recently reported methods for the determination of inorganic arsenic in foods[28,36-38].

Table 3. Analytical Figures of Merit of the Method(n=3)
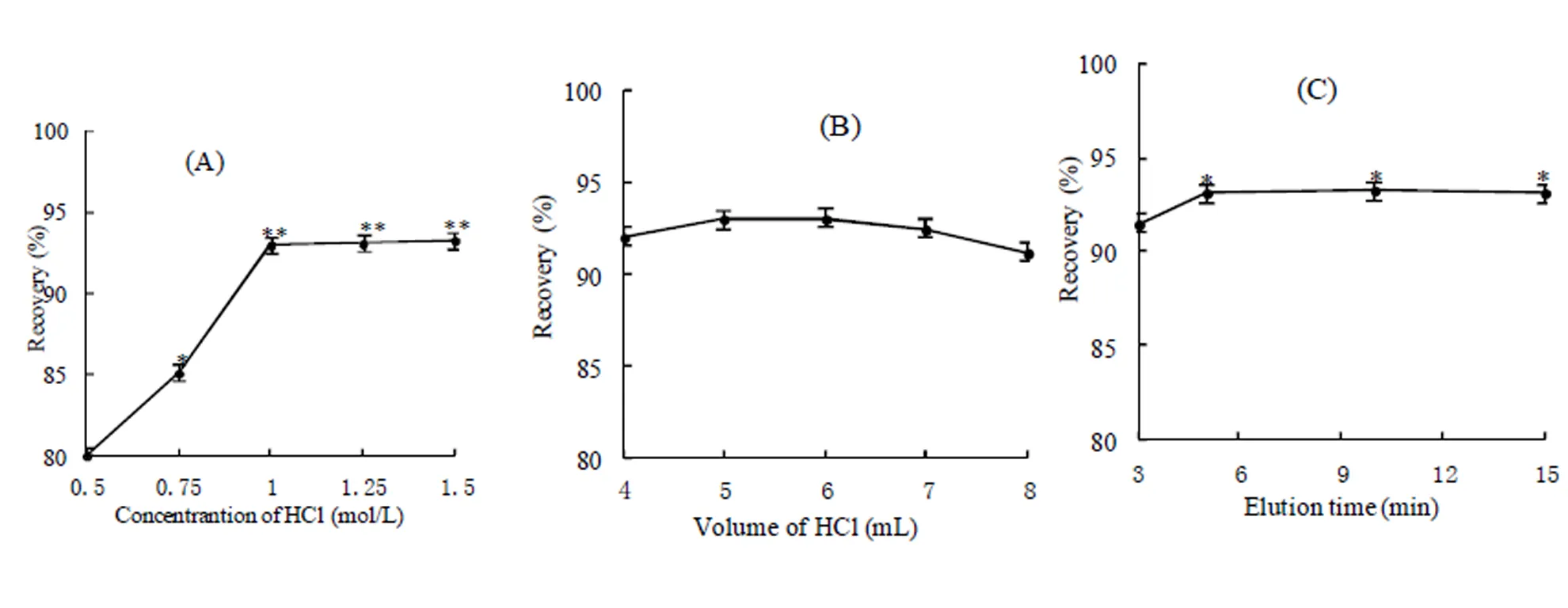
Fig. 7. Effect of desorption conditions on the recovery of inorganic arsenic content in SRM1568b rice flour.
