Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
YIN Guo-Jie CHU Xi-Jie ZHANG To MAO Hi-Fng WANG Cho-Yng YANG Yong-Fu
Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
YIN Guo-Jiea②CHU Xi-JieaZHANG TaoaMAO Hai-FangbWANG Chao-YangbYANG Yong-Fuc
a(471023)b(201418)c(471023)
A new boron compound [C27H21BN4O3] based on 2-(2΄-hydroxyphenyl)-1H- benzimidazole has been synthesized and characterized by single-crystal X-ray diffraction, and its crystal crystallizes in the monoclinic system, space group21/with= 9.6544(5),= 14.1558(8),= 16.4314(9) Å,= 97.730°,M= 460.29,= 2225.2(2) Å3,= 4,D= 1.374 g/cm3,= 0.74 mm-1,= 1.051,(000) = 960, the final= 0.0643 and= 0.1569 for2233 observed reflections (> 2()). The title compound is a B(III) center mononuclear molecule in the asymmetric unit. The typical structural characteristic of the title compound is the methanol group adopting a2-bridging mode to link two different adjacent chelating modes though two types of hydrogen bonds to form aone-dimensional supramolecular structure.Additionally,aromaticstacking interactions between adjacent benzimidazolyl groups lead to a three-dimensional network. Furthermore, the stability and fluorescence property revealed the potential applications in the organic photoelectric material.
synthesis, crystal structure, stability, fluorescence property;
1 INTRODUCTION
Leonardo da Vinci has ever said that “The sensation closest to the sense organs responds most quickly, and such sensation is vision, the chief of all the sensations”. Thus, it can be seen that the most important information source is visually-accepted information. Luminescent materials can be used as important carrier for information owning to their luminescent properties, which can not only be widely used in some conventional fields like fluore- scent pigments, fluorescent paints and fluorescent brighteners, but also showed broad applications in prospects in some high-tech fields like fluorescence molecular probes[1, 2], logic gates[3-6]and electro-luminescent diodes. Therefore, the design and syn- thesis of new luminescent materials have become one of the research focuses, such as benzimidazole compounds.
Benzimidazole compounds have a unique-conju- gated structure. When the carbon atom on the nitro- gen heterocyclic ring connected to 2-hydroxyphenyl, a strong fluorescence phenomenon occurred, such as 2-(2΄-hydroxyphenyl)-1H-benzimidazole (HPBI), because in such compounds the phenolic hydroxyl groups are linked to other heteroatoms (e.g., nitrogen) via intramolecular hydrogen bonds. After absorbing the energy, the molecule is energetically hydro- genated from the ground state enantiomeric N···H–O hydrogen bonds, and isomerized to keto form N–H···O hydrogen bonds to generate the excited state intramolecular proton transfer (ESIPT) Fluore- scence (Scheme 1)[7-9]. The ESIPT fluorescence has advantages in large Stokes shift, high fluorescent quantum’s yield and good light stability. Therefore, the application of such substances, especially those high in the fluorescence yield of HBI, on the fields of organic laser dye and bioprobe has aroused great attention. Simultaneously, the proton transfer rate of ESIPT molecule with bistable state and optical nonlinearity can reach the fs order of magnitude and the proton transfer process is reversible. Therefore, such compounds have become preferred materials in the use of optical devices like photoswitches, optical limiting (OL) devices and real-time optical storage devices[10-16]. Moreover, since the 2-(2΄-hydroxy- phenyl)benzoazole compound can complex with metal ion in the form of an inner complex salt to form a stable six-membered chelate ring, a stable light-emitting compound can be obtained. The 2-(2΄-hydroxyphenyl)benzoazole organic compounds have attracted extensive attention in the fields of metal ion probes and organometallic luminescent materials[17-26].
