Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
ZHANG Jing YU Wei-Ling ZHOU Sheng-Hu LI Yi ZHANG Yong-Fn CHEN Wen-Ki, b, c
Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
ZHANG JingaYU Wei-LingaZHOU Sheng-HuaaLI YiaZHANG Yong-FanaCHEN Wen-Kaia, b, c②
a(350116)b(350116)c(610005)
The gold atoms on the Au20clusterhad been substituted by the palladium and platinum atoms to obtain the doped clusters with more stable geometries as a function of the bind energy and interaction energy in the previous study. Therefore, we investigated the catalytic activities of the Au19Pd and Au19Pt clusters for CO oxidation along the Langmuir-Hinshelwood mechanism. It is found that the coadsorption of CO and O2on the doped clusters is obviously stronger than on the Au20cluster, especially on the doped atom, which makes potential energy of the transition state lower than the total energy of the reactants so that it can promote CO oxidation. The reaction on these doped clusters with the heteroatom on the vertex is more difficult. However, the Au19Pd (S) is more prone to catalyzing the CO oxidation, in which the rate-limiting step has thelower energy barrier of 38.84 kJ/mol for this study. Therefore, the single atom can be modified to change the catalytic activity of the cluster for the CO oxidation. Meanwhile, the different sites on the clusters have different strengths of activity for the reaction.
bimetallic cluster, catalytic activity, CO oxidation, density functional theory;
1 INTRODUCTION
Note that the noble metals are widely used as catalysts in recent years. However, it is a very tough problem how to use them efficiently because of their limited resources[1]. It is found that bimetallic cataly- sts can largely improve the efficiency and selectivity of catalytic process instead of monometallic cataly- sts, which can be explained by the concepts of “ensemble” or “geometric” and “ligand” or “electronic” effect in electrochemistry and heterogenous cataly- sis[1, 2]. Therefore, the surface composition of bime- tallic alloys hinders the formation of inhibited species in the reaction, and the bimetallic systempossesses a special overall catalytic activity via the modification of electronic structure. Meanwhile, it is feasible to make full use of noble metals and improve their catalytic activities by means of size control[3, 4]. Recently, metallic nanocluster with so unique shape and size exhibits unusual physical and chemical properties that have many applications in the fields of magnetic, optical and electronic mate-rials, photocatalyts, catalysts, drug delivery and so on, which have been of great interest and intensely researched[5-9]. It is especially found that bimetallic nanoclusters have been particularly attractive because of the improvement of catalytic pro- perties[10-12]. And bimetallic catalysts also have been investigated to obtain the relationship between the metal structure and catalytic activity[13].
The gold clusters with metal impurity have attracted considerable attention on the basis of experimental and theoretical researches, and there are many potential applications in catalysis, mole- cular electronics, material science, and biomedical diagnosis[14-21]. The presence of heteroatom in the doped gold clusters coordinates the electronic and geometric properties of these bimetallic clusters so as to alter their chemical reactivity in a desirable manner[22, 23]. The theoretical studies illustrate the influence of heteroatoms on the chemical reactivity of these bimetallic clusters, which has been proved by experiments[15, 16, 24-31]. Moreover, it is found that alkali or transition metal atoms can beselected as heteroatoms doped in the cluster, which can signifi- cantly improve the catalytic activity of the host gold cluster[25, 26, 28-32].
Goldcan be in conjunction with transition metals like platinum[9, 33-44]or palladium as a useful alloying metal because of its relatively low reactivity in many catalytic reactions. Platinum has many applications including CO/NOoxidation, syngas reformation, and petroleum refinement as an excellent catalyst. And also palladium served as catalyst for CO oxidation and Suzuki reaction in the form of monometal and bimetal[45, 46]. It is found that gold nanoparticles possess high catalytic activity for oxidation reactions[47-51], which is demonstrated by the study about the CO oxidation on the gold nanocluster under low temperature in 1989[49]. The catalytic activities of Au–Pt nanoparticles are superior to those nanoclusters containing gold or platinum alone, which is indicated by recent theoretical studies[52]. Therefore, Pt-doped gold clusters have attracted special attention because of many potential application in catalysis[53]and high catalytic activities for a lot of reactions. There are several studies about the interaction between CO molecule and Pt-doped small gold clusters with up to 13 atoms[20, 21, 27, 28, 54]. Note that many studies indicate that the presence of platinum atom could promote the CO adsorption on the gold cluster, in which the platinum site is more prone to CO adsorption. In general, the platinum in gold clusters enhances the catalytic activity for CO oxidation. Among various bimetallic systems, Au–Pd alloy nanoparticles have also attracted particular attention as catalysts in a number of reactions like CO oxidation, acetylene cyclotrimerization, the synthe- sis of vinyl acetate monomer, and selective oxidation of alcohols to aldehydes[55-57]. Thereby, we have rationally improved the catalytic activity of Au–Pd alloy as a function of a deeper understanding of physical reasons.