(A) concentration of HCl; (B) elution time; (C) volume of HCl
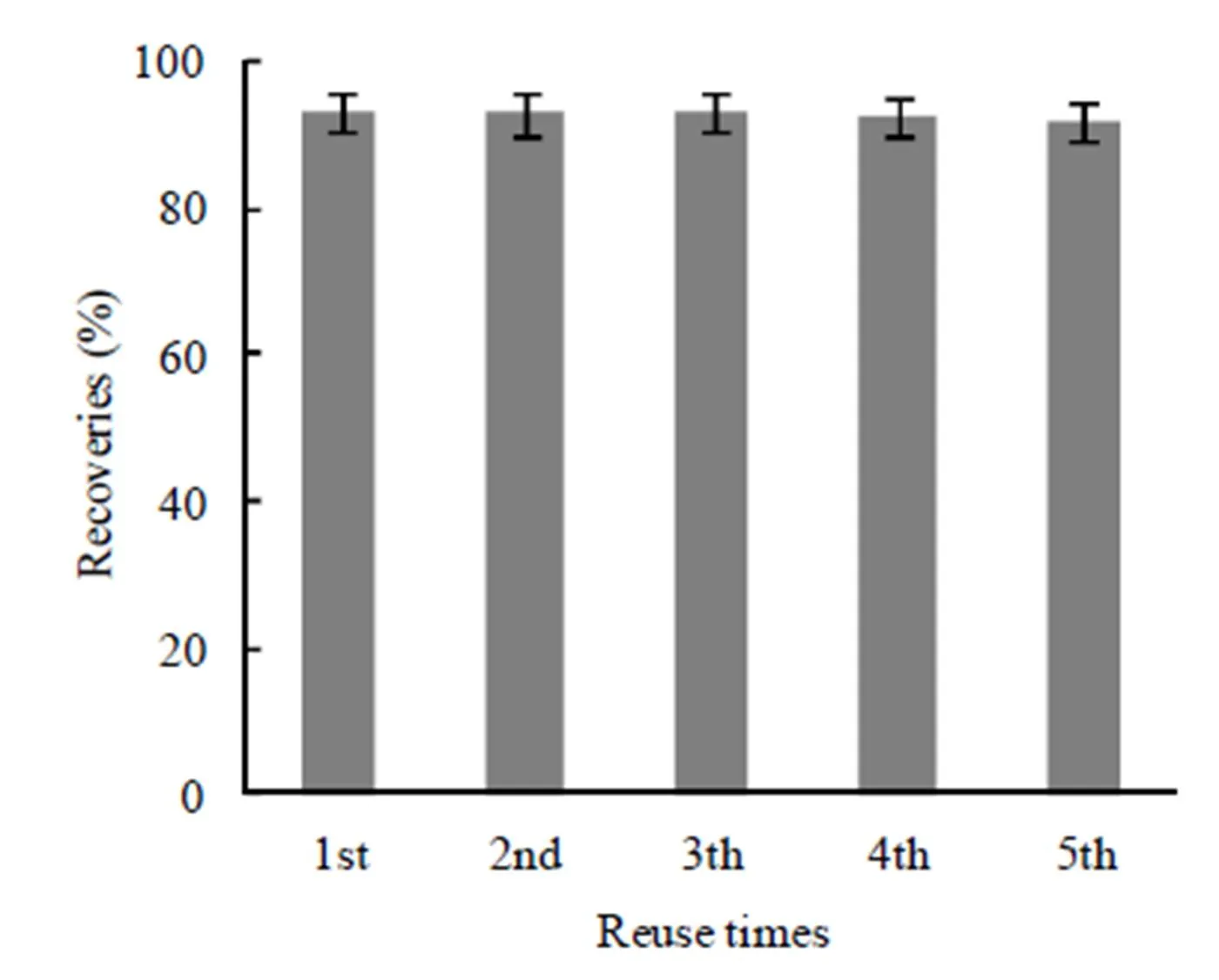
Fig. 8. Effect of reuse times of 1-MIIL@SBA15 adsorbent on recovery of inorganic arsenic in SRM1568b rice flour
3. 7 Rice sample analysis
This method was applied to iAs determination in rice (Table 4). The contents of iAs in rice samples were in the range of 67~190g/kg; while those for brown rice (150~190g/kg) are higher than the corresponding values for the white rice(96~116g/kg) because of higher iAs distribution in aleurone layer, in consistence with previous reports[16,39-43]. The results of SRM 1568b are in good agreement with the certified values, validating this method for iAs determination in rice.