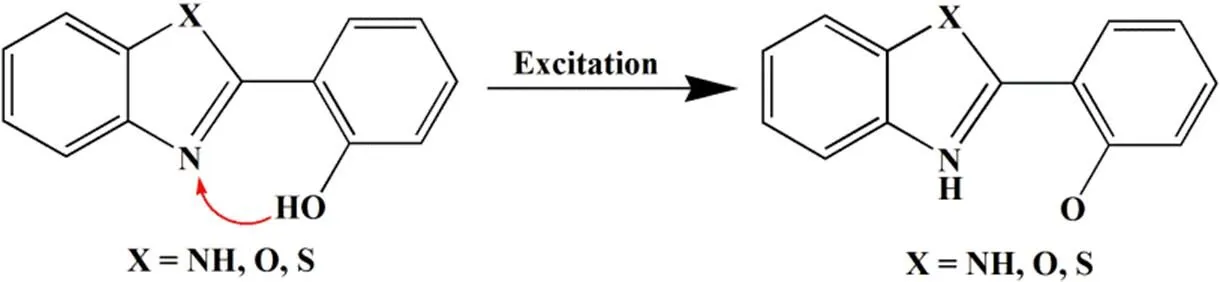
Scheme 1. Excited state intramolecular proton transfer (ESIPT) of benzimidazole compounds
Considering these in mind, we chose 2-(2-hy- droxyphenyl)-1H-benzimidazole (HPBI) as the pre- cursor ligand and used HPBI to react with tri- phenylborane to synthesize compound [C27H21BN4O3] and then discussed its structure and fluorescence property.
2 EXPERIMENTAL
All the reagents and solvents used for synthesis were purchased from Aldrich and used without further purification. The ligand HPBI was synthe- sized in a one-step reductive cyclization process usingsodium hydrosulfite as the reducing agent according to the published procedure[27, 28]. The boron complex[C27H21BN4O3]was synthesized according to the literature[26]. The IR spectra were obtained as KBr disks on a Shimadzu IR 435 spectrometer. Boron analyses were carried out by conventional chemical titration. The C, H, O and N elemental analyses were performed on an ELEMENTAR vario EL elemental analyzer. 1H NMR spectra were recorded on a Bruker DPX- 400MHz spectrometer. Thermogravimetric analysis (TGA) was performed on Netzsch (DSC/DTA-TG) STA 449 F3 Jupiter thermal analyzers under nitrogen atmosphere at a heating rate of 10 ℃/min. Absorption and photoluminescence (PL) emission spectra of the title compound were measured using an Edinburghinstruments FS5 fluorescence spec- trometer.
2. 1 Synthesis of 2-(2΄-hydroxyphenyl)- 1H-benzimidazole (HPBI, 1)
The ligand HPBI was prepared according to the procedure shown in Scheme 2[27, 28]. To a solution of 2-nitroaniline (1.38 g, 10 mmol) and salicylal- dehyde (1.2 mL, 11.26 mmol) in a mixed solvent of ethanol and water (5/1, 60 mL) was added sodium dithionite (5.25 g, 30.17 mmol). The reaction solution was stirred at 90 ℃with thin layer chromatography (TLC) tracking the reaction and filtered after reaction finished, the filtered solution was poured into distilled water, and then the solvent was removed in vacuo to yield the crude product. Purification by silica gel chromatography using 100~200 mesh ZCX II eluted by petroleum ether-ethyl acetate (1/2, V/V) gave compound 1 (4.52 g, 91%). 1H NMR (300 MHz, DMSO):= 13.21 (m, 2H), 8.08 (d,) 7.7 Hz, 1H), 7.67 (m, 2H), 7.39 (tr,) 7.5 Hz, 1H), 7.29 (m, 2H), 7.03 (m, 2H) ppm. FT-IR (KBr:bar) 3322 (m), 3245 (s), 3054 (m), 1593 (s), 1492 (s), 1417 (s), 1319 (m), 1259 (s), 1130 (w), 1037 (w), 840 (s), 798 (w), 734 (s), 522 (m), 468 cm-1(w). Elemental analysis calcd. (%) for C13H10N2O: C, 74.27; H, 4.79; N, 13.33. Found (%): C, 74.22; H, 4.81; N, 13.49.