In the magical world of gold clusters, there is a gold cluster containing 20 gold atoms (Au20) which is enough chemically inert and a highly stable cluster with a tetrahedral pyramidal structure on the basis ofinitio DFT-based calculation[58, 59]and experimental studies like far-infrared vibrational spectroscopy and photoelectron spectroscopy. Fur- thermore, we have consulted review paper written by Kryachko and Remacle, regarding the properties of magic gold cluster Au20. There is a large energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) for Au20cluster, which is superior to that of C60to manifest that Au20cluster should be a chemically inert and stable cluster.Moreover, the gold atom located at the surface, vertex and edge of the Au20cluster with a tetrahedral structure could be substituted with the platinum and palladium atoms, respectively, which could not obviously change the original geometry of the Au20cluster and obtain more stable geometries on the basis of the previous study.
In this study, we have firstly obtained the Au19Pt and Au19Pd clusters, in which the platinum and palladium atoms substitute with the gold atom of Au20cluster, respectively. Then we have detailedly investigated the catalytic activities of Au19Pt and Au19Pd clusters for CO oxidation along the Langmuir-Hinshelwood reaction pathway. Therefore, the calculated results involved in the reaction to the Au19Pt and Au19Pd clusters are obtained via DFT-based calculation so as to compare with that on the Au20cluster to obtain some information about the influence of different doped atoms located at different sites on the catalytic activity of cluster.
2 COMPUTATIONAL METHOD
To investigate the catalytic activities of the Au19Pt and Au19Pd clusters for CO oxidation, the program package Dmol3of Materials Studio of Accelrys[60]Inc has been performed to optimize geometries and searchtransition states. Generalized gradient appro- ximation (GGA) with exchange-correlation func- tional proposed by Perdew, Burke and Ernzerhof (PBE) is performed.The DFT semicore pseudopo- tential is employed for the core electrons of gold, platinum and palladium atoms, and the double- numerical basis with polarization functions (DNP) has been also employed in the calculation. During geometrical optimization, the energy, maximum force, and maximum displacement for convergence tolerance are 2.0´10-5Hartree, 0.004 Hartree/Å and 0.005 Å, respectively.The transition states are determined by the complete LST/QST method, which means linear synchronous transition and quadratic synchronous transition, respectively. And the RMS convergence, charge mixing and spin mixing are 0.01, 0.1 and 0.2, respectively, in the process of transition state search. Furthermore, every transition state structure has a single imaginary frequency, which is in accordance with the reaction pathway.The Fermi smearing method for a window size is set as 0.005 Hartree, and it is 4.5 Å for the orbital cutoff range, which could accelerate the convergence. Meanwhile, every atom on the cluster is relaxed in the calculation.
And also we calculate the adsorption energy of gas molecule on the cluster using the following equation:ads=system–(cluster+CO/O2), whereadsis the adsorption energy for the system,systemmeans the total energy of the substrate and gas molecule together,clusterpresents the energy of the substrate, andCO/O2is the energy of the CO or O2molecule alone.
3 RESULTS AND DISCUSSION
A gold atom on the Au20cluster with a tetrahedral pyramidal structure could be modified to palladium or platinum atom, as shown in Fig. 1, which does not obviously change the geometry after optimization and also makes the structure more stable as a function of the bind energy and interaction energy in the previous study. Although the stabilities of Au19Pd clusters increase in the order of Au19Pd (S) > Au19Pd (E) > Au19Pd (V), it is in accordance with the stabilities of Au19Pt clusters via DFT-based calculation. Therefore, we have investigated the catalytic activities of these clusters for CO oxidation as a function of the Langmuir-Hinshelwood mecha- nism in order to compare with the Au20cluster.
