Surface Modification of Titanium Oxide by Indium for Efficient Photocatalytic Hydrogen Generation①
XUE Chui-Bing LI Guo-Jing GONG Ci GUO Wng HUANG Ji-Qun
Surface Modification of Titanium Oxide by Indium for Efficient Photocatalytic Hydrogen Generation①
XUE Chui-Binga, bLI Guo-Jinga, cGONG Caia, bGUO WangaHUANG Ji-Quana②
a(350002)b(350117)c(100039)
We fabricated indium ion-modified TiO2nanoparticles. The results revealed that indium presents on TiO2surface in the form of fixed ion, by coordinating with hydroxyl groups or terminal oxygen atoms at the surface of TiO2, which resulted in smaller grain size, larger surface area, and mesoporous structure relative to pure titanium dioxide. Compared with pure TiO2, indium ion-modified TiO2showed great enhancement of photocatalytic activity to hydrogen generation. Owing to electronic capture capability of indium, the excited electrons can rapidly transfer from TiO2conduction band to indium, resulting in the separation of electron-hole pairs. The optimal H2evolution rate was 277.8mmol·g-1·h-1, which was about 23 times higher than that of Degussa P25.
indium modification, hydrogen generation, photocatalytic activity;
1 INTRODUCTION
Since the discovery of water photolysis on TiO2photoanode in 1970s[1], TiO2has promoted extensive investigations for photocatalytic hydrogen generation from water decomposition. However, presently, the reactivity of bare TiO2for water splitting is still low, mainly due to the high recombination rate of photoexcited electron-hole pairs, the fast backward reaction, and the large bandgap.
To this end, metal or non-metal doping has been developed, for example, Nb, Fe, Co, Rh, Cr, C and N doped TiO2[2-8]. Most doped TiO2photocatalystes are responsive to visible light due to the formation of impurity level or the narrowing of the band gap. However, doping impurity levels and oxygen vacancy could serve as recombination centers, leading to the decrease of photocatalytic activity[9].
Recently, another strategy, i.e., metal ion modifi- cation, has drawn much attention[10-17]. In this method, metal modifier is not doped in lattices but just fixed on the surface of TiO2in the forms of metal ion or molecular metal oxide (oxyhydroxide, chloride,) species, and charge transfers occur between the metal modifiers and TiO2[18, 19]. The metal modifier may inject electrons into the conduction band of TiO2. Some accept electrons from the valence band and others work as electron acceptors from the conduction band of TiO2like a cocatalyst[13]. Notably, the separation of electron-hole pairs is greatly promoted, thereby improving the photocatalytic activity. The Cu(II)-grafted TiO2exhibited a high quantum efficiency for 2-propanol decomposition under visible light[11]. Fe(III)/TiO2with amorphous FeO(OH)-like structure was assigned to the interfacial charge transfer from the valence band of TiO2to the surface Fe(III) species[12]. The platinum(III) species as a promoter for O2reductions and an electron injector to the conduction band of TiO2for response to visible light[14, 20]. In addition, analogous method could be applied to other semiconductors[21-23]. In general, surface modifica- tion is an effective strategy to design highly active photocatalyst systems, which can be applied to a variety of semiconductor materials.
Herein, we report the fabrication of indium ions modified TiO2(In-TiO2) as an efficient photocatalyst by the sol-gel method. It is found that the intro- duction of indium modifier efficiently enhanced photocatalytic performance of TiO2. The maximum hydrogen production rate reached 277.8 µmol·g-1·h-1, which is about 23 times higher than that of P25. This improvement in photocatalytic performance can be contributed to the fixed indium ions on TiO2surface, rather than the absorption of visible light.