Scheme 2. Schematic drawing for the synthetic route of HPBI
2. 2 Synthesis of [C27H21BN4O3] (2)

Scheme 3. Synthesis procedure of compound 2 [C27H21BN4O3]
2. 3 X-ray crystallographic studies of [C27H21BN4O3] (2)
A yellow crystal of the title compound with dimensions of 0.30mm × 0.24mm × 0.20mm was selected and mounted on a glass fiber. The X-ray diffraction data were collected on a SMART APEX diffractometer (graphite-monochromated, Curadiation,--scan technique,= 0.71073 Å). The intensity data were integrated by the SAINT program[29]. SADABS[30]was used to perform area- detector scaling and absorption corrections. The structures were solved by direct methods and refined on2using all reflections with the SHELXTL package[31]. All non-hydrogen atoms were refined anisotropically. H atoms in compound 2 were placed in calculated positions and refined in the “riding model”. The final full-matrix least- squares refinement gave= 0.0643,= 0.1569 for 2233 observed reflections (2()), (Δ/)max= 0.001,= 1.05, (Δ)max= 0.38 and (Δ)min= −0.14 e/Å3. Details of selected bond lengths and bond angles for the crystals of the title compound are shown in Tables 1 and 2, respectively.
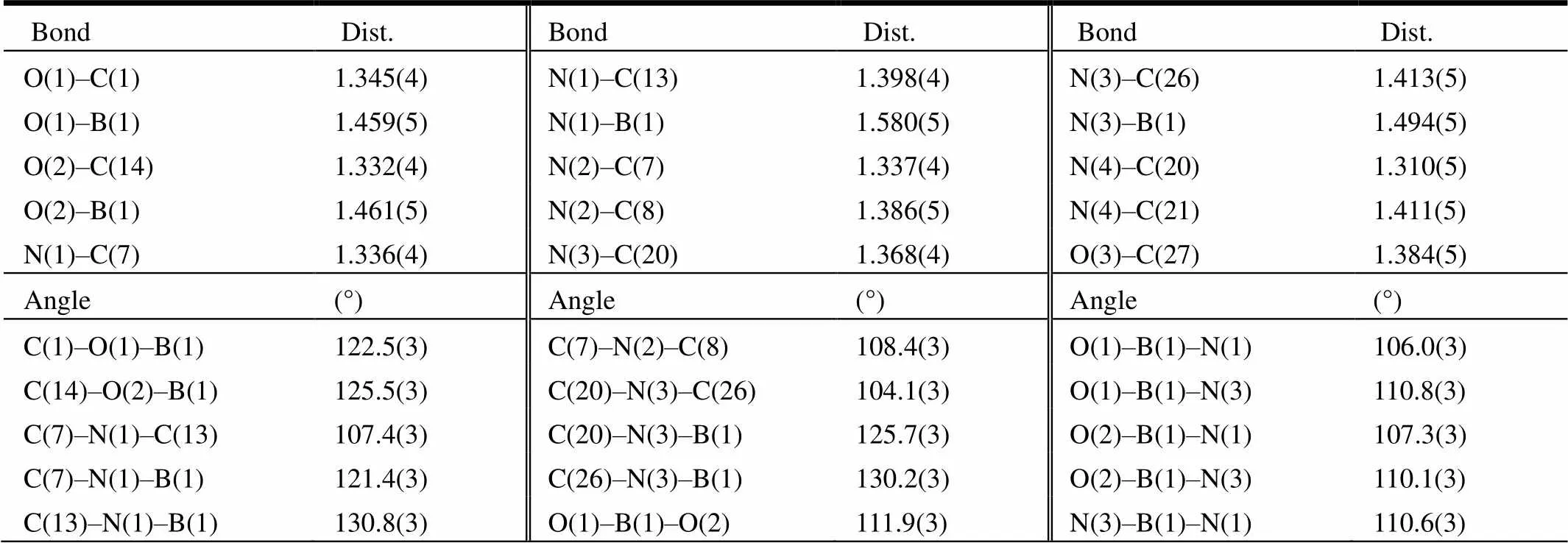
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for the Crystals of the Title Compound
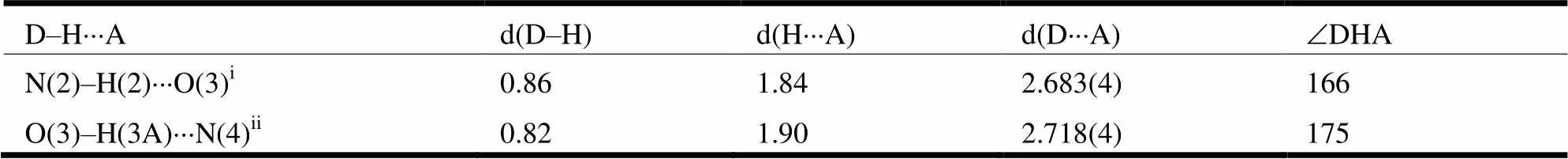
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry codes: (i) −, −, −+1; (ii) −+1/2,−1/2, −+1/2
3 RESULTS AND DISCUSSION
Compound 2 crystallizes in monoclinic with space group21/. As shown in Fig. 1, the B atom is four-coordinated by two hydroxyl oxygen atoms (B(1)–O(1)1.460(5) Å, B(1)–O(2)1.463(5)Å) and two benzimidazolyl nitrogen atoms(B(1)–N(1)1.579(5), B(1)–N(3)1.492(5) Å) of the ligands. The