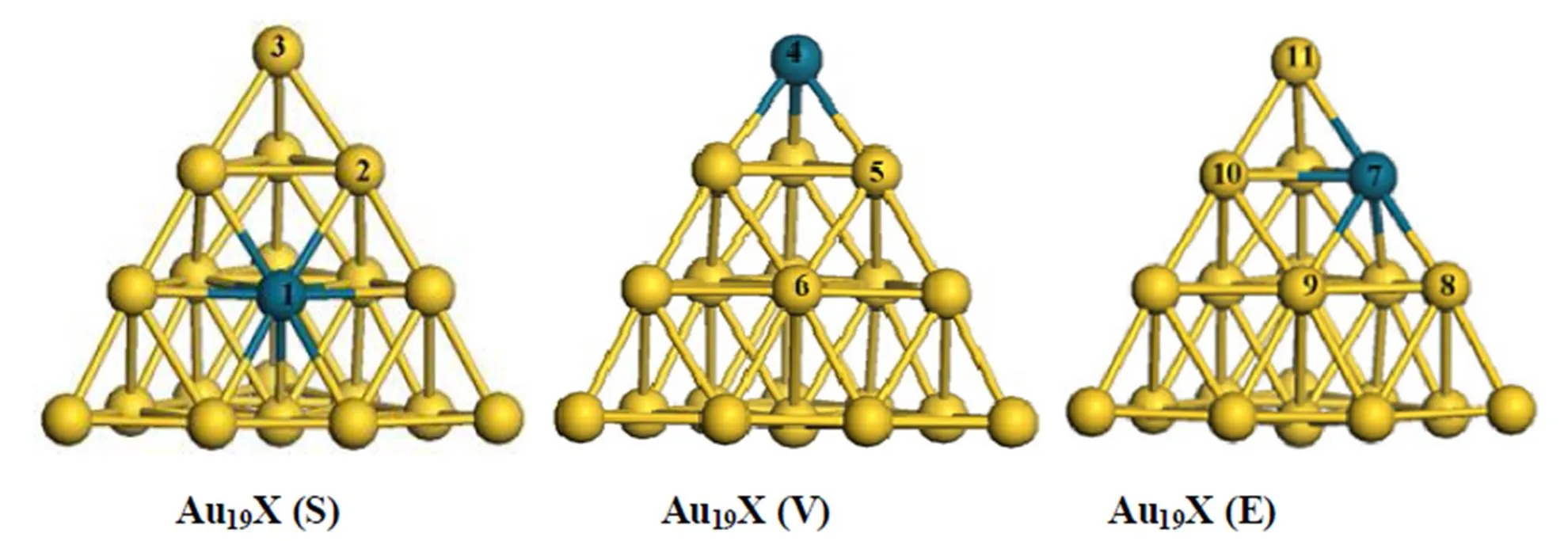
Fig. 1. Optimized geometries of Au19X (X = Pt, Pd) clusters
Note that CO adsorption on these doped clusters is an elementary step in this reaction, which has been firstly studied according to Langmuir-Hinshel- wood channel. The CO adsorption energies on the doped clusters have been indicated in Table 1. It is found that CO adsorption on the heteroatom is much stronger than that on other gold atoms of the doped cluster, which is in accordance with the previous study. And also it is noted that the CO is more prone to be on the vertex of the Au20cluster with the adsorption energy to be –83.94 kJ/mol, which is much weaker than that on the heteroatom located at the doped cluster, as shown in Fig. 1. Therefore, both of the palladium and platinum atoms on these clusters can promote CO adsorption in this study, especially the latter. Furthermore, the doped atoms at different sites of the cluster influence the CO adsorption, which suggests that CO is more prone to be on the heteroatom located at the vertex and edge of the doped cluster, respectively. Instead, CO adsorption in the reaction can’t totally determine catalytic activity of the cluster. The coadsorption for CO and O2on the cluster is also a crucial step for CO oxidation as a function of the previous study. Thereby, we have further studied the catalytic activities of these clusters via DFT-based calculation.