2 EXPERIMENTAL
2. 1 Preparation of the photocatalysts
In-TiO2nanoparticles were prepared by the sol-gel process. At room temperature, a certain amount of InCl3solution (0.1 mol/L) was mixed with 15 mL of absolute ethanol. Subsequently, tetrabutyl titanate (0.01 mol) was added to the above solution under vigorous stirring. Then, 0.5 mL distilled water was added into the transparent solution, while hydro- chloric acid was used to adjust the pH. The obtained sol was stirred until a transparent gel was formed. After aging for 1 h, the gels were dried at 75 ℃ for 24 h. Subsequently, the dry gels were ground and then calcined at 450 ℃ for 2 h. The samples were named as% In-TiO2, where“%” represents the molar percentage of In3+ions in all metal ions (In3+and Ti4+) in TiO2. The synthesis procedure for pure TiO2is similar to that of In-TiO2, except for the absence of InCl3solution.
2. 2 Characterization of the photocatalysts
The crystal structures of the samples were deter- mined by X-ray diffractometer (Miniflex 600, Rigaku, Japan), and the morphology was characte- rized by Transmission Electron Microscopy (Tecnai G2 F20, FEI, USA). The surface areas and pore structures of the samples were analyzed by Pore Analyzer (ASAP2020, Mike, USA). The reflectance spectra of the samples were measured by a UV-Vis spectrophotometer (Lambda 950, PerkinElmer, USA) with an integrating sphere in the range of 250~800 nm and standard BaSO4powder was used as a reference. The surface analyses were determined by X-ray photoelectron spectroscopy (XPS) (ESCALAB 250, Thermo Scientific, USA).
2. 3 Photocatalytic activity testing
The hydrogen generation was tested using a photocatalytic testing system (CEL-SPH2N, AULTT, China). 100 mg photocatalysts were placed at the bottom of a 500 mL Pyrex reactor with a quartz window containing 100 mL methanol-water solution (10 vol.% methanol). The ambient temperature was kept at 28℃while the reaction liquid was maintained at 6 ℃ by circulating cooling water to prevent solution evaporation. The light source was a 300 W Xe lamp. The photocatalytic reaction time was 10 h. Hydrogen generation was determined using a gas chromatograph (SP7800, N2carrier, AULTT, China).
The photocurrent and electrochemical impedance spectroscopies of the electrodes were measured in a 0.1 M Na2SO4electrolyte solution in a regular three-electrode electrochemical cell on potentiostat (CHI760E). A Xe lamp (PLS-SXE300C) was used as the source of sunlight. Pt wire and an Ag/AgCl electrode were used as the counter and reference electrodes, respectively.
3 RESULTS AND DISCUSSION
3. 1 Photocatalyst characterization
The X-ray diffraction (XRD) patterns of In–TiO2and pure TiO2are shown in Fig. 1a. It is evident that all the samples displayed typical XRD pattern of TiO2with anatase structure. No characteristic diffraction peaks of indium species (metal or oxide phase) and any other secondary or impurity phases were observed, indicating the absence of crystalline indium oxides. Besides, with the increase of indium, there was no shift in the diffraction peaks, and the calculated lattice parameters keep nearly constant (see Fig. 1b~1c and Table 1). Given that the effec- tive ionic radius (with coordination number of 6) is 0.080 nm for In3+and 0.0605 nm for Ti4+, substi- tution of Ti4+by In3+will lead to the lattice expan- sion, reflecting as the shift of the peak positions to lower diffraction angles. Therefore, it can deduce that there was no doped In3+in TiO2lattices, or the doping concentration was very low. The difficult substitution of In3+for Ti4+can be mainly attributed to the large mismatching in the radius (> 30%) between In3+and Ti4+. Therefore, it is presumed that the In3+ions may exist in the form of amorphous nanoclusters[24, 25], or chemisorbed on the surface of TiO2as a complex-like form coordinated by the surface hydroxyl group[26]. Additionally, both crystallinity and particle size were decreased by the introduction of indium (see Fig. 1). The crystallite size of the samples was calculated by employing the Scherrer equation and shown in Table 1. The crystal size was about 18 nm for the pristine TiO2, and smaller than 10 nm for the indium ion-modified TiO2samples.