overall geometry around the B center is a distorted tetrahedron. A typical feature of the compound is the solvent methanol molecule in every asymmetric unit, as shown in Fig. 2, of which each methanol group adopts a2-bridging mode to link two different adjacent chelating modes though two types of hydrogen bond (N(2) –H(2)···O(3) (N/O 2.683(4)Å, H···O 1.84 Å,ÐNHO, 166°), O(3)–H(3A)···N(4) (O/N 2.718(4)Å, H···O 1.90 Å,ÐOHO, 175°)} to form aone-dimensional supramolecular structure.
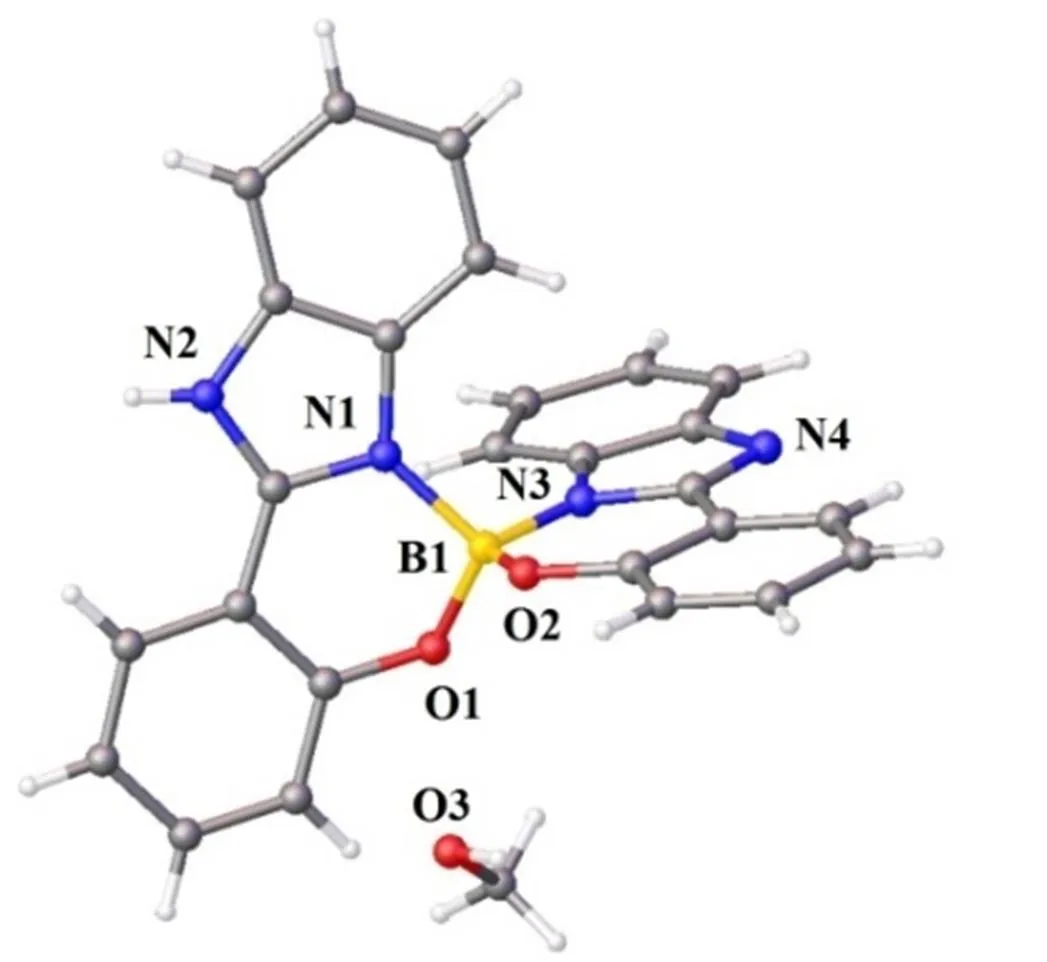
Fig. 1. Coordination environment of the B atom in compound [C27H21BN4O3]
Furthermore, the two benzimidazolyl planesbelonging to the adjacent molecules are almost parallel. The aromatic-stacking interaction[32]between adjacent benzimidazolyl groups not only leads to increased thermodynamic stability but also effectively fills the void space of this one-dimen- sional structure. As a result, the existence of hydrogen bond and-stacking interactionlead to a three-dimensional network (as shown in Fig. 3) and the stability of the crystal structure.

Fig. 2. Hydrogen-bonded one-dimensional network in compound 2 (Hydrogen atoms are omitted for clarity)
Fig. 3. Due to the hydrogen bond and-stacking interaction, perspective view of the network of compound 2 along theaxis (Hydrogen atoms connected to the carbon atoms are omitted for clarity)
In order to reveal the stability of the crystal structure, thermal gravimetric analysis (TGA) was performed for compound 2 under nitrogen atmos- phere in the range from room temperature to 850 ℃. As depicted in Fig. 4, the initial weight loss of 6.99% from room temperature to 65.3 ℃corresponds to the release of CH3OH guest molecu- les (calcd. 6.96%). After that, there is a long plateau region up to 400.3 ℃, and then the weight loss is attributable to the decomposition of the coordina- tion framework. The final residue of 7.59% for compound 2 is in agreement with the percentage of B2O3in all cases (calcd. 7.56%).