Table 1. Adsorption Energies of CO Molecule on the Au19Pd and Au19Pt Clusters, in Which the Adsorption Sites Are as a Function of Fig. 1, Respectively
Therefore, Figs. 2 and 3 present the energy profiles without considering the energy of the bare substrates and reaction coordinates for the first CO oxidation on the Au19Pd and Au19Pt clusters, respectively, in which the bond lengths of the molecules on the clusters are changing along the reaction channel and the calculated results are shown in Table 3 in detail. The bond distances of CO and O2are 1.14 and 1.23 Å, respectively, which are elongated to 1.16and 1.24 Å so that both CO and O2are activated after adsorption on the Au19Pd clusters. And also it is found that O2adsorption has no obvious influence on the bond distance of CO on the clusters, but can make the systems more stable. Meanwhile, the O2adsorption on the Au20cluster with CO could lower the stability of the system and coadsorption of CO and O2on the Au20cluster is much weaker than that on the Au19Pd clusters. Furthermore, the Au19Pd (V) is more prone to enhance the coadsorption of CO and O2than other two Au19Pd clusters. Meanwhile, the coadsorption of CO and O2on the Au19Pt clusters is in accordance with that on the Au19Pd clusters. However, it has been obtained that the Au19Pt clusters can facilitate the coadsorption of CO and O2more obviously, especially the Au19Pt (V) cluster, in comparison with the Au19Pd clusters. In general, these clusters shown in Fig. 1 can distinctly enhance the coadsorption of CO and O2, which make potential energy of the transition state lower than the total energy of the reactants so that it is enough to promote the first CO oxidation. Then the middle state is firstly generated by the CO and O2coadsorption on these clusters, in which the doped cluster with the heteroatom on the surface or edge catalyzes the formation of the middle state in the reaction more obviously. And also the catalytic activity of the palladium-doped cluster on the clusters is similar to that of the platinum-doped cluster for the production of the middle state. Furthermore, we have obtained that the Au20cluster possesses higher catalytic activity for the formation of the middle state with the energy barrier of 11.58 kJ/mol than those doped clusters shown in Fig. 1, in which theenergy barriers are 38.84 and 35.10 kJ/mol on Au19Pd (S) and Au19Pt (E) clusters, respectively. However, the decomposition of the middle state on the Au20cluster needs to surmount the energy barrier of 41.49 kJ/mol, which is a rate-limiting step in a complete reaction process and obviously more difficult than on the Au19Pd (S) and Au19Pt (S) with the energy barriers of 2.18 and 16.16 kJ/mol, respectively. Thereby, the palladium or platinum on the surface of the cluster is more prone to facilitating the decomposition of the middle state, especially the Au19Pd(S). That indicates that the single atom on the cluster is modified to change the catalytic activity of the substrate. Moreover, the doped atom located at different sites of the cluster has obvious influence on the activity of the substrate in the reaction process.

Fig. 2. Energy profile and reaction coordinates for the first CO molecule oxidation on the Au19Pd clusters
Fig. 3. Energy profile and reaction coordinates for the first CO molecule oxidation on the Au19Pt clusters
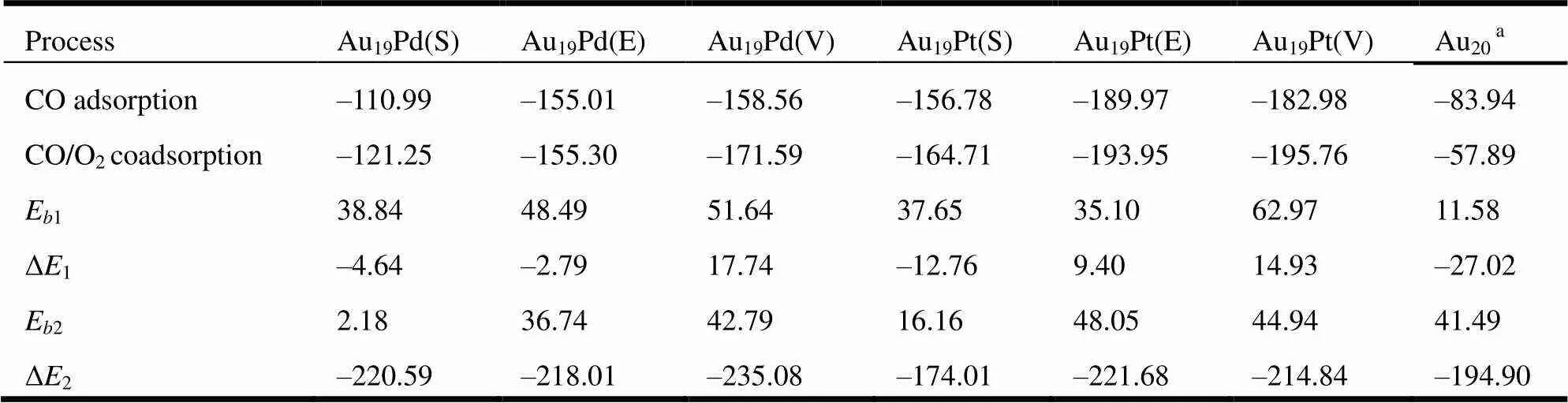
Table 2. Comparison of the Adsorption Energy, Energy Barrier (Eband Eb2) and Reaction Energy (ΔEr1and ΔEr2) in the Process of the Intermediate States Producing and Decomposing, Respectively, with Literature Values along the Corresponding Reaction Pathways, in Which All Energies Are Given in kJ/mol
adenotes these results obtained by Gao Y.[59]
The second CO reacts with the residual oxygen atom along the LH reaction mechanism, as illustra- ted in Figs. 4 and 5. It is noted that the formation of the second CO2on the Au20cluster is spontaneous along the ER channel. Meanwhile, it is found that the second CO adsorption is strong as the first CO adsorption on these clusters, which promotes the CO oxidation. Furthermore, both Au19Pd (V) and Au19Pt (V) with the residual oxygen atoms are more prone to facilitating the CO adsorption. However, it is found that the second CO oxidation on Au19Pt (V) and Au19Pd (V) needs to surmount very high energy barriers of 217.94 and 257.21 kJ/mol, respectively, which are obviously higher than that on other clusters so as to hinder the reaction. Therefore, the doped cluster with heteroatom located at the surface and edge is better to promote the second CO oxida- tion, especially the Au19Pd (S) with a low energy barrier of 34.92 kJ/mol and high exothermicity of 218.64 kJ/mol.
