A Solvothermal Synthesis and Characterization of a New Compound (PyH)[Cu2(ttc)]①
LI Fei LIU Yn ZHANG Ren-Chun JI Min ANYong-Lin
A Solvothermal Synthesis and Characterization of a New Compound (PyH)[Cu2(ttc)]①
LI FeiaLIU YanaZHANG Ren-ChunbJI MinaANYong-Lina②
a(116024)b(()455000)
solvothermal synthesis, 1,3,5-triazine-2,4,6-trithione, mineralizer;
1 INTRODUCTION
Enormous current attention has been paid towards the synthesis and characterization of transition metal compounds bridged by ligands not only due to their fascinating structures but also to versatile applica- tions in biology, pharmacology, industry and analy- tical chemistry[1-4]. Organic ligands containing N and S donor atoms are widely used in the construction of new compounds, especially the heterocyclic thiones. Heterocyclic thiones are potentially ambidentate or multifunctional donors, due to its abundant coor- dination modes using exocyclic sulfur or heterocyclic nitrogen atoms. They are easily linked with the soft acid like Cu+, Ag+and Cd2+, and thus various novel compounds have been synthesized. For example, with the pyridine-2-thione(2-SC5H4NH) as a ligand, the Cu(I)-containing linear polymeric {Cu6(3- SC5H4NH)4(2-SC5H4NH)2(I4)(-I)2}·2CH3CN has been synthesized[5]. By using pymt (pyrimidine-2- thione) as linkers, 3polymers [Cu(pymtH)Cl]and [Cu(pymtH)Br]have been obtained[6]. Chereported the complex [(CuPPh3)6(ttc)2] constructed by the ligand ttcH3(trithiocyanuric acid), and the striking feature was in the virtually parallel orienta- tion of two triazine rings[7]. Huhas assembled a 0complex [Zn(ttcH2)2(3-bpt)2]·2H2O based on the two mixed-ligand of ttcH3and 4-amino-3,5- bis(pyridine-3-yl)-1,2,4-triazole(3-bpt)[8]. In order to access new multi-dimensional coordination polymers, we choose ttcH3as ligand, which can exist in either thiol or thione tautomeric forms[8-11]acting as a potentially multifunctional bridging ligand[12](Scheme 1). The deprotonated ttcH3(ttc3-) molecule has three N-donor atoms and three S-donor atoms which can behave as either a chelating or a bridging ligand. However, no multi-dimensional networks based on deprotonated ttcH3(ttc3-) only with transi- tion metal cations have been reported so far because the less soluble transition metal sulfides prefer to form during the syntheses[13, 14].
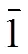
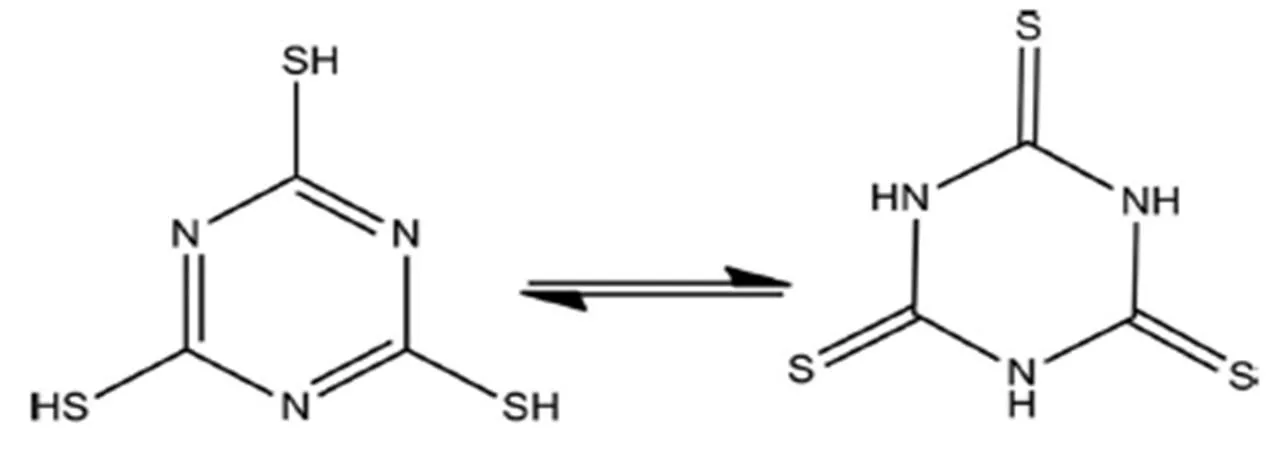
Scheme 1. ttcH3in either thiol or thione form
2 EXPERIMENTAL
2. 1 Materials and instruments
All the chemicals with analytical grade were purchased from commercial sources and used without any further purification. The energy disper- sive spectroscopy (EDS) was carried out using a JEOL JSM-5600LV scanning electronic microscope. The morphology and dimensions of the compound (PyH)[Cu2(ttc)] were observed by scanning electron microscopy (SEM, SU8200). Infrared (IR) spectrum was measured using KBr pellet technique and recorded on a Nicolet 6700 spectrometer from 4000 to 400 cm-1under room temperature. The UV-vis spectrum was obtainedby the JASCO V-550UV/Vis. The TGA was carried outon a TA-Q50 thermogravi- metric analyzer under nitrogen atmosphere at a heating rate of 10 ℃/min. Power X-ray diffraction (XRD) was recorded with a D/MAX-2400 diffrac- tometer with Moradiation (= 0.71073 nm) at 40 kV and 30 mA. Fluorescence measurements were performed by a Perkin-Elmer LS55 fluorescence spectrophotometer.