It has been established that coordination polymers with conjugated organic ligands are promising candidates for photoactive materials due to their luminescent properties, and the optics studies on the ligand HPBI have been reported for several years[12, 13, 26, 33-35]. Consequently, absorption (Abs) and photoluminescence (PL) emission properties of compound2 have been investigated at room temperature in CH3OH (10-6mol/L). As shown in Fig. 5, compared with the ligand HPBI[26], the absorption spectra of the compound are similar to that of the ligand, but the fine structure of the absorption spectrum of the compound was much clearer than its ligand HPBI and the spectra red-shifts for about 19 nm. It means that in their dilute solutions the intermolecular interaction between compounds is much weaker than that between HPBI because the chelate structure may decrease the intermolecular interaction. Compared with the ligand[26], compound 2 exhibits a strong emission band at 385 nmupon 350 nm excitation (Stokes shift: 35 nm). The emission blue-shifted remarkably (43 nm for 2 to HPBI), and the full width at half-ma- ximum (fwhm, 53 nm) was much smaller than that of the ligand (81 nm). After forming the chelate, compared with the free ligand emission originated fromESIPT in the dilute solution, the PL spectra are blue-shifted due to the higher band gap of*-transitions of the ligand itself. Those characteristics of compound 2 mentioned above reveal the potential applications in the organic photoelectric materials.