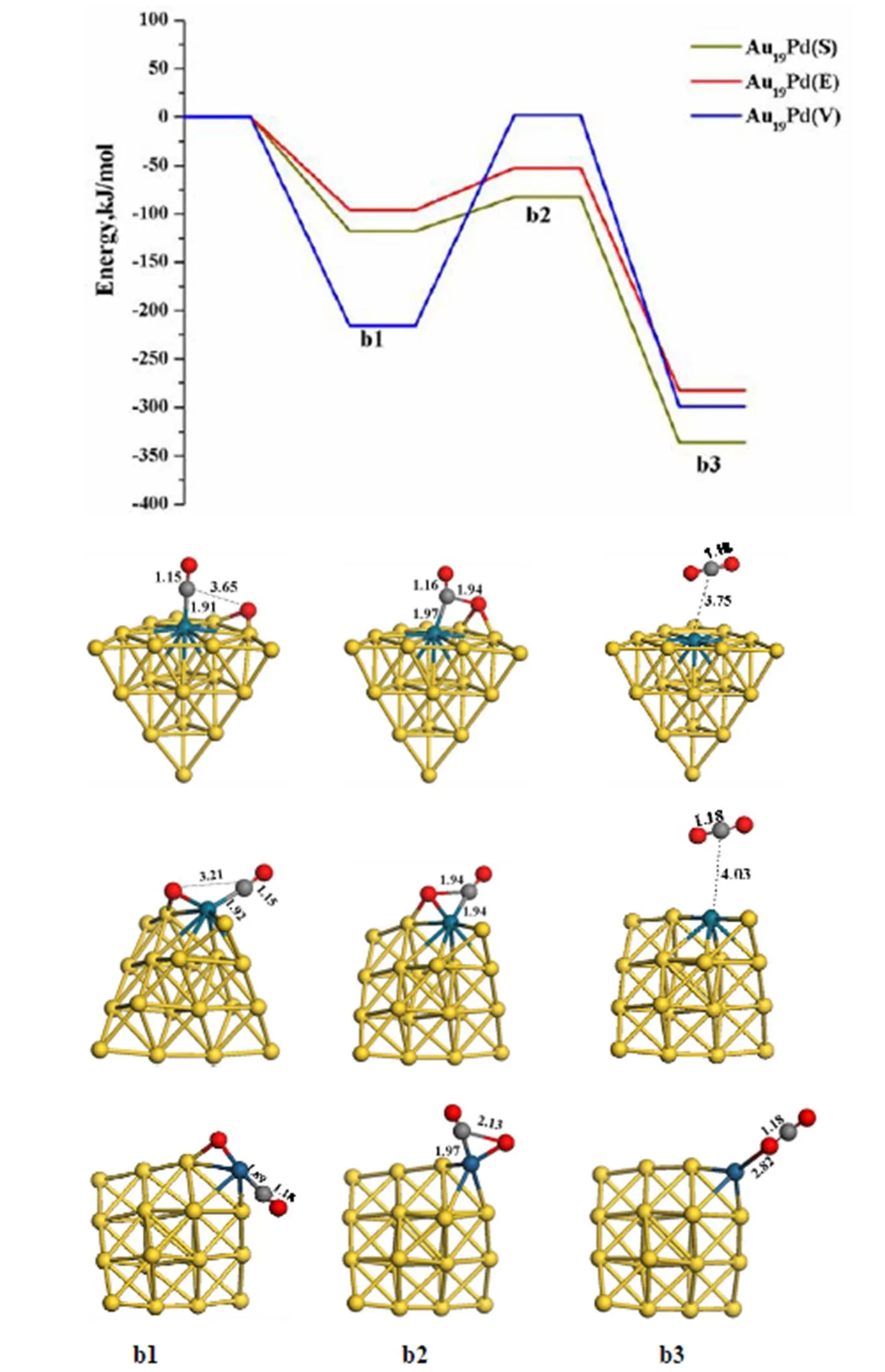
Fig. 4. Energy profile and reaction coordinates for the second CO molecule oxidation on the Au19Pd clusters
Fig. 5. Energy profile and reaction coordinates for the second CO molecule oxidation on the Au19Pt clusters

Table 3. Calculated Adsorption Energy, Energy Barrier (Eb) and Reaction Energy (ΔEr) along the LH Reaction Pathway for the Second CO Molecule Oxidation on the Cluster, in Which All Energies Are Given in kJ/mol
4 CONCLUSION
In summary, the gold atoms on the Au20cluster had been modified to the palladium or platinum atom to obtain a stable geometry via interaction energy in the previous study. Therefore, we have investigated the catalytic activities of Au19Pd and Au19Pt clusters for CO oxidation along the LH reaction channel. Firstly, the CO and O2coadsorp- tion on these doped clusters is stronger than that on the Au20cluster. And also the rate-limiting step on the Au20cluster is the intermediate state decom- position with an energy barrier of 41.5 kJ/mol. Instead, the formation of the middle state on the Au19Pd (S) cluster is the rate-limiting step with the lower energy barrier of 38.84 kJ/mol in this study, which is accelerated by the strong coadsorption of gas molecules and high exothermicity. Therefore, the Au19Pd (S) possesses higher catalytic activities than the Au20cluster in a complete CO oxidation. Note that the Au19Pt clusters can be more prone to enhance the adsorption of CO and O2. However, the reaction on these clusters is more difficult than on the Au19Pd clusters. Thus, the single atom on the cluster is modified to change the catalytic activity of the substrate in the reaction. Furthermore, the doped clusters with the heteroatom located at the vertexpromote the coadsorption of CO and O2but hinder the reaction with larger energy barrier. It has been shown that the doped atom on different sites of the clusterhave obvious influence on the activity of the substrate. Finally, the Au19Pd with the palladium atom on the surface of the cluster is a superior catalyst for CO oxidation. Moreover, it hopes that our theoretical study provides a clue for further investigation on the catalytic activities of Au19Pd and Au19Pt clusters on the suitable support for CO oxidation.