Fig. 1. (a) X-ray diffraction patterns of pure TiO2and indium ion-modified TiO2. (b) and (c) are the enlarged patterns of (101) and (200) peaks of anatase TiO2, respectively
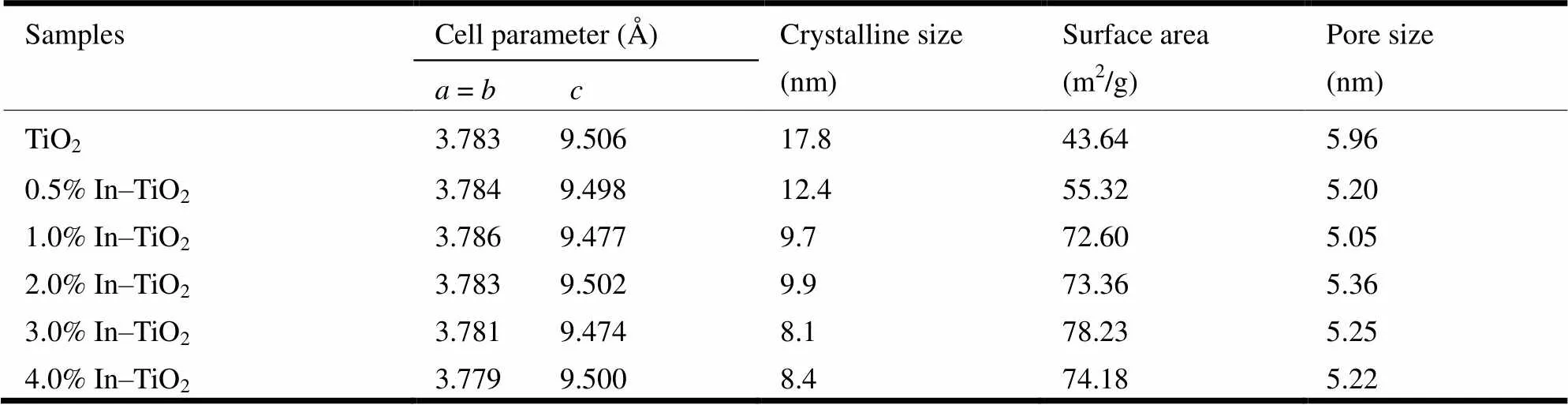
Table 1. Cell Parameters, Crystallite Size and Surface Area of Pure TiO2 and Indium Ion-modified TiO2
The TEM and HRTEM images of pristine TiO2and 2 % In–TiO2are shown in Fig. 2. The particles were roughly spherical in shape, and the average crystal sizes are about 18 and 9 nm for the pure TiO2and In–TiO2powders, respectively, which was consistent with the XRD results. The HRTEM images (Fig. 3b and 3d) reveal an interplanar spacing of 0.35 nm, corresponding to the (101) plane of anatase TiO2. Besides, EDX elemental mapping ( Fig. 2e~2f) of 2% In–TiO2sample clearly revealed the homogeneous distribution of Ti, O, and In elements in the entire nanoparticle, highlighting the formation of In3+without the second phases or local aggrega- tion, excluding the formation of amorphous In2O3nanoparticles. Therefore, the indium species may be existed in the form of fixed ion, by coordinating with hydroxyl groups or terminal oxygen atoms at the surface of TiO2[27-29].
Fig. 2. Typical TEM (a, c) and HRTEM (b, d) images of TiO2(a, b) and In-TiO2(c, d). (e~h) STEM-EDS mapping of Ti, O, and In element for 2% In–TiO2sample
Optical response of the samples was measured by diffuse reflection spectrometer at room temperature. The band gap energy values calculated usingequation were 3.17, 3.17, 3.18, 3.15, 3.19, and 3.17 eV for pure TiO2, 0.5, 1, 2, 3, and 4% In-TiO2, respectively, indicating that the band gap of In–TiO2was almost unchanged after the introduction of indium. Besides, additional visible absorption was not observed after the introduction of indium (see Fig. 3). In principle, indium ions doping can form impurity energy levels and reduce the value of. Similarly, the recombination of indium oxide and titanium dioxide can also result in visible light absorption due to the lower band gap of indium oxide (= 2.8 eV)[31]. Therefore, the UV-Vis reflectance spectrum indicated that indium neither exists in the lattice nor in the form of In2O3particles, which further demon- strated the presence of indium in the state of ions by hydroxyl or Ti–O terminal on the surface of TiO2[27-29].