Table 4. Analytical Results for iAs in Real Rice Samples
4 CONCLUSION
In this work, 1-MIIL@SBA15 was successfully synthesized as a DSPE adsorbent and applied to iAs separation from rice matrix. This DSPE metho- dology gained selectivity, sensitivity, operation, and cost advantages. The resulting DSPE-HG-AFS methodology proved an effective alternative for HPLC-ICP-MS for quantification of iAs in rice. This study also demonstrated a real-world application of mesoporous materials.
(1) Cubadda, F.; Jackson, B. P.; Cottingham, K. L.; Van Horne, Y. O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: state of knowledge, gaps and uncertainties.2017, 579, 1228–1239.
(2) Smith, A. H.; Ercumen, A.; Yuan, Y.; Steinmaus, C. M. Increased lung cancer risks are similar whether arsenic is ingested or inhaled.2009, 19, 343–348.
(3) Cheng, Y. Y.; Huang, N. C.; Chang, Y. T.; Sung, J. M.; Shen, K. H.; Tsai, C. C.; Guo, H. R. Associations between arsenic in drinking water and the progression of chronic kidney disease: a nationwide study in Taiwan.2017, 321, 432–439.
(4) Rasheed, H.; Kay, P.; Slack, R.; Gong, Y. Y. Arsenic species in wheat, raw and cooked rice: exposure and associated health implications.2018, 634, 366–373.
(5) Llorente-Mirandes, T.; Rubio, R.; Lopez-Sanchez, J. F. Inorganic arsenic determination in food: areview of analytical proposals and quality assessment over the last six years.2017, 71, 25–69.
(6) Qu, H. O.; Mudalige, T. K.; Linder, S. W. Arsenic speciation in rice by capillary electrophoresis/inductively coupled plasma mass spectrometry: enzyme-assisted water-phase microwave digestion.2015, 63, 3153–3160.
(7) Islama, S.; Rahman, M. M.; Islam, M. R.; Naidu, R. Arsenic accumulation in rice: consequences of rice genotypes and management practices to reduce human health risk.2016, 96, 139–155.
(8) Amaral, C. D. B.; Nobrega, J. A.; Nogueira, A. R. A. Sample preparation for arsenic speciation in terrestrial plants-a review.2013, 115, 291–299.
(9) Commission, C. A. The report of the eighth session of the Codex Committee on contaminants in foods.,2014.
(10) Jung, M. Y.; Kang, J. H.; Jung, H. J.; Ma, S. Y. Inorganic arsenic contents in ready-to-eat rice products and various Korean rice determined by a highly sensitive gas chromatography-tandem mass spectrometry.2018, 240, 1179–1183.
(11) Huber, C. S.; Vale, M. G. R.; Dessuy, M. B.; Svoboda, M.; Musil, S.; Dedina, J. Sample preparation for arsenic speciation analysis in baby food by generation of substituted arsines with atomic absorption spectrometry detection.2017, 175, 406–412.
(12) Shibata, T.; Meng, C.; Umoren, J.; West, H. Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the US.2016, 13.
(13) Chen, S. Y.; Yuan, B. A.; Xu, J. J.; Chen, G. T.; Hu, Q. H.; Zhao, L. Y. Simultaneous separation and determination of six arsenic species in Shiitake () mushrooms: method development and applications.2018, 262, 134–141.
(14) Choi, J. Y.; Khan, N.; Nho, E. Y.; Choi, H.; Park, K. S.; Cho, M. J.; Youn, H. J.; Kim, K. S. Speciation of arsenic in rice by high-performance liquid chromatography-inductively coupled plasma mass spectrometry.2016, 49, 1926–1937.
(15) Lai, G. X.; Chen, G. Y.; Chen, T. W. Speciation of As-III and As-V in fruit juices by dispersive liquid-liquid microextraction and hydride generation-atomic fluorescence spectrometry.2016, 190, 158–163.
(16) Chen, G. Y.; Chen, T. W. SPE speciation of inorganic arsenic in rice followed by hydride-generation atomic fluorescence spectrometric quantification.2014, 119, 202–206.
