A New Cadmium(II) Coordination Polymer Extended through Hydrogen Bonds and π-π Stacking Interactions: Synthesis and Photoluminescence Property①
XIAO Guo-Bin FANG Zi-Han YAO Xiao-Qiang
A New Cadmium(II) Coordination Polymer Extended through Hydrogen Bonds andStacking Interactions: Synthesis and Photoluminescence Property①
XIAO Guo-Bin FANG Zi-Han YAO Xiao-Qiang②
(730070)
A new coordination polymer, {[Cd(OPY)2(tdc)(H2O)]·H2O}n(OPY = 4,4΄-(oxy- bis(4,1-phenylene))dipyridine, H2tdc = thiophene-2,5-dicarboxylic acid), has been synthesized hydrothermally based on a V-shaped ligand OPY. The structure was fully characterized by elemental analysis, FT-IR spectroscopy, and X-ray single-crystal diffraction analysis. In 1, two OPY ligands and one water molecule acted as terminal ligands coordinating to Cd2+cation to form [Cd(OPY)2H2O]2+units, which are then linked by tdc2−ligands to generate a one-dimensional chain. Every two adjacent chains linked by extensive O–H···O hydrogen bonds constitute one-dimensional double-chains, and such chains are extended into two-dimensional layersO–H···N hydrogen bonds. These layers are further connected to form a three-dimensional supramolecular architecture-stacking interactions. In addition, the thermal stability and solid state fluorescence property of 1 were also investigated.
crystal structure, hydrogen bond,-stacking interaction, solid state fluorescence property;
1 INTRODUCTION
With the rapid development of supramolecular chemistry and crystal engineering, synthesis and characterization of coordination polymers (CPs) have attracted more and more attention, not only because of their intriguing variety of structures, but also of their potential applications in many fields such as gas storage, nonlinearoptics, molecular magnetism, selective separation, catalysis and luminescence[1-4]. However, the rational design and assembly of coordination polymers with desired structure are still a challenge. It is well known that the structures can be influenced by many factors, such as ligand structure, metal ions, solvents, pH value and reaction temperature[5-7]. Besides that, the weak interactions including hydrogen bonds and-interactions also play an important role in the assemble process[8-10]. Numerous examples of expanding the low-dimen- sional building block into higher dimensional structure through weak interactions have been repor- ted[11, 12]. The symmetric N-containing ligands are wildly used to construct CPs for their good coordina- tion ability, and in most cases, all the coordination atoms participate in coordination. The V-shaped ligand OPY with two pyridine rings are well suitable for constructing CPs, and many CPs based on OPY ligand have been reported[13-15]. In all known exam-ples, both two pyridine rings of OPY ligand participate in coordination, while in this article, only one pyridine N atom of OPY coordinates to the Cd(II) ion, and the other pyridine ring is uncoordinated. Despite this, the uncoordinated pyridine ring as hydrogen-bond acceptor still plays a crucial role in expanding the structure.
2 EXPERIMENTAL
2. 1 Materials and measurements
All chemicals of analytical reagent grade were commercially purchased and used as received. OPY was prepared according to the previously reported method[16]. Elemental (C, H, N) analyses were performed on a Perkin-Elmer 2400 element analyzer. The phase purity of the synthesized complex has been examined by powder X-ray diffraction (PXRD). PXRD pattern was collected using a Philips PW 1710-BASED diffractometer. IR spectra were recorded with a Perkin-Elmer Spectrum One FT-IR spectrophotometer on KBr pellets in the range of 4000~400 cm–1. The thermal analysis was perfor- med under nitrogen with a Netzsch STA449C thermal analyzer. The solid state fluorescence pro- perty was measured at room temperature on a Hitachi F-2700 fluorescence spectrophotometer.