2. 2 Synthesis of (PyH)[Cu2(ttc)]
A mixture of CuSPh (5 mg, 0.0289 mmol), ttcH3(5 mg, 0.0282 mmol) as reactants was placed in a Pyrex glass tube(12 cm length and 7 mm i.d.), to which the mixed solvent of thiophenol and pyridine with a ratio (16mg/364mg) was added. Then, the tube was sealed and placed in a stainless-steel autoclave and heated at 100 ℃ for 5 days, followed by cooling naturally to ambient temperature. After washing several times with ethanol and drying in air, dark-red blocks were obtained with the yield of 50% based on Cu.
Thiophenol (PhSH) plays an important role as the mineralizer in the synthesis of the compound (PyH)[Cu2(ttc)]. Thiophenol can dissolve copper sulfide under amine alkaline conditions and can speed up crystallization. Otherwise, the pure product will not be obtained[12]. We attempted to use ethyl- enediamine (H2NCH2CH2NH2) instead of pyridine (C5H5N) to serve as solvent, but a large quantity of copper sulfide was formed probably due to decomposition of the ligand. We also have failed to carry out the reaction with CuI instead of CuSPh, and from this it can be sure that PhS-groups play a mineralizer role.
2. 3 X-ray structure determination
A single crystal of compound (PyH)[Cu2(ttc)] was selected and mounted on a Bruker Smart APEX II diffractometer equipped with graphite-monochromi- tized Moradiation (= 0.71073Å) for intensity data collection at room temperature. The structure was solved by direct methods and refined by direct methods with SHELXTL[16-18]. 2224 reflections were collected in total, and 1558 were observed with> 2() using an-scan mode (–9≤≤9, –9≤≤9, –12≤≤12). The final= 0.0654 and= 0.1936. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were added in the riding model without refinement. The selected bond dis- tances and bond angles of the compound (PyH)[Cu2(ttc)] are listed in Table 1.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
Symmetry transformations used to generate the equivalent atoms: A: –, –+1, –;
3 RESULTS AND DISCUSSION
3. 1 Structural description
In compound(PyH)[Cu2(ttc)], each S atom in a-2 bridging mode links adjacent Cu+ions, thereby generating a 2layer structure, in which pyH+(protonated pyridine) ions reside (Fig. 2). The structure of an anionic layer is shown in Fig. 3. Interestingly, two triazine rings donating S1 and N1 atoms to the same Cu(I) are virtually parallel. The distance between triazine parallel rings is 3.09 Å, which is shorter than that found in [Cu4(dppn)4][CF3SO3]4(3.47 Å)[22].