(17) Khaligh, A.; Mousavi, H. Z.; Shirkhanloo, H.; Rashidi, A. Speciation and determination of inorganic arsenic species in water and biological samples by ultrasound assisted-dispersive-micro-solid phase extraction on carboxylated nanoporous graphene coupled with flow injection-hydride generation atomic absorption spectrometry.2015, 5, 93347–93359.
(18) Ben Issa, N.; Rajakovic-Ognjanovic, V. N.; Marinkovic, A. D.; Rajakovic, L. V. Separation and determination of arsenic species in water by selective exchange and hybrid resins.2011, 706, 191–198.
(19) Abbaszadeh, A.; Tadjarodi, A. Speciation analysis of inorganic arsenic in food and water samples by electrothermal atomic absorption spectrometry after magnetic solid phase extraction by a novel MOF-199/modified magnetite nanoparticle composite.2016, 6, 113727–113736.
(20) Guivar, J. A. R.; Bustamante, A.; Gonzalez, J. C.; Sanches, E. A.; Morales, M. A.; Raez, J. M.; Lopez-Munoz, M. J.; Arencibia, A. Adsorption of arsenite and arsenate on binary and ternary magnetic nanocomposites with high iron oxide content.2018, 454, 87–100.
(21) Rashid, M.; Sterbinsky, G. E.; Pinilla, M. A. G.; Cai, Y.; O'Shea, K. E. Kinetic and mechanistic evaluation of inorganic arsenic species adsorption onto humic acid grafted magnetite nanoparticles.2018, 122, 13540–13547.
(22) Wen, S. P.; Zhu, X. S. Speciation of inorganic arsenic(III) and arsenic(V) by a facile dual-cloud point extraction coupled with inductively plasma-optical emission spectrometry.2018, 181, 265–270.
(23) Huong, P. T. L.; Tu, N.; Lan, H.; Thang, L. H.; Quy, N. V.; Tuan, P. A.; Dinh, N. X.; Phan, V. N.; Le, A. T. Functional manganese ferrite/graphene oxide nanocomposites: effects of graphene oxide on the adsorption mechanisms of organic MB dye and inorganic As(V) ions from aqueous solution.2018, 8, 12376–12389.
(24) (24)Perez-Moreno, F.; Prieto-Garcia, F.; Rojas-Hernandez, A.; Marmolejo-Santillan, Y.; Salinas-Rodriguez, E.; Patino-Cardona, F. Study of arsenic removal with ionic exchange resins in drinking water from Zimapan, Hidalgo State, Mexico.2006, 42, 391–395.
(25) Cai, J. H.; Wang, X. Y.; Zhou, Y.; Jiang, L.; Wang, C. R. Selective adsorption of arsenate and the reversible structure transformation of the mesoporous metal-organic framework MIL-100(Fe).2016, 18, 10864–10867.
(26) Zou, Z. R.; Wang, S. L.; Jia, J.; Xu, F. J.; Long, Z.; Hou, X. D. Ultrasensitive determination of inorganic arsenic by hydride generation-atomic fluorescence spectrometry using Fe3O4@ZIF-8 nanoparticles for preconcentration.2016, 124, 578–583.
(27) Kim, S.; Park, S.; Han, Y.; Choi, J.; Park, J. Adsorption of Co(II) and Mn(II) ions on mesoporous silica SBA15 functionalized with amine groups.2014, 55, 1494–1499.
(28) Shirkhanloo, H.; Ghazaghi, M.; Rashidi, A.; Vahid, A. Arsenic speciation based on amine-functionalized bimodal mesoporous silica nanoparticles by ultrasound assisted-dispersive solid-liquid multiple phase microextraction.2017, 130, 137–146.
(29) Wang, X.; Li, G.; Row, K. H. Graphene and graphene oxide mModified by deep eutectic solvents and ionic liquids supported on silica as adsorbents for solid-phase extraction.2017, 38, 251–257.
(30) Grijalba, A. C.; Escudero, L. B.; Wuilloud, R. G. Ionic liquid-assisted multiwalled carbon nanotube-dispersive micro-solid phase extraction for sensitive determination of inorganic As species in garlic samples by electrothermal atomic absorption spectrometry.2015, 110, 118–123.