Fig. 4a shows the N2adsorption-desorption isotherms of TiO2and In–TiO2samples. The four isotherms were similar to typecurve withhysteresis loops, suggesting the highly mesoporous structure[32].Fig. 4b shows the Barrett-Joyner- Halenda () pore size distribution. The average pore size was obtained from the maximum of pore size distribution curves for the synthesized samples. The distribution curve revealed the mesopores of 4.5~6.5 nm. Furthermore, the BET surface area was 43.64 m2/g for TiO2, and 55.32, 72.60, 73.36, 78.23, and 74.18 m2/g for 0.5, 1, 2, 3, 4% In-TiO2samples, respectively (see Table 1). Obviously, this is related to the decrease of particle size. In other words, indium was attached to the surface of titanium dioxide, which hinders the growth of grain and thus increases the specific surface area of the samples.
Fig. 3. (a) UV-Vis reflectance spectrum and (b) relationship between (F(R)hν)0.5and band energy () of the In–TiO2samples. F(R) = (1 –)2/2whereis the reflectance
Fig. 4. (a) N2adsorption-desorption isotherms and (b) pore size distribution curves of TiO2and In–TiO2
In order to determine the chemical environment of Ti, In and O, XPS analysis was carried out. The selected spectra for Ti 2, O 1, and In 3are shown in Fig. 5. For Ti 2spectra, the peaks of Ti located at 458.6 and 464.5 eV were assigned to Ti 23/2and Ti 21/2, respectively (Fig. 5a), and the splitting between Ti 21/2and Ti 23/2was 5.7 eV, indicating a normal state of Ti4+in In–TiO2samples. As shown in Fig. 5b, O 1peak was fitted with two components at 529.7 and 531.3 eV, which could be assigned to the lattice oxygen bound to anatase Ti4+and surface hydroxyl groups, respectively[33]. For the In 3spectra (Fig. 5c), the peaks with binding energy located at 443.8 and 451.3 eV were assigned to In 35/2and In 33/2respectively, which indicated the indium exists in the form of ionic state[34]. It can be seen that with the increase of indium, the peaks of O 1gradually move slightly to the high binding energy (an offset of about 0.13 eV between TiO2and 4% In-TiO2is shown in Fig. 5b). Since the Fermi level was calibrated as 0 eV and set as the reference point for all core levels in XPS, the chemical shift of the core levels (typically, oxide O 1) could be considered to be caused by the variation in the Fermi level[35]. Thus, it can be seen that the absolution energy position of theEof TiO2became more negative () by introducing indium species.
3. 2 Photocatalytic activity
The photocatalytic activity of the samples was investigated in the aqueous CH3OH solution under the Xe lamp irradiation for 10 h. As shown in Fig. 6a, for all the samples, the amount of generated H2increased almost linearly with the irradiation time, implying the stability of photocatalysts. Under the applied experimental conditions, pure TiO2did not exhibit observable photocatalytic activity. However, indium modified titanium dioxide presented remarkable improvement on hydrogen production. Fig. 6b depicts the dependence of H2evolution rate on the indium contents. With the incorporation of a small amount of indium, the activity of 0.5% In-TiO2sample was markedly enhanced. The H2evolution rate of the samples further increased with increasing the indium content from 0.5% to 2.0%. 2% In-TiO2sample exhibited the highest H2evolution rate, viz. 277.8 µmol·g-1·h-1, which was about 23 times higher than that of P25 (12 µmol·g-1·h-1, experimental data were not shown in Fig. 6). After that, further increasing the indium contents led to a decline of the photocatalytic activity. Most likely, excess indium attached to TiO2surface may cover its surface, resulting in the decrease of light absorption of titanium dioxide[36].