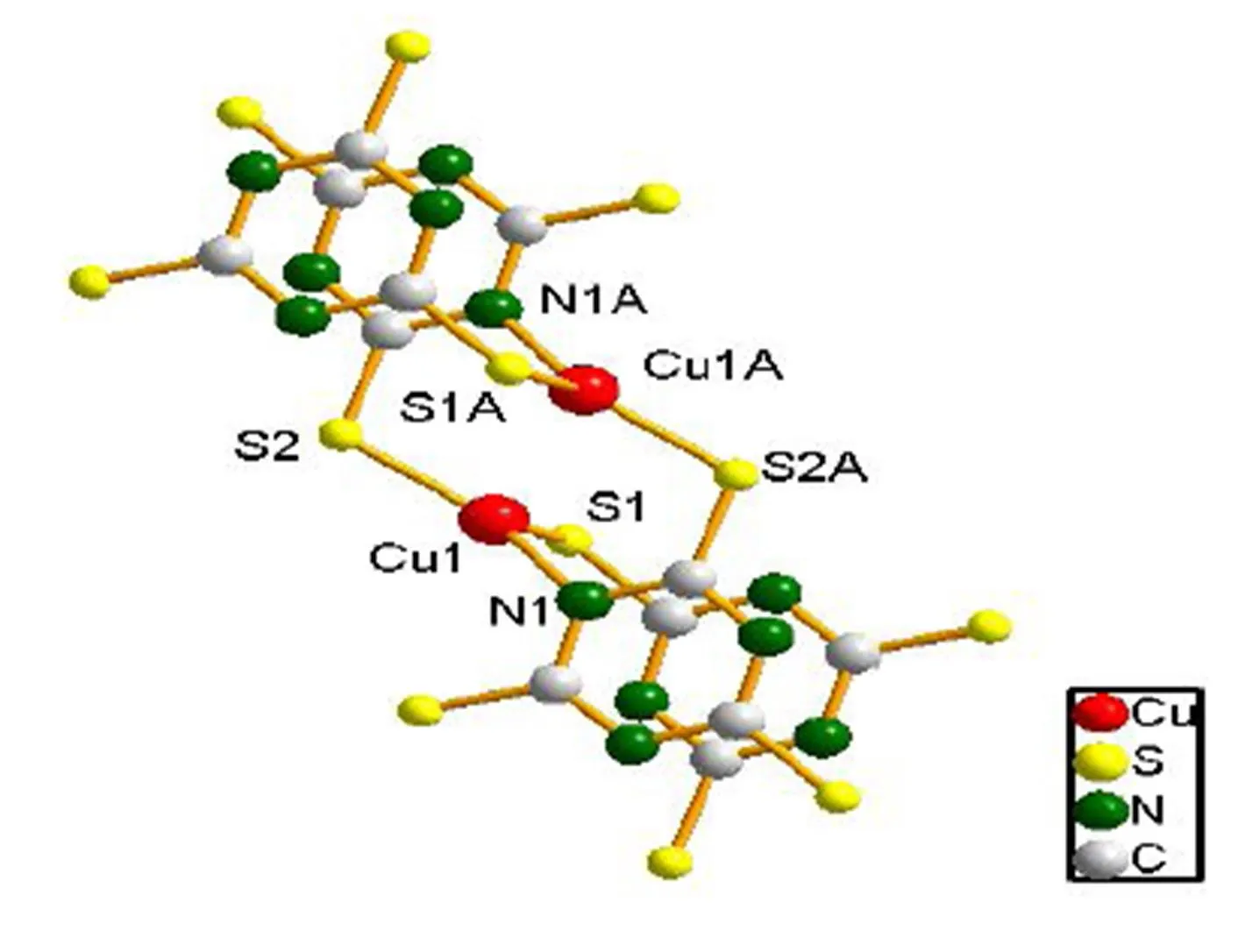
Fig. 1. Compound (PyH)[Cu2(ttc)] coordination environment diagram
Fig. 2. 2stacking of the compound (PyH)[Cu2(ttc)]. Hydrogen atoms are omitted for clarity
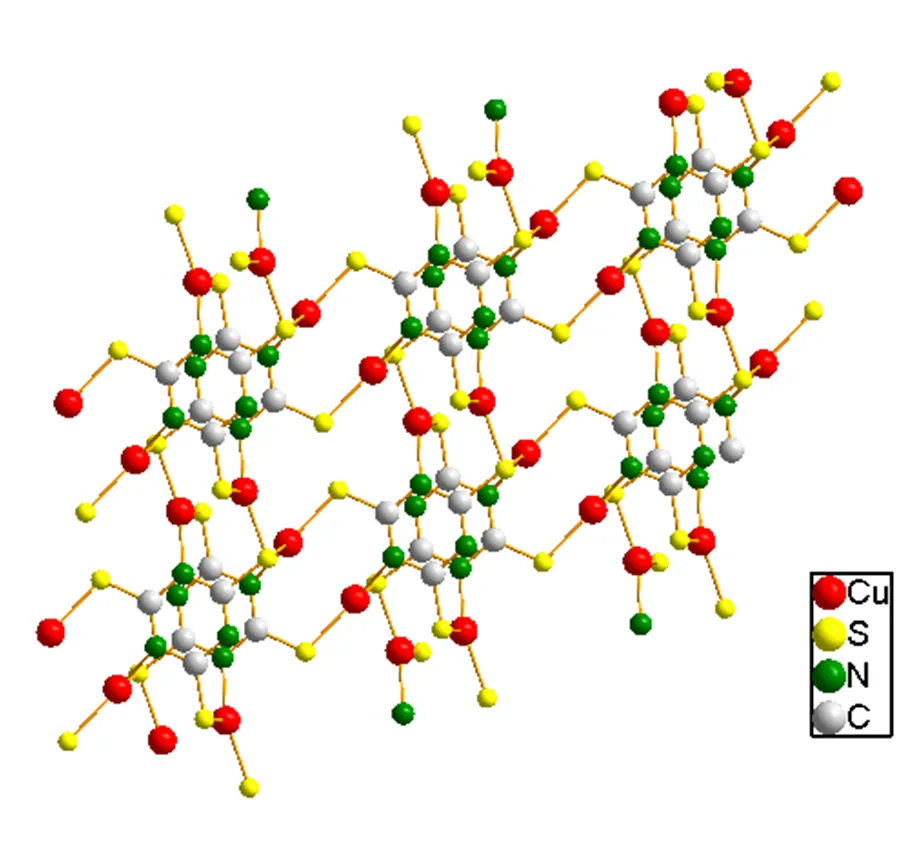
Fig. 3. An anionic layer of [Cu2ttc]-
3. 2 Characterizations
3. 2. 1 Powder X-ray diffraction
The crystalline phase purity of compound (PyH)[Cu2(ttc)] was confirmed by powder X-ray dif- fraction. Obviously, the experimental XRD pattern of this compound is in good agreement with that simulated from single-crystal X-ray data (Fig. 4). Kept in dark place, the compound (PyH)[Cu2(ttc)] is quite stable towards air, which was confirmed by powder X-ray diffraction.
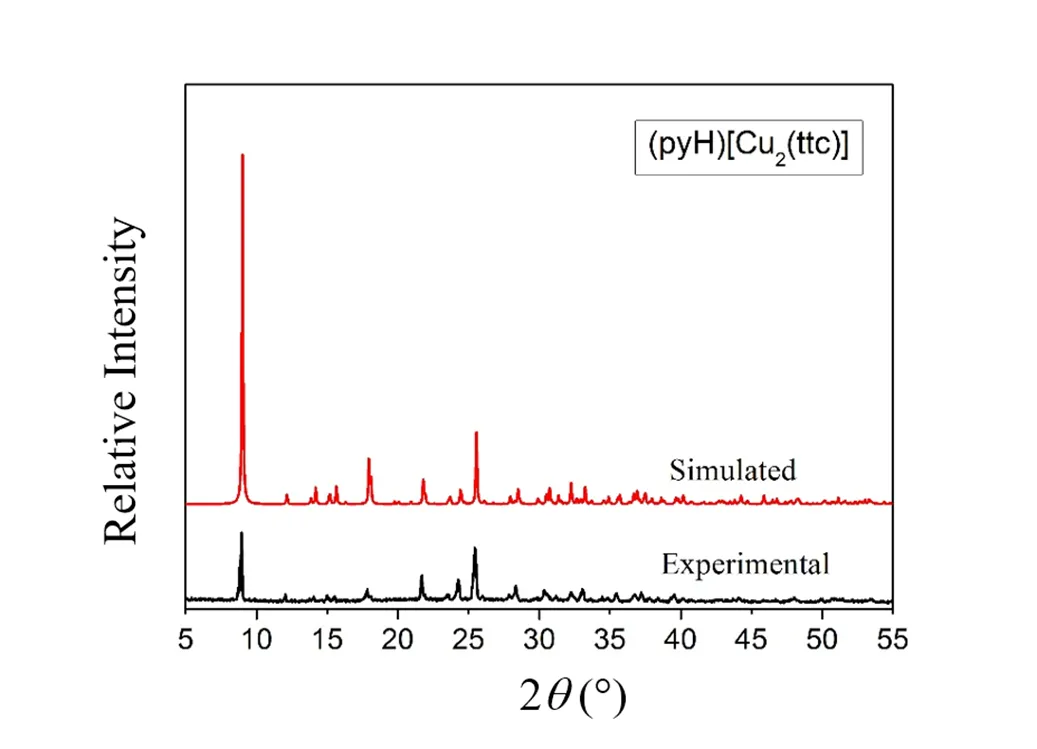
Fig. 4. Experimental and simulated PXRD patterns of compound (PyH)[Cu2(ttc)]

Fig. 5. SEM photograph of the compound (PyH)[Cu2(ttc)]
Fig. 6. EDS analysis of the compound(PyH)[Cu2(ttc)]
3. 2. 3 IR spectroscopy
Fig. 7 provides the information of IR spectrum. The absorption peakappearsat 3070 cm-1, corres- ponding to the (C–H)PyH+stretching vibration of pyridine[23]. The peak at 1600 cm-1is attributed to the stretching vibration of cyanuric ring vibrations[23]. The peak at 1550 cm-1isusually associated with the deformation vibration of (N–H)PyH+. The band near 1150 cm-1can overlap with the bands of (C=S)ttc3-stretching vibration[24]. Two peaks at 780 and 740 cm-1belong to the stretching vibration of S-triazine ring[25].