2. 2 Synthesis of {[Cd(OPY)2(tdc)(H2O)]·H2O}n(1)
A mixture of Cd(NO3)2·4H2O (50 mg, 0.16 mmol), H2tdc (36 mg, 0.21 mmol) and OPY (68 mg, 0.21 mmol) was dissolved in the mixture of 3 mL DMF (N, N-dimethylformamide) and 6 mL H2O in a Teflon-lined stainless-steel vessel and heated at 368 K for three days, then cooled slowly to room tempe- rature. The white prismatic crystals (yield 25 mg, 50%, based on OPY) were obtained. Elemental analysis calculated for C50H38CdN4O8S: C, 62.03; H, 3.93; N, 5.79%. Found: C, 62.08; H, 3.95; N, 5.83%. IR (KBr pellet, cm−1): 3393 (m), 1596 (s), 1554 (m), 1514 (m), 1486 (s), 1387 (s), 1340 (s), 1222 (s), 1166 (w), 1086 (s), 855 (w), 821 (m).
2. 3 X-ray structure determination
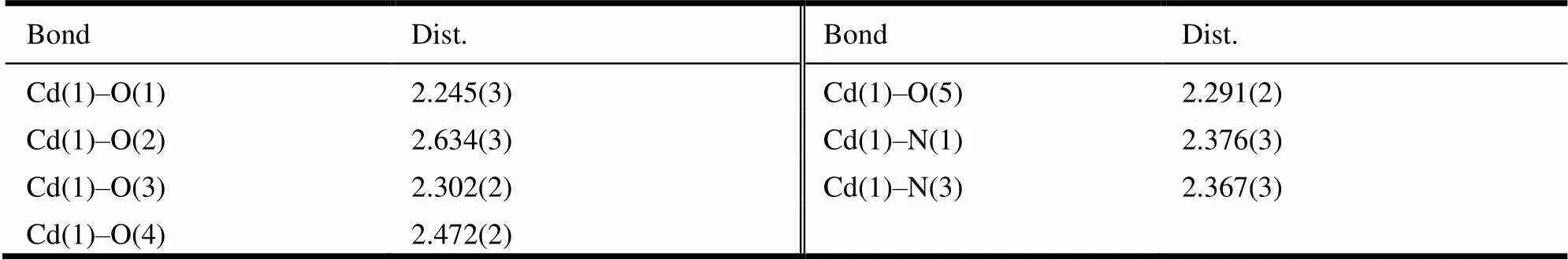
Table 1. Selected Bond Lengths (Å) for Compound 1
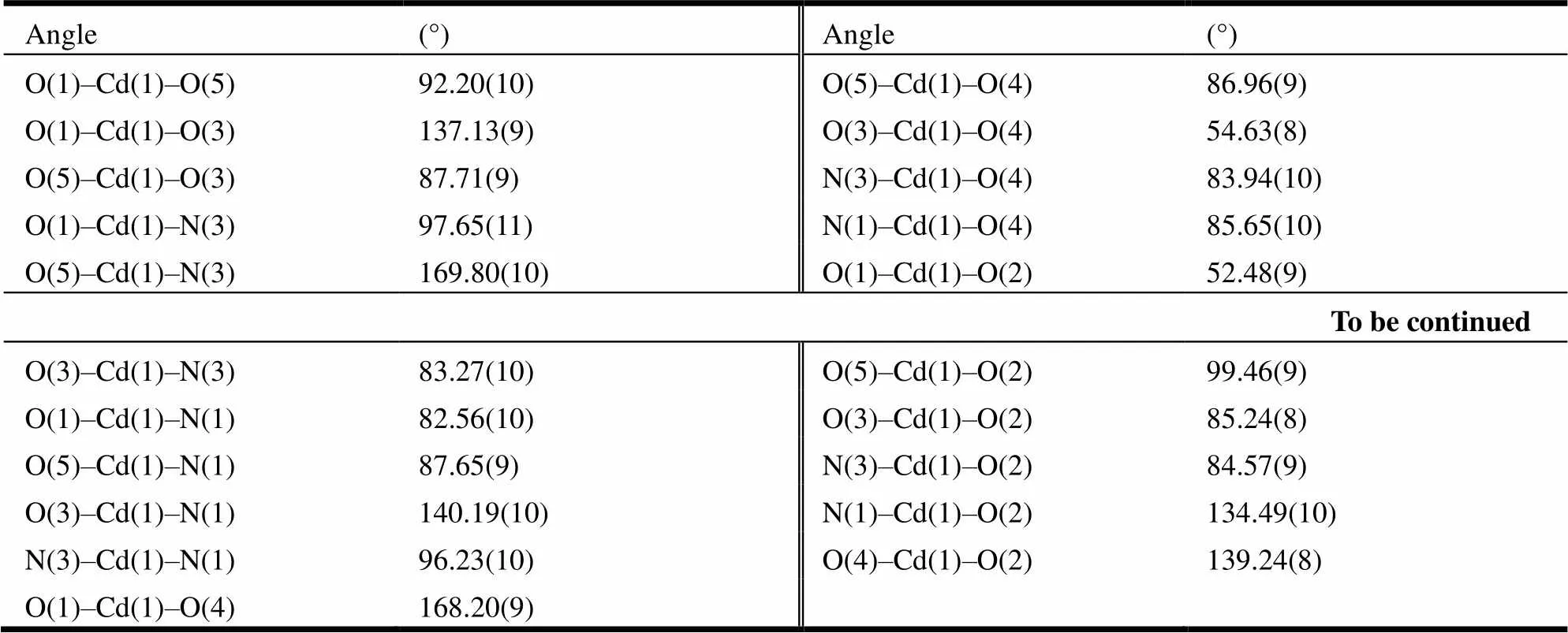
Table 2. Selected Bond Angles (°) for Compound 1
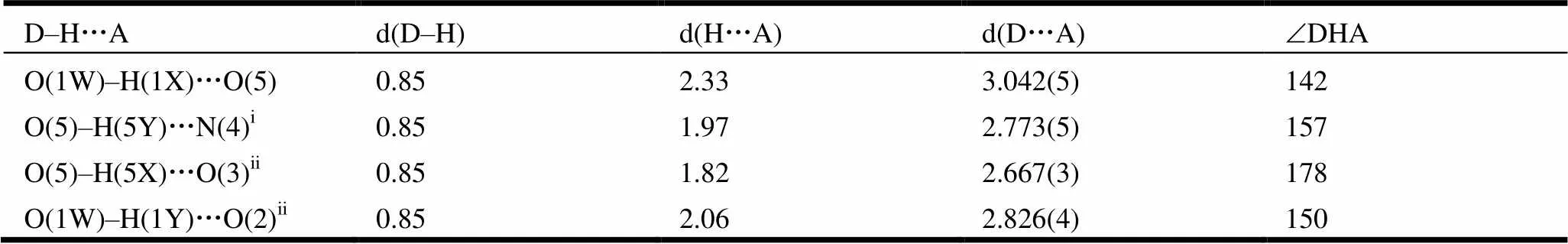
Table 3. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry codes: (i)–2,–1,; (ii) –+1, –, –
3 RESULTS AND DISCUSSION
3. 1 Crystal structure
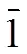
3. 2 Phase purity and thermogravimetric analysis
The phase purity of compound 1 was confirmed by powder X-ray diffraction (PXRD) at room tempera- ture. As shown in Fig. 6, the peak positions of the simulated and experimental PXRD patterns are consistent with each other, indicating the phase pu- rity of the compound.
The thermogravimetric analysis (TGA) was carried out to investigate the thermal stability of compound 1. The polycrystalline samples were heated from room temperature to 800 ℃ under a flow of nitrogen with a heating rate of 10 ℃ min−1(Fig. 7). The TGA curve suggests that a little weight loss of 3.84% (calcd. 3.72%) from 25 ℃ to 120 ℃ ascribes to the release of coordinated and lattice water molecules. Then further weight loss observed at ~260 ℃is due to the collapse of the framework. During the period, weak interactions contribute to stabilizing the framework.