Fig. 5. XPS of In-TiO2samples. (a) Ti 2, (b) O 1and (c) In 3
Fig. 6. (a) Time course of photocatalytic H2evolution. (b) Dependence of H2evolution rate on the Indium contents
3. 3 Photoelectrochemical characterization
In order to further ascertain the reasons for the enhanced photocatalytic ability, electrochemical measurements were carried out. Fig. 7a shows the current-time() curves of the TiO2and In–TiO2electrodes. The transient photocurrent responses of the samples were recorded under intermittent light on and off. Strikingly, considerable rise in the current intensity of In–TiO2electrodes was observed relative to bare TiO2electrodes, suggesting that the boosted separation efficiency of photogenerated electron-hole pairs was afforded for In–TiO2[37]. Electrochemical impedance spectroscopy () was measured to evaluate the electrochemical characteristics of bare TiO2and In–TiO2electrodes (Fig. 7b). The charge transfer resistance of samples can be deduced from the Nyquist plots of TiO2and In–TiO2[38]. Obviously, In–TiO2sample shows a lower resistance than that of bare TiO2, indicating that the modification of indium can greatly promote the efficiency of charge transfer.
Fig. 7. (a) Time courses of photoresponse using bare TiO2and In–TiO2electrodes. (b) Electrochemical impednce spectra of bare TiO2and In–TiO2electrodes
3. 4 Photocatalysis mechanism
According to the above results, the improved hydrogen production capacity of TiO2by indium modification can be explained as follows. Pure TiO2does not exhibit detectable photocatalytic activity due to its wide band gap energy and high photo- generated carrier recombination rate. However, for indium ion-modified TiO2, when TiO2nanoparticles are irradiated by photons equal to or larger than the band gap energy, the electrons in the valence band will transfer to the conduction band to form photo- generated electrons (e-), while photogenerated holes (h+) produced in the valence band accordingly. Subsequently, the excited electrons can be transferred rapidly from the conduction band to indium due to the ability of indium to trap electrons, resulting in the separation of electron-hole pairs[39]. The photoge- nerated holes (h+) react with absorbed H2O to generate hydrogen ions (H+) and hydroxyal radical (•OH), while electrons convert H+into H2(see the schematic Fig. 8). As a result, photogenerated elec- trons and holes can be separated efficiently via the presence of In3+over the surface, and then contribute to the photocatalytic reactions. Moreover, In modification can lead to smaller grains and larger surface area, thereby reducing the migration distance of the electrons in the bulk TiO2(i.e., reducing the recombination) and increasing the surface active sites[40]. Therefore, indium ion-modified TiO2shows much higher photocatalytic activities than pure TiO2under UV-Vis light irradiation.
Fig. 8. Schematic diagram of photocatalytic mechanism on indium ion-modified TiO2under simulated sunlight
4 CONCLUSION
In summary, we have successfully fabricated indium ion-modified TiO2nanoparticles with remarkable photocatalytic performance for hydrogen generation from water splitting. It is found that indium was distributed over TiO2in the form of fixed ion, by coordinating with hydroxyl groups or terminal oxygen atoms at the surface of TiO2. Due to surface modification of indium, the synthesized samples have smaller grain size, larger surface area, and mesoporous structure. The sample with 2% In exhibits high efficiency for hydrogen generation, corresponding to the value of 277.8 µmol·g-1·h-1. The presence of indium over TiO2surface is conductive to the separation and transport of photogenerated carriers and thereby responsible for the high photocatalttic efficiency.
(1) Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode.1972, 238, 37-38.
(2) Hoffmann, M. R.; Martin, S. T.; Choi, W.; Bahnemann, D. W. Environmental applications of semiconductor photocatalysis.1995, 95, 69-96.