(31) Yuan, C. Y.; Huang, Z. W.; Chen, J. Basic ionic liquid supported on mesoporous SBA-15: an efficient heterogeneous catalyst forepoxidation of olefins with H2O2as oxidant.2012, 24, 56–60.
(32) Zhang, J.; Zhao, G. F.; Popovic, Z.; Lu, Y.; Liu, Y. Pd-porphyrin functionalized ionic liquid-modified mesoporous SBA-15: an efficient and recyclable catalyst for solvent-free Heck reaction.2010, 45, 1648–1653.
(33) Zhao, L. Y.; Wang, S. C.; Wu, Y.; Hou, Q. F.; Wang, Y.; Jiang, S. M. Salicylidene Schiff base assembled with mesoporous silica SBA-15 as hybrid materials for molecular logic function.2007, 111, 18387–18391.
(34) Kumar, S. R.; Jayavignesh, V.; Selvakumar, R.; Swaminathan, K.; Ponpandian, N. Facile synthesis of yeast cross-linked Fe3O4nanoadsorbents for efficient removal of aquatic environment contaminated with As(V).2016, 484, 183–195.
(35) Yan, Z. M.; Wu, M.; Hu, B. Q.; Yao, M. N.; Zhang, L.; Lu, Q. M.; Pang, J. Electrospun UiO-66/polyacrylonitrile nanofibers as efficient sorbent for pipette tip solid phase extraction of phytohormones in vegetable samples.2018, 1542, 19–27.
(36) Magoda, C.; Nomngongo, P. N.; Mabuba, N. Magnetic iron-cobalt/silica nanocomposite as adsorbent in micro solid-phase extraction for preconcentration of arsenic in environmental samples.2016, 128, 242–247.
(37) Siangproh, W.; Chailapakul, O.; Songsrirote, K. Simple and fast colorimetric detection of inorganic arsenic selectively adsorbed onto ferrihydrite-coated silica gel using silver nanoplates.2016, 153, 197–202.
(38) Fazelirad, H.; Taher, M. A. A study on the synthesis and application of magnetic nanosorbent for the simultaneous preconcentration and measurement of toxic metals in different real and standard samples.2017, 97, 23–28.
(39) Lee, S. G.; Kim, D. H.; Lee, Y. S.; Cho, S. Y.; Chung, M. S.; Cho, M.; Kang, Y.; Kim, H.; Kim, D.; Lee, K. W. Monitoring of arsenic contents in domestic rice and human risk assessment for daily intake of inorganic arsenic in Korea.2018, 69, 25–32.
(40) dos Santos, G. M.; Pozebon, D.; Cerveira, C.; de Moraes, D. P. Inorganic arsenic speciation in rice products using selective hydride generation and atomic absorption spectrometry (AAS).2017, 133, 265–271.
(41) Signes-Pastor, A. J.; Carey, M.; Meharg, A. A. Inorganic arsenic in rice-based products for infants and young children.2016, 191, 128–134.
(42) Yim, S. R.; Park, G. Y.; Lee, K. W.; Chung, M. S.; Shim, S. M. Determination of total arsenic content and arsenic speciation in different types of rice.2017, 26, 293–298.
(43) Rahman, M. A.; Rahman, M. M.; Reichman, S. M.; Lim, R. P.; Naidu, R. Arsenic speciation in Australian-grown and imported rice on sale in Australia: implications for human health risk.2014, 62, 6016–6024.
28 September 2018;
6 November 2018
①Financially supported by the National Natural Science Foundation of China (No. 31701708), and the Outstanding Youth Foundation Project of Fujian Agriculture and Forestry University of China (No. xjq201710)
Chen Shao-Jun, E-mail: fjzxcsj@163.com; Chen Guo-Ying, E-mail: guoying.chen@ars.usda.gov
10.14102/j.cnki.0254-5861.2011-2201
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design
- Surface Modification of Titanium Oxide by Indium for Efficient Photocatalytic Hydrogen Generation①