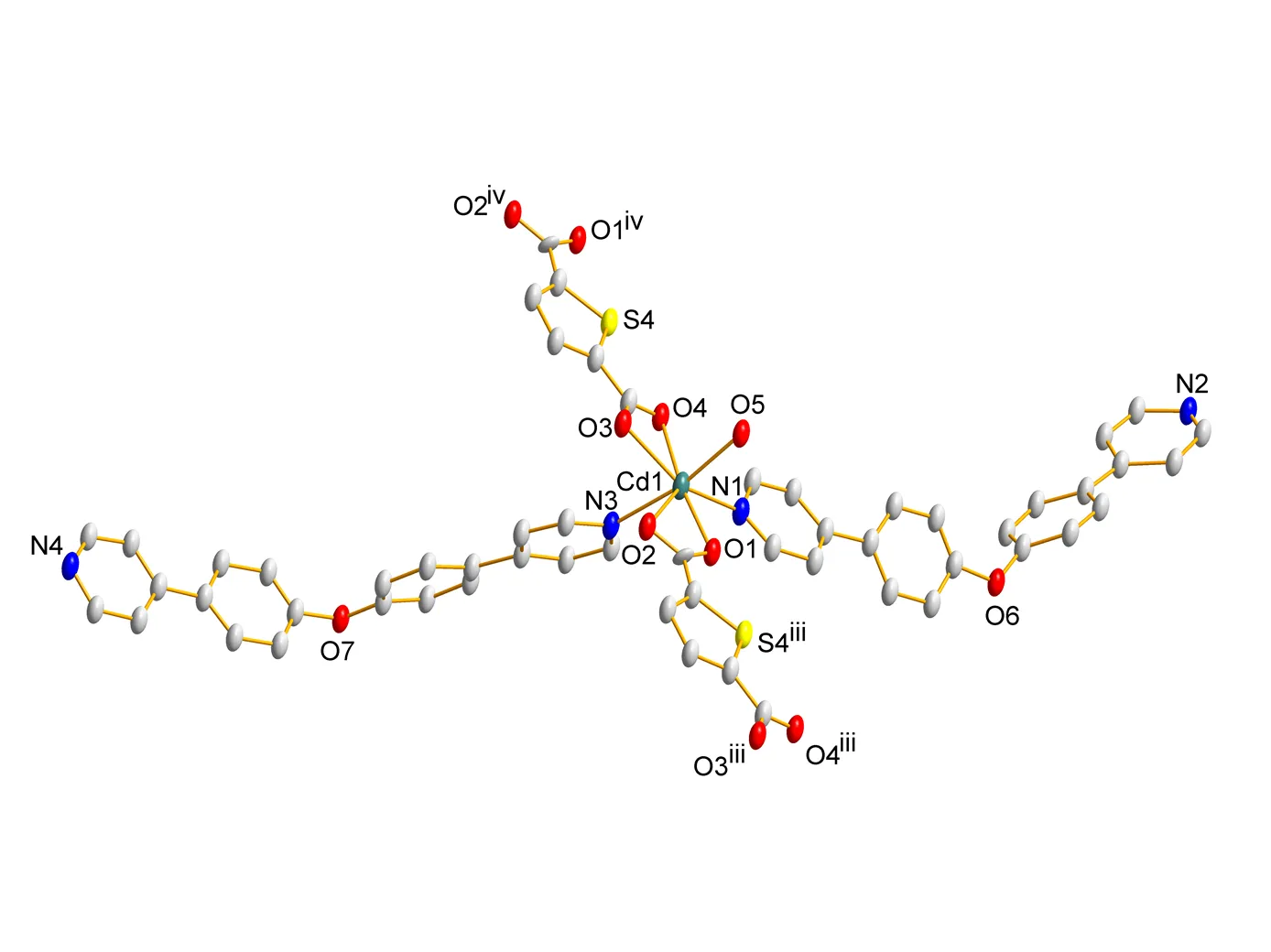
Fig. 1. Coordination environment for the Cd(II) atom in compound 1. All H atoms have been omitted for clarity. Symmetry codes: (iii),– 1,; (iv),+ 1,
Fig. 2. A view of the one-dimensional [Cd(OPY)2(tdc)(H2O)]nchain of 1
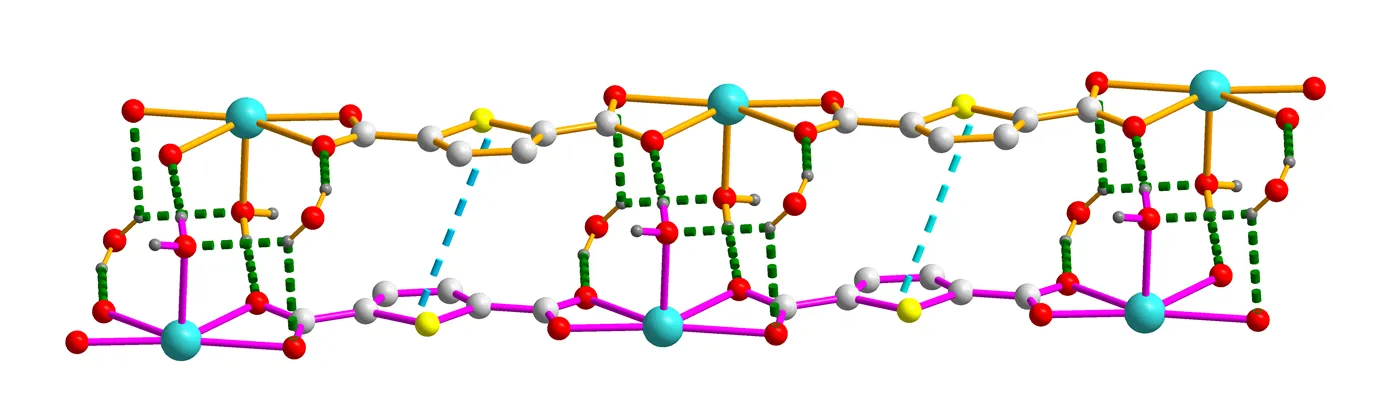
Fig. 3. A view of the one-dimensional double-chain of 1 generated by hydrogen bonds and-stacking interactions (O(1W)–H(1X)···O(5), O(5)–H(5X)···O(3)ii, O(1W)–H(1Y)···O(2)ii). Symmetry codes: (ii) –+ 1, –, −; (v) –+ 1, –+ 1, –
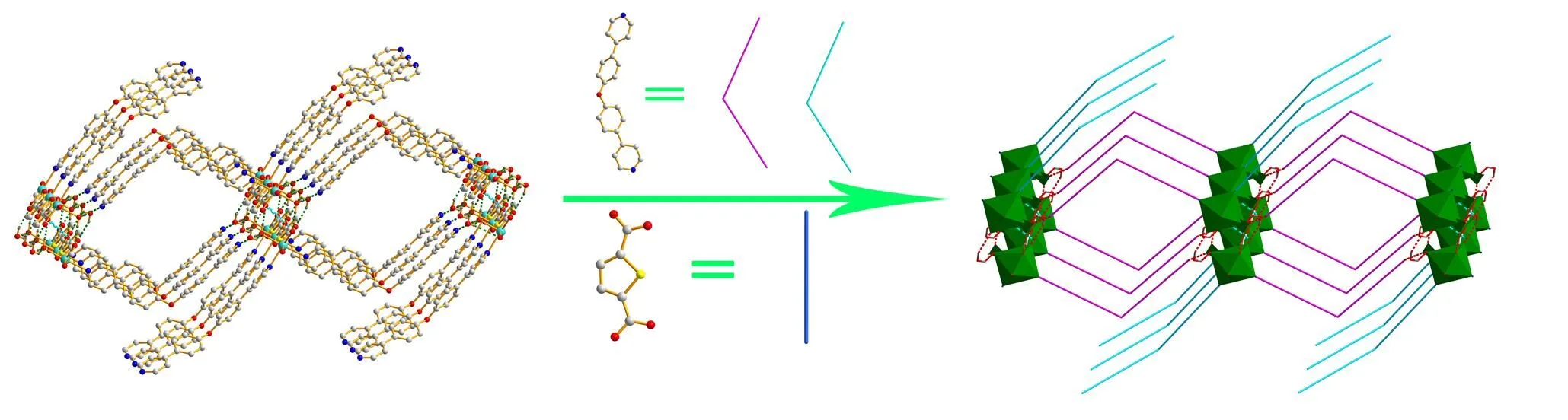
Fig. 4. A view of the two-dimensional layer structure of 1 generated by hydrogen bonding interactions (O(5)–H(5Y)···N(4)i). Symmetry code: (i)– 2,– 1,
Fig. 5. A view of the three-dimensional structure of 1 generated by hydrogen bonds and-stacking interactions
Fig. 6. Experimental and simulated PXRD patterns of compound 1
Fig. 7. TGA curve of compound 1
3. 3 Photoluminescence properties
The photoluminescence properties of compound 1, ligands OPY and H2tdc were investigated in the solid state at room temperature (Fig. 8). Compound 1 exhibits an emission peak maximum at 401 nm upon excitation at 273 nm, the pure H2tdc ligand with emission at 353 nm (ex= 280 nm) and emission band for free OPY at 351 nm (ex= 270 nm). Compound 1 exhibits large red-shift in emission peaks with respect to both OPY and H2tdc ligands, which might be ascribed to cooperative effects of intraligand as well as ligand-to-ligand charge transi- tion[22, 23].