(3) Chen, X.; Shen, S.; Guo, L.; Mao, S. S. Semiconductor-based photocatalytic hydrogen generation.2010, 110, 6503-6570.
(4) Wang, L.; Zhang, S.; Zhu, Y.; Patlolla, A.; Shan, J.; Yoshida, H.; Takeda, S.; Frenkel, A. I.; Tao, F. Catalysis andstudies of Rh/Co3O4nanorods in reduction of NO with H2.2013, 3, 1011-1019.
(5) Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide.2003, 42, 4908-4911.
(6) Park, J. H.; Kim, S.; Bard, A. J. Novel carbon-doped TiO2nanotube arrays with high aspect ratios for efficient solar water splitting.2006, 6, 24-28.
(7) Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides.2001, 293, 269-271.
(8) Chen, X.; Burda, C. The electronic origin of the visible-light absorption properties of C-, N-and S-doped TiO2nanomaterials.2008, 130, 5018-5019.
(9) Ni, M.; Leung, M. K. H.; Leung, D. Y. C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2for hydrogen production.. 2007, 11, 401-425.
(10) Irie, H.; Shibanuma, T.; Kamiya, K.; Miura, S.; Yokoyama, T.; Hashimoto, K. Characterization of Cr(III)-grafted TiO2for photocatalytic reaction under visible ligh.2010, 96, 142-147.
(11) Irie, H.; Miura, S.; Kamiya, K.; Hashimoto, K. Efficient visible light-sensitive photocatalysts: grafting Cu(II) ions onto TiO2and WO3photocatalysts.2008, 457, 202-205.
(12) Yu, H.; Irie, H.; Shimodaira, Y. An efficient visible-light-sensitive Fe(III)-grafted TiO2photocatalyst.2010, 114, 16481-16487.
(13) Kitano, S.; Murakami, N.; Ohno, T.; Mitani, Y.; Nosaka, Y.; Asakura, H.; Teramura, K,; Tada, T.; H.; Hashimoto, K.; Kominam, H. Bifunctionality of Rh3+Modifier on TiO2and working mechanism of Rh3+/TiO2photocatalyst under irradiation of visible light.2013, 117, 11008-11016.
(14) Kitano, S.; Tanaka, A.; Hashimoto, K.; Kominami, H. Metal ion-modified TiO2photocatalysts having controllable oxidative performance under irradiation of visible light.2016, 521, 202-207.
(15) Kisch, H.; Zang, L.; Lange, C.; Maier, W. F.; Antonius, C.; Meissner, D. Modified, amorphous titania—a hybrid semiconductor for detoxification and current generation by visible light.1998, 37, 3034-3036.
(16) Tada, H.; Jin, Q.; Nishijima, H.; Yamamoto, H.; Fujishima, M.; Okuoka, S.; Hattori, T.; Sumida, Y.; Kobayashi, H. Titanium (IV) dioxide surface-modified with iron oxide as a visible light photocatalyst.2011, 50, 3501-3505.
(17) Jin, Q.; Ikeda, T.; Fujishima, M.; Tada, H. Nickel(II) oxide surface-modified titanium(IV) dioxide as a visible-light-active photocatalyst.. 2011, 47, 8814-8816.
(18) Kumar, S. G.; Devi, L. G. Review on modified TiO2photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics.2011, 115, 13211-13241.
(19) Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2for environmental photocatalytic applications: a review.2013, 52, 3581-3599.
(20) Dai, Z. M.; Burgeth, G.; Parrino, F.; Kisch, H. Visible light photocatalysis by a titania-rhodium(III) complex.2009, 694, 1049-1054.
(21) Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system.2006, 252, 5227-5232.
(22) Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, P.; Zhang, Y.; Ye, J. Surface-alkalinization-induced enhancement of photocatalytic H2evolution over SrTiO3-based photocatalysts.2012, 134, 1974-1977.