Fig. 4. TGA curve of compound 2
Fig. 5. Abs (dashed lines) and PL (solid lines) spectra of the compound in CH3OH
Generally speaking, the HOMO is mainly distri- buted on electron-rich groups, while LUMO mainly on the electron-deficient group. For the energy of HOMO, the size of the conjugated substituent group has a significant effect. With the increase in delo- calized electrons, the energy of HOMO shows an increasing tendency[36]. Furthermore, the HOMO- LUMO gap is an important parameter to characterize the electronic structure of molecule. It is well known that the energy gap retains close connection to some molecular properties[37]. In order to obtain the electronic density distribution of compound 2, the energy type of the initial spatial configuration was investigated at the B3LYP[38]/3-21G level with density functional theory[39]based on the ground state by using the Gaussian 09 program package[40]. As shown in Fig. 6, the HOMO energy level of –5.14 ev is distributed on the group of ligand where N4 atom is located, while the LUMO energy level of –2.09 ev is distributed on the group of ligand where N2 atom is located and the energy gap of which is 3.05 ev. It can be seen that3-B obstacles theelectron flowing in the whole molecule, and the hole and electron have the transmission path of each own. Such charge separation situation indicates that this compound has great potential of bipolar transport capacity, which is consistent with the purpose of the design.
4 CONCLUSION
In conclusion, a novel B(Ⅲ) coordination com- pound was successfully synthesized with 2-(2-hy- droxyphenyl)benzimidazole ligand and triphenyl- borane, which was a B(III) center mononuclear molecule in the asymmetric unit. In the crystal, the methanol group adopts a2-bridging mode to link two different adjacent chelating modes though two types of hydrogen bonds to form aone-dimensional supramolecular structure.Additionally, thearomatic-stacking interaction between adjacent benzimidazolyl groups leads to a three-dimensional network. Finally, this compound reveals potential applications in the organic photoelectric material.
(1) Xu, H.; Wang, Z.; Li, Y.; Ma, S.; Hu, P.; Zhong, X. A quantum dot-based “off-on” fluorescent probe for biological detection of zinc ions.2013, 138, 2181-2191.
(2) Xiang, Y.; Lu, Y. DNA as sensors and imaging agents for metal ions.2014, 53, 1925-1942.
(3) De Silva, A. P.; Gunaratne, H. Q. N.; McCoy, C. P. Molecular photoionic and logic gates with bright fluorescence and “off-on” digital action.1997, 119, 7891-7892.
(4) De Silva, A. P.; Gunaratne, H. Q. N.; McCoy, C. P. A molecular photoionic and gate based on fluorescent signalling.1993, 364, 42-44.
(5) Liu, G.; Pu, S.; Wang, R. Photochromism of asymmetrical diarylethenes with a pyrrole unit: effects of aromatic stabilization energies of aryl rings.2013, 15, 980-983.
(6) Norsten, T. B.; Branda, N. R. Photoregulation of fluorescence in a porphyrinic dithienylethene photochrome.2001, 123, 1784-1785.
(7) Padalkar, V.; Tathe, A.; Gupta, V.; Patil, V.; Phatangare, K.; Sekar, N. Synthesis and photo-physical characteristics of esipt inspired 2-substituted benzimidazole, benzoxazole and benzothiazole fluorescent derivatives.2012, 22, 311-322.
(8) Ríos, M. A.; Ríos, M. C.study of ground and excited state proton transfer in 2-(2΄-hydroxyphenyl)benzoxazole.1995, 99, 12456-12460.
(9) Ríos, M. A.; Ríos, M. C.study of the hydrogen bond and proton transfer in 2-(2΄-hydroxyphenyl)benzothiazole and 2-(2΄-hydroxyphenyl) bezimidazole.1998, 102, 1560-1567.