Fig. 8. Fluorescence spectra of 1 and the free OPY and H2tdc ligands at room temperature
4 CONCLUSION
In summary, a new Cd(II) coordination polymer has been synthesized and structurally characterized. The structure was extended by hydrogen bonds and-stacking interactions. Interestingly, the exobiden- tate ligand OPY just adopts a monodentate coordina- tion mode, with only one pyridine N atom coordina- ting to the Cd(II) ion, while the other one remains uncoordinated, which plays a double role in the compound, not only building a two-dimensional network with hydrogen bonds, but also extending into a three-dimensional supramolecular structure with-stacking interactions.
(1) Wang, J. G.; Huang, C. C.; Huang, X. H.; Liu, D. S. Three-dimensional lanthanide thiophenedicarboxylate framework with an unprecedented (4,5)-connected topology.2008,8, 795-798.
(2) Qin, L.; Ju, Z. M.; Wang, Z. J.; Meng, F. D.; Zheng, H. G.; Chen, J. X. Interpenetrated metal-organic framework with selective gas adsorption and luminescent properties.2014,14, 2742-2746.
(3) Wu, M. K.; Yi, F. Y.; Fang, Y.; Xiao, X. W.; Wang, S. C.; Pan, L. Q.; Zhu, S. R.; Tao, K.; Han, L. An ultrastable metal-organic framework with open coordinated sites realizing selective separation toward cationic dyes in aqueous solution.2017, 17, 5458-5464.
(4) Jiang, H. X.; Wang, Q. Y.; Wang, H. Q.; Chen, Y. F.; Zhang, M. H. MOF-74 as an efficient catalyst for the low-temperature selective catalytic reduction of NOwith NH3.. 2016, 8, 26817-26826.
(5) Mu, Y. J.; Han, G.; Li, Z.; Liu, X. T.; Hou, H. W.; Fan, Y. T. Effect of organic polycarboxylate ligands on the structures of a series of zinc(II) coordination polymers based on a conformational bis-triazole ligand.. 2012, 12, 1193-1200.
(6) Li, X. J.; Yu, Z. J.; Li, X. X.; Guo, X. F. Solvent-mediated transformation from achiral to chiral nickel(II) metal-organic frameworks and reassembly in solution.. 2015, 21, 16593-16600.
(7) Li, Q. Q.; Zhang, W. Q.; Ren, C. Y.; Fan, Y. P.; Li, J. L.; Liu, P.; Wang, Y. Y. Reaction-determined assemblies of 0D to 3D complexes: structural diversities and luminescence properties.. 2016, 18, 3358-3371.
(8) Wu, H.; Yang, J.; Su, Z. M.; Batten, S. R.; Ma, J. F. An exceptional 54-fold interpenetrated coordination polymer with 103-srs network topology.2011, 133, 11406-11409.
(9) Chen, B. L.; Ma, S. Q.; Zapata, F.; Fronczek, F. R.; Lobkovsky, E. B.; Zhou, H. C. Rationally designed micropores within a metal-organic framework for selective sorption of gas molecules.2007, 46, 1233-1236.