(23) Yu, H.; Xu, L.; Wang, P.; Wang, P.; Yu, J. Enhanced photoinduced stability and photocatalytic activity of AgBr photocatalyst by surface modification of Fe(III) cocatalyst.2014, 144, 75-82.
(24) Tagliente, M. A.; Mattei, G.; Tapfer, L.; Antisari, M.; Mazzoldi, P. Thermal behavior of indium nanoclusters in ion-implanted silica.2004, 70, 075418.
(25) Shi, F. F.; Bulkowski, M.; Hsieh, K. C. Synthesis of indium nanoclusters and formation of thin film contacts on plastic substrates for organic and flexible electronics applications.2007, 18, 265301.
(26) Jeon, M. K.; Kang, M. Synthesis and characterization of indium-tin-oxide particles prepared using sol-gel and solvothermal methods and their conductivities after fixation on polyethyleneterephthalate films.2008, 62, 676-682.
(27) Wang, E.; Yang, W.; Cao, Y. Unique surface chemical species on indium doped TiO2and their effect on the visible light photocatalytic activity.2009, 113, 20912-20917.
(28) Yu, Y.; Wang, E.; Yuan, J.; Cao, Y. Enhanced photocatalytic activity of titania with unique surface indium and boron species.2013, 273, 638-644.
(29) Myilsamy, M.; Mahalakshmi, M.; Murugesan, V.; Subha, N. Enhanced photocatalytic activity of nitrogen and indium co-doped mesoporous TiO2nanocomposites for the degradation of 2,4-dinitrophenol under visible light.. 2015, 342, 1-10.
(30) Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium.1966, 15, 627-637.
(31) Mu, J.; Chen, B.; Zhang, M.; Guo, Z.; Zhang, P.; Zhang, Z.; Sun, Y.; Shao, C.; Liu, Y. Enhancement of the visible-light photocatalytic activity of In2O3-TiO2nanofiber heteroarchitectures.2011, 4, 424-430.
(32) Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials.2001, 13, 3169-3183.
(33) Dickinson, T.; Povey, A. F.; Sherwood, P. M. A. Dissolution and passivation of nickel. An X-ray photoelectron spectroscopic study.1977, 73, 327-343.
(34) Powell, C. J. Recommended Auger parameters for 42 elemental solids.2012, 185, 1-3.
(35) Glover, E. N. K.; Ellington, S. G.; Sankar, G.; Palgrave, R. G. The nature and effects of rhodium and antimony dopants on the electronic structure of TiO2: towards design of Z-scheme photocatalysts.2016, 4, 6946-6954.
(36) Ismail, A. A.; Bahnemann, D. W.; Al-Sayari, S. A. Synthesis and photocatalytic properties of nanocrystalline Au, Pd and Pt photodeposited onto mesoporous RuO2-TiO2nanocomposites.2012, 431, 62-68.
(37) Sun, M.; Chen, Z. Enhanced photoelectrochemical cathodic protection performance of the In2O3/TiO2composite.2015, 162, C96-C104.
(38) Kern, R.; Sastrawan, R.; Ferber, J.; Stangl, R.; Luther, J. Modeling and interpretation of electrical impedance spectra of dye solar cells operated under open-circuit conditions.2002, 47, 4213-4225.
(39) Tahir, M.; Amin, N. A. S. Indium-doped TiO2nanoparticles for photocatalytic CO2reduction with H2O vapors to CH4.2015, 162, 98-109.
(40) Li, Z.; Dong, T.; Zhang, Y.; Studies on In(OH)Ssolid solutions: syntheses, characterizations, electronic structure, and visible-light-driven photocatalytic activities.2007, 111, 4727-4733.
13 April 2018;
5 June 2018
the Natural Science Foundation of Fujian Province (2015j01231), the Chunmiao Project of Haixi Institute of Chinese Academy of Sciences (CMZX-2014-005), and the National Key Research and Development Program of China (2016YFB0701003)
. Huang Ji-Quan. E-mail: hjq@fjirsm.ac.cn
10.14102/j.cnki.0254-5861.2011-2046
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