(10) Costela, A.; Garcı́a-Moreno, I.; Mallavia, R.; Amat-Guerri, F.; Barroso, J. Proton-transfer lasers based on solid copolymers of modified 2-(2΄-hydroxyphenyl)benzimidazoles with methacrylate monomers.1998, 152, 89-95.
(11) Sakai, K.; Tsuzuki, T.; Motoyoshiya, J.; Inoue, M.; Itoh, Y.; Ichikawa, M.; Fujimoto, T. Using proton-transfer laser dyes for organic laser diodes.2005, 86, 081103.
(12) Wu, F.; Ma, L.; Zhang, S.; Geng, Y.; Lü, J.; Cheng, X. Two-photon-induced intramolecular excited-state proton transfer process and nonlinear optical properties of HBI in ethanol solution.2012, 519, 141-144.
(13) Wu, F.; Ma, L.; Zhang, S.; Wang, Z.; Cheng, X. The nonlinear optical properties of HBI in different solvents.2014, 116, 231-234.
(14) Costela, A.; Muñoz, J. M.; Douhal, A.;Figuera, J. M.; Acufia, A. U. Experimental test of a four-level kinetic model for excited-state intramolecular proton transfer dye lasers.1989, 49, 545-552.
(15) Costela, A.; Amat, F.; Catalán, J.; Douhal, A.; Figuera, J. M.; Muñoz, J. M.; Acuña, A. U. Phenylbenzimidazole proton-transfer laser dyes: spectral and operational properties.1987, 64, 457-460.
(16) Acuña, A. U.; Amat, F.; Catalán, J.; Costela, A.; Figuera, J. M.; Muñoz, J. M. Pulsed liquid lasers from proton transfer in the excited state.1986, 132, 567-569.
(17) Roh, S. G.; Kim, Y. H.; Seo, K. D.; Lee, D. H.; Kim, H. K.; Park, Y. I.; Park, J. W.; Lee, J. H. Synthesis, photophysical, and electroluminescent device properties of Zn(II)-chelated complexes based on functionalized benzothiazole derivatives.2009, 19, 1663-1671.
(18) Chu, Q.; Medvetz, D. A.; Panzner, M. J.;Pang, Y. A fluorescent bis(benzoxazole) ligand: toward binuclear Zn(II)-Zn(II) assembly.2010, 39, 5254-5259.
(19) Katkova, M. A.; Balashova, T. V.; Ilichev, V. A.; Konev, A. N.; Isachenkov, N. A.; Fukin, G. K.; Ketkov, S. Y.; Bochkarev, M. N. Synthesis, structures, and electroluminescent properties of scandium n, o-chelated complexes toward near-white organic light-emitting diodes.2010, 49, 5094-5100.
(20) Tian, Y.; Chen, C. Y.; Yang, C. C.; Young, A. C.; Jang, S. H.; Chen, W. C.; Jen, A. K. 2-(2′-hydroxyphenyl)benzoxazole-containing two-photon- absorbing chromophores as sensors for zinc and hydroxide ions.2008, 20, 1977-1987.
(21) Nah, M. K.; Rho, S. G.; Kim, H. K.; Kang, J. G. Sensitized near-IR luminescence of In(III) complexes with benzothiazole derivatives.2007, 111, 11437-11443.
(22) Kwon, J. E.; Lee, S.; You, Y.; Baek, K. H.; Ohkubo, K.; Cho, J.; Fukuzumi, S.; Shin, I.; Park, S. Y.; Nam, W. Fluorescent zinc sensor with minimized proton-induced interferences: photophysical mechanism for fluorescence turn-on response and detection of endogenous free zinc ions.2012, 51, 8760-8774.
(23) Henary, M. M.; Wu, Y.; Fahrni, C. J. Zinc(II)-selective ratiometric fluorescent sensors based on Inhibition of excited-state intramolecular proton transfer.2004, 10, 3015-3025.
(24) Taki, M.; Wolford, J. L.; O΄Halloran, T. V. Emission ratiometric imaging of intracellular zinc: design of a benzoxazole fluorescent sensor and its application in two-photon microscopy2003, 126, 712-713.