(10) Kong, Z. G.; Liu, D. X.; Song, M. Y.; Zhang, L. Y.; Feng, S. Y.; Xu, Z. L. A two-dimensional supramolecular complex constructed by 1,10-phenanthroline derivative ligand: synthesis, structure and luminescence.2017, 36, 1859-1863.
(11) Singh, R.; Ahmad, M.; Bharadwaj, P. K. Coordination polymers of copper and zinc ions with a linear linker having imidazole at each end and an azo moiety in the middle: pedal motion, gas adsorption, and emission studies.. 2012, 12, 5025-5034.
(12) Chen, X. C.; Han, S. M.; Wang, R. Y.; Li, Y. Four supramolecular isomers of dichlorido-bis(1,10-phenanthroline)cobalt(II): synthesis, structure characterization and isomerization.2016, C72, 6-13.
(13) Xie, H.; Yao, X. Q.; Ma, H. C.; Lei, Z. Q.; Liu, J. C. A pillar-layered-like metal-organic framework built from dinuclear cobalt chain and a V-shaped bis-pyridyl ligand with different coordination modes.. 2017, 80, 15-18.
(14) Hu, J. S.; Zhu, C. L.; Song, X. M.; He, J. A three-folded interpenetrated metal-organic framework constructed by H-bonding interaction.. 2012, 22, 220-221.
(15) Li, Y.; Yao, X. Q.; Xiao, G. B.; Ma, H. C.; Yang, Y. X.; Liu, J. C. Two isostructural cobalt(II) coordination polymers with both polyrotaxane and polycatenane features assembled with a V-shaped rigid ligand.. 2015, 1089, 16-19.
(16) Zhang, Z. L.; Yao, X. Q.; An, N.; Ma, H. C.; Lei, Z. Q.; Liu, J. C. A homochiral Cu(II) coordination polymer built from helical motif based on two V-shaped ligands.2014, 45, 127-130.
(17) Sheldrick, G. M.and,. University of Göttingen, Germany 1997.
(18) Zhu, M. A.; Guo, X. Z.; Xiao, L.; Chen, S. S. A new Cd(II) coordination compound based on 4-(1,2,4-triazol-4-yl)phenylacetic acid: synthesis, structure and photoluminescence property.2018, 37, 437-444.
(19) Li, L. N.; Wang, S. Y.; Chen, T. L.; Sun, Z. H.; Luo, J. H.; Hong, M. C. Solvent-dependent formation of Cd(II) coordination polymers based on a2-symmetric tricarboxylate linker.. 2012, 12, 4109-4115.
(20) Sun, D.; Han, L. L.; Yuan, S.; Deng, Y. K.; Xu, M. Z.; Sun, D. F. Four new Cd(II) coordination polymers with mixed multidentate N-donors and biphenyl-based polycarboxylate ligands: syntheses, structures, and photoluminescent properties.. 2013, 13, 377-385.
(21) Zhao, S.; Hao, X. M.; Wang, H.; Wu, Y. B.; Guo, W. L. Crystal structures and luminescent properties of three coordination polymers based on 2,5-furandicarboxylic acid ligand and 1,10-phenanthroline.2018, 37, 131-139.
(22) Shen, Y.; Fan, C. C.; Wei, Y. Z.; Du, J.; Zhu, H. B.; Zhao, Y. Construction of noninterpenetrating and interpenetrating (4-fold and 8-fold) 3-D Cd(II) networks with tris(1,2,4-triazol-4-yl)phenyl)amine modulated by aromatic dicarboxylate coligands.2016, 16,5859-5868.
(23) Kan, W. Q.; Liu, B.; Yang, J.; Liu, Y. Y.; Ma, J. F. A series of highly connected metal-organic frameworks based on triangular ligands and10metals: syntheses, structures, photoluminescence, and photocatalysis.2012, 12, 2288-2298.
18 March 2018;
1 June 2018 (CCDC 1001494)
the National Natural Science Foundation of China (Nos. 21361023 and 21461023)
. Yao Xiao-Qiang, associate professor. E-mail: yxq@nwnu.edu.cn
10.14102/j.cnki.0254-5861.2011-2008
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