(1) Chen, M.;Kumar, D.;Yi, C. W.;Goodman, D.W.The promotional effect of gold in catalysis by palladiumgold2005,310,291-293.
(2) Bligaard, T.;Nørskov, J.K.Ligand effects in heterogeneous catalysis and electrochemistry2007,52,5512-5516.
(3) Haruta, M.;Yamada, N.;Kobayashi, T.;Iijima, S.Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide1989,115,301-309.
(4) Valden, M.;Lai, X.;Goodman, D.W.Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties1998,281,1647-1650.
(5) Burda, C.;Chen, X.;Narayanan, R.;El-Sayed, M.A.Chemistry and properties of nanocrystals of different shapes2005,105,1025-1102.
(6) Jin, R.;Cao, Y.;Mirkin, C.A.;Kelly, K.;Schatz, G.C.;Zheng, J.Photoinduced conversion of silver nanospheres to nanoprisms2001,294,1901-1903.
(7) Sulman, E.;Matveeva, V.;Doluda, V.;Nicoshvili, L.;Bronstein, L.;Valetsky, P.;Tsvetkova, I.Nanostructured catalysts for the synthesis of vitamin intermediate products2006,39,187-190.
(8) Schmid, G.Large clusters and colloids:metals in the embryonic state1992,92,1709-1727.
(9) Lee, A.F.;Baddeley, C.J.;Hardacre, C.;Ormerod, R.M.;Lambert, R.M.;Schmid, G.;West, H.Structural and catalytic properties of novel Au/Pd bimetallic colloid particles: EXAFS, XRD, and acetylene coupling1995,99,6096-6102.
(10) Toshima, N.;Yonezawa, T.;Kushihashi, K.Polymer-protected palladium-platinum bimetallic clusters: preparation, catalytic properties and structural considerations1993,89,2537-2543.
(11) Harada, M.;Asakura, K.;Toshima, N.Catalytic activity and structural analysis of polymer-protected gold/palladium bimetallic clusters prepared by the successive reduction of hydrogen tetrachloroaurate(III) and palladium dichloride1993,97,5103-5114.
(12) Wang, Y.;Toshima, N.Preparation of Pd-Pt bimetallic colloids with controllable core/shell structures1997,101,5301-5306.
(13) Sinfelt, J.H.Structure of bimetallic clusters1987,20,134-139.
(14) Li, X.;Kiran, B.;Cui, L. F.;Wang, L. S.Magnetic properties in transition-metal-doped gold clusters: M@ Au6(M = Ti, V, Cr)2005,95,253401,1-4.
(15) De Haeck, J.;Veldeman, N.;Claes, P.;Janssens, E.;Andersson, M.;Lievens, P.Carbon monoxide adsorption on silver doped gold clusters2011,115,2103-2109.