(25) Xu, Y.; Liu, Q.; Dou, B.; Wright, B.; Wang, J.; Pang, Y. Zn2+binding-enabled excited state intramolecular proton transfer: a step toward new near-infrared fluorescent probes for imaging applications.2012, 1, 485-492.
(26) Xu, H.; Xu, Z. F.; Yue, Z. Y.; Yan, P. F.; Wang, B.; Jia, L. W.; Li, G. M.; Sun, W. B.; Zhang, J. W. A novel deep blue-emitting Zn(II) complex based on carbazole-modified 2-(2-hydroxyphenyl)benzimidazole: synthesis, bright electroluminescence, and substitution effect on photoluminescent, thermal, and electrochemical properties.2008, 112, 15517-15525.
(27) Zhu, H.; Huang, W. D.; Pu, J. Q. Synthesis of benzimidazoles from-nitroanilines in a one-step reductive cyclization process.2007, 13, 77–81.
(28) Yang, D. L.; Fokas, D.; Li, J. Z.; Yu, L. B.; Baldino, C. M. A versatile method for the synthesis of benzimidazoles from-nitroanilines and aldehydes in one step via a reductive cyclization.2005, 1, 0047-0056.
(29) Bruker.. Bruker AXS, Madison, WI, USA 2000.
(30) Sheldrick, G. M.Bruker AXS, Madison, WI, USA 1998.
(31) Sheldrick, G. M.. Bruker AXS: Madison, WI, USA 2000.
(32) Główka, M. L.; Martynowski, D.; Kozàowska, K. Stacking of six-membered aromatic rings in crystal.1999, 474, 81-89.
(33) Najafpour, M. M.; Tabrizi, M. A.; Haghighi, B.; Govindjee. A 2-(2-hydroxyphenyl)-1-benzimidazole-manganeseoxide hybrid as a promising structural model for thetyrosine 161/histidine 190-manganese cluster inphotosystem II.2013, 42, 879-884.
(34) Filik, H.; Tavman, A. A new cloud-point preconcentration approach for the spectrophotometric determination of-aminophenol in the presence of paracetamol with 2-(2-hydroxyphenyl)-1H-benzimidazole as a coupling reagent.2007, 62, 530-535.
(35) Machura, B.; Wolff, M.; Kusz, J.; Kruszynski, R. Reactivity of [ReOX3(PPh3)2] and [ReOX3(AsPh3)2] towards 2-(2-hydroxyphenyl)-1H-benzimida- zole: synthesis, X-ray studies, spectroscopic characterization and DFT calculations for [ReOX2(hpb)(EPh3)] and [ReO(OMe)(hpb)2]·MeCN.2009, 28, 2949-2964.
(36) Wong, K. T.; Chen, H. F.; Fang, F. C. Novel spiro-configured pet chromophores incorporating 4,5-diazafluorene moiety as an electron acceptor.2006, 8, 3501-3504.
(37) Pearson, R. G. Absolute electronegativity and hardness: application to inorganic chemistry.1988, 27, 734-740.
(38) Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density.1988, 37, 785-789.
(39) Dreizler, R. M.; Gross E. U. K.. Heidelberg, Germany: Springer-Verlag 1990.
(40) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.Gaussian, Inc., Pittsburgh PA 2009
1 April 2018;
11 June 2018 (CCDC 1845839 for 2)
① The research was supported by the Scientific and Technological Project of Henan Province (No. 182102210102), the Key Scientific Research Project of Colleges and Universities of Henan Province (No. 15A150061), and the National Natural Science Foundation of China (Nos. 21371154, 61405054 and 21601156)
.Yin Guo-Jie, born in 1981, majoring in functional coordination chemistry. E-mail: 591941522@qq.com
10.14102/j.cnki.0254-5861.2011-2026
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design
- Surface Modification of Titanium Oxide by Indium for Efficient Photocatalytic Hydrogen Generation①