(16) Lin, L.;Lievens, P.;Nguyen, M.T.Theoretical study of CO adsorption on yttrium-doped gold clusters AuY (=1~9)2010,498,296-301.
(17) Chen, H.T.;Chang, J.G.;Ju, S.P.;Chen, H.L.First-principle calculations on CO oxidation catalyzed by a gold nanoparticle2010,31,258-265.
(18) Koyasu, K.;Mitsui, M.;Nakajima, A.;Kaya, K.Photoelectron spectroscopy of palladium-doped gold cluster anions; AuPd(= 1~4)2002,358,224-230.
(19) Zhou, S. H.; Yu, W. L.; Zhang, J.; Li, Y.; Zhang, Y. F.; Chen, W. K. A density functional study for the reaction mechanism of CO oxidation on the copper cluster.2018, 37, 1379-1392.
(20) Tian, W.Q.;Ge, M.;Gu, F.;Yamada, T.;Aoki, Y.Binary clusters aupt and Au6Pt: structure and reactivity within density functional theory2006,110,6285-6293.
(21) Yuan, D.;Wang, Y.;Zeng, Z.Geometric, electronic, and bonding properties of AuM (= 1~7, M = Ni, Pd, Pt) clusters2005,122,114310,1-11.
(22) Mondal, K.;Ghanty, T.K.;Banerjee, A.;Chakrabarti, A.;Kamal, C.Density functional investigation on the structures and properties of Li atom doped Au20cluster2013,111,725-734.
(23) Qian, H.;Jiang, D. E.;Li, G.;Gayathri, C.;Das, A.;Gil, R.R.;Jin, R.Monoplatinum doping of gold nanoclusters and catalytic application2012,134,16159-16162.
(24) Molina, L.;Hammer, B.The activity of the tetrahedral Au20cluster: charging and impurity effects2005,233,399-404.
(25) Nhat, P.V.;Tai, T.B.;Nguyen, M.T.Theoretical study of AunV-CO,= 1~14: the dopant vanadium enhances CO adsorption on gold clusters2012,137,164312,1-12.
(26) Ge, Q.;Song, C.;Wang, L.A density functional theory study of CO adsorption on Pt–Au nanoparticles2006,35,247-253.
(27) Morrow, B.H.;Resasco, D.E.;Striolo, A.;Nardelli, M.B.CO adsorption on noble metal clusters: local environment effects2011,115,5637-5647.
(28) Beletskaya, A.V.;Pichugina, D.A.;Shestakov, A.F.;Kuz’menko, N.E.Formation of H2O2on Au20and Au19Pd clusters: understanding the structure effect on the atomic level2013,117,6817-6826.
(29) Le, H.T.;Lang, S.M.;De Haeck, J.;Lievens, P.;Janssens, E.Carbon monoxide adsorption on neutral and cationic vanadium doped gold clusters2012,14,9350-9358.
(30) Torres, M.B.;Fernández, E.M.;Balbas, L.C.Theoretical study of oxygen adsorption on pure Aun+and doped MAun+cationic gold clusters for M = Ti, Fe and= 3~72008,112,6678-6689.
(31) Jena, N.K.;Chandrakumar, K.;Ghosh, S.K.Theoretical investigation on the structure and electronic properties of hydrogen-and alkali-metal-doped gold clusters and their interaction with CO: enhanced reactivity of hydrogen-doped gold clusters2009,113,17885-17892.
(32) Toshima, N.;Harada, M.;Yamazaki, Y.;Asakura, K.Catalytic activity and structural analysis of polymer-protected gold-palladium bimetallic clusters prepared by the simultaneous reduction of hydrogen tetrachloroaurate and palladium dichloride1992,96,9927-9933.
(33) Turkevich, J.;Kim, G.Palladium: preparation and catalytic properties of particles of uniform size1970,169,873-879.
(34) Mizukoshi, Y.;Okitsu, K.;Maeda, Y.;Yamamoto, T.A.;Oshima, R.;Nagata, Y.Sonochemical preparation of bimetallic nanoparticles of gold/palladium in aqueous solution1997,101,7033-7037.
(35) Mizukoshi, Y.;Fujimoto, T.;Nagata, Y.;Oshima, R.;Maeda, Y.Characterization and catalytic activity of core-shell structured gold/palladium bimetallic nanoparticles synthesized by the sonochemical method2000,104,6028-6032.
(36) Schmid, G.;Lehnert, A.;Malm, J.O.;Bovin, J.O.Ligand-stabilized bimetallic colloids identified by hrtem and edx1991,30,874-876.
(37) Fajín, J.L.;Cordeiro, M.N.D.;Gomes, J.R.DFT study of the CO oxidation on the Au (321) surface2008,112,17291-17302.
(38) Davis, R.J.;Boudart, M.Structure of supported pdau clusters determined by X-ray absorption spectroscopy1994,98,5471-5477.
(39) Boennemann, H.;Endruschat, U.;Tesche, B.;Rufinska, A.;Lehmann, C.W.;Wagner, F.E.;Filoti, G.;Parvulescu, V.;Parvulescu, V.I.An SiO2embedded nanoscopic Pd/Au alloy colloid2000,2000,819-822.
(40) Yonezawa, T.;Toshima, N.Mechanistic consideration of formation of polymer-protected nanoscopic bimetallic clusters1995,91,4111-4119.
(41) Toshima, N.;Yonezawa, T.Bimetallic nanoparticles—novel materials for chemical and physical applications1998,22,1179-1201.
(42) Guczi, L.;Beck, A.;Horvath, A.;Koppány, Z.;Stefler, G.;Frey, K.;Sajo, I.;Geszti, O.;Bazin, D.;Lynch, J.AuPd bimetallic nanoparticles onTiO2: XRD, TEM, in situ EXAFS studies and catalytic activity in CO oxidation2003,204,545-552.
(43) Miyaura, N.;Suzuki, A.Palladium-catalyzed cross-coupling reactions of organoboron compounds1995,95,2457-2483.
(44) Henglein, A.Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles1989,89,1861-1873.
(45) Leutwyler, W.K.;Bürgi, S.L.;Burgl, H.Semiconductor clusters, nanocrystals, and quantum dots1996,271,933-937.
(46) Haruta, M.;Daté, M.Advances in the catalysis of au nanoparticles2001,222,427-437.
(47) Daniel, M. C.;Astruc, D.Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology2004,104,293-346.
(48) Astruc, D.;Lu, F.;Aranzaes, J.R.Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis2005,44,7852-7872.
(49) Bond, G.C.The electronic structure of platinum-gold alloy particles2007,51,63-68.
(50) Sadek, M.M.;Wang, L.Effect of adsorption site, size, and composition of Pt/Au bimetallic clusters on the CO frequency: a density functional theory study2006,110,14036-14042.
(51) Baddeley, C.J.;Tikhov, M.;Hardacre, C.;Lomas, J.R.;Lambert, R.M.Ensemble effects in the coupling of acetylene to benzene on a bimetallic surface: a study with Pd {111}/Au1996,100,2189-2194.
(52) Enache, D.I.;Edwards, J.K.;Landon, P.;Solsona-Espriu, B.;Carley, A.F.;Herzing, A.A.;Watanabe, M.;Kiely, C.J.;Knight, D.W.;Hutchings, G.J.Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2catalysts2006,311,362-365.
(53) Zeng, Q.S.;Sun, B.Z.;Zhao, W.N.;Lin, H.X.;Li, Y.;Chen, W.K.Adsorption of Co2B2and Ni2B2clusters on the TiO2(110) surface: a density functional study2013,32,341-348.
(54) Li, J.;Li, X.;Zhai, H. J.;Wang, L. S.Au20: a tetrahedral cluster2003,299,864-867.
(55) Wang, J.;Wang, G.;Zhao, J.Structures and electronic properties of Cu20, Ag20, andAu20clusters with density functional method2003,380,716-720.
(56) Gruene, P.;Rayner, D.M.;Redlich, B.;van der Meer, A.F.;Lyon, J.T.;Meijer, G.;Fielicke, A.Structures of neutral Au7, Au19, and Au20clusters in the gas phase2008,321,674-676.
(57) Kryachko, E.S.;Remacle, F.The magic gold cluster Au202007,107,2922-2934.
(58) Mondal, K.;Banerjee, A.;Ghanty, T.K.Structural and chemical properties of subnanometer-sized bimetallic Au19Pt cluster2014,118,11935-11945.
(59) Gao, Y.;Shao, N.;Pei, Y.;Chen, Z.;Zeng, X.C.Catalytic activities of subnanometer gold clusters (Au16–Au18, Au20, and Au27–Au35) for CO oxidation2011,5,7818-7829.
(60) Delley, B. From molecules to solids with the DMol3 approach.2000, 18, 7756-7764.
4 April 2018;
19 September 2018
the National Natural Science Foundation of China (Nos. 51574090, 21773030) and Natural Science Foundation of Fujian Province (2017J01409)
. Professor, majoring in computation chemistry. E-mail: wkchen@fzu.edu.cn
10.14102/j.cnki.0254-5861.2011-1943
- 结构化学的其它文章
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design
- Surface Modification of Titanium Oxide by Indium for Efficient Photocatalytic Hydrogen Generation①