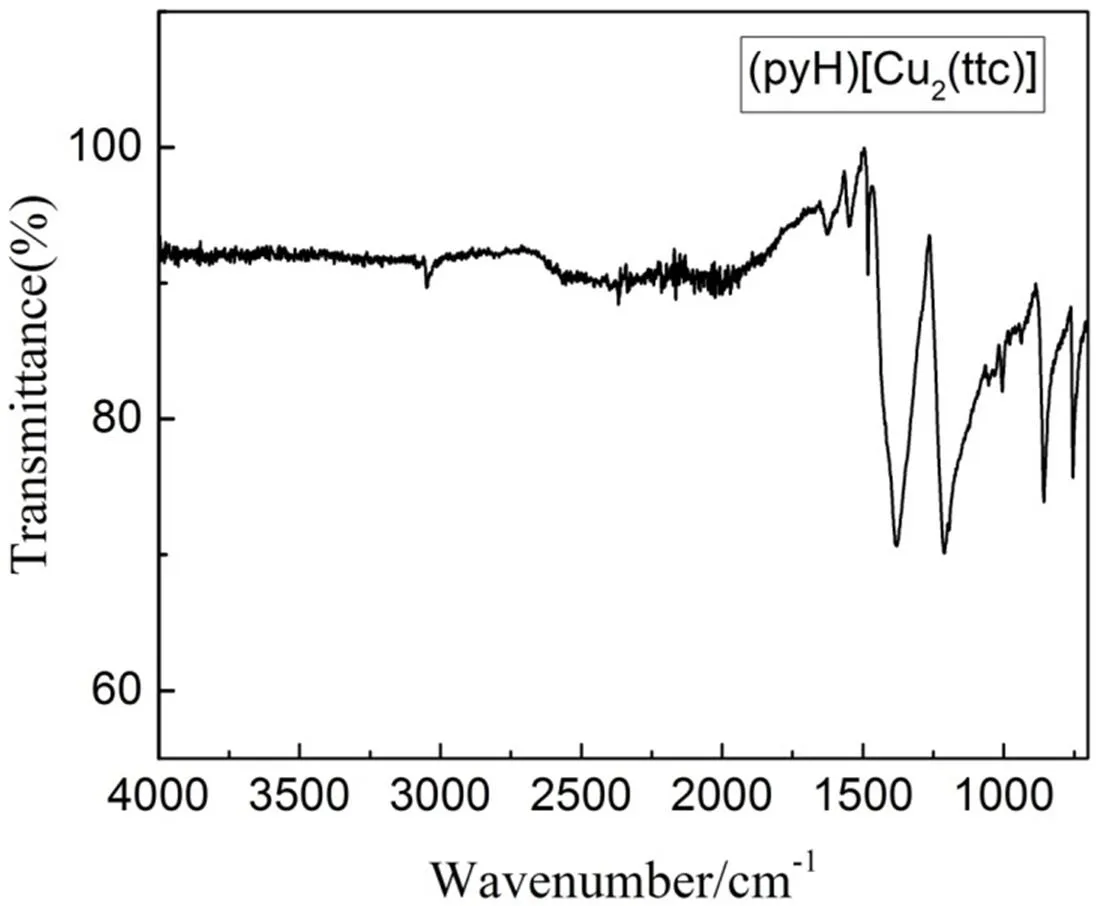
Fig. 7. Absorption spectrum of the compound (PyH)[Cu2(ttc)]
3. 2. 4 Thermogravimetric analysis
To characterize the thermal stability of compound (PyH)[Cu2(ttc)], TG curves have been obtained from crystalline samples in the flowing nitrogen (40 mL/min) at a heating rate of 10 ℃/min (Fig. 8). As expected, compound (PyH)[Cu2(ttc)] exhibits several steps of weight loss. The first weight loss process is from 100 to 270 ℃ with a corresponding weight of 19%, which is basically consistent with the theoretical calculation value of pyridine loss (20.7%). The loss processes ranging from 270 to 610 ℃ show a corresponding weight loss of 39%, which is close to the theoretical value of part ligand ttc3-loss (37.1%) to yield Cu2S.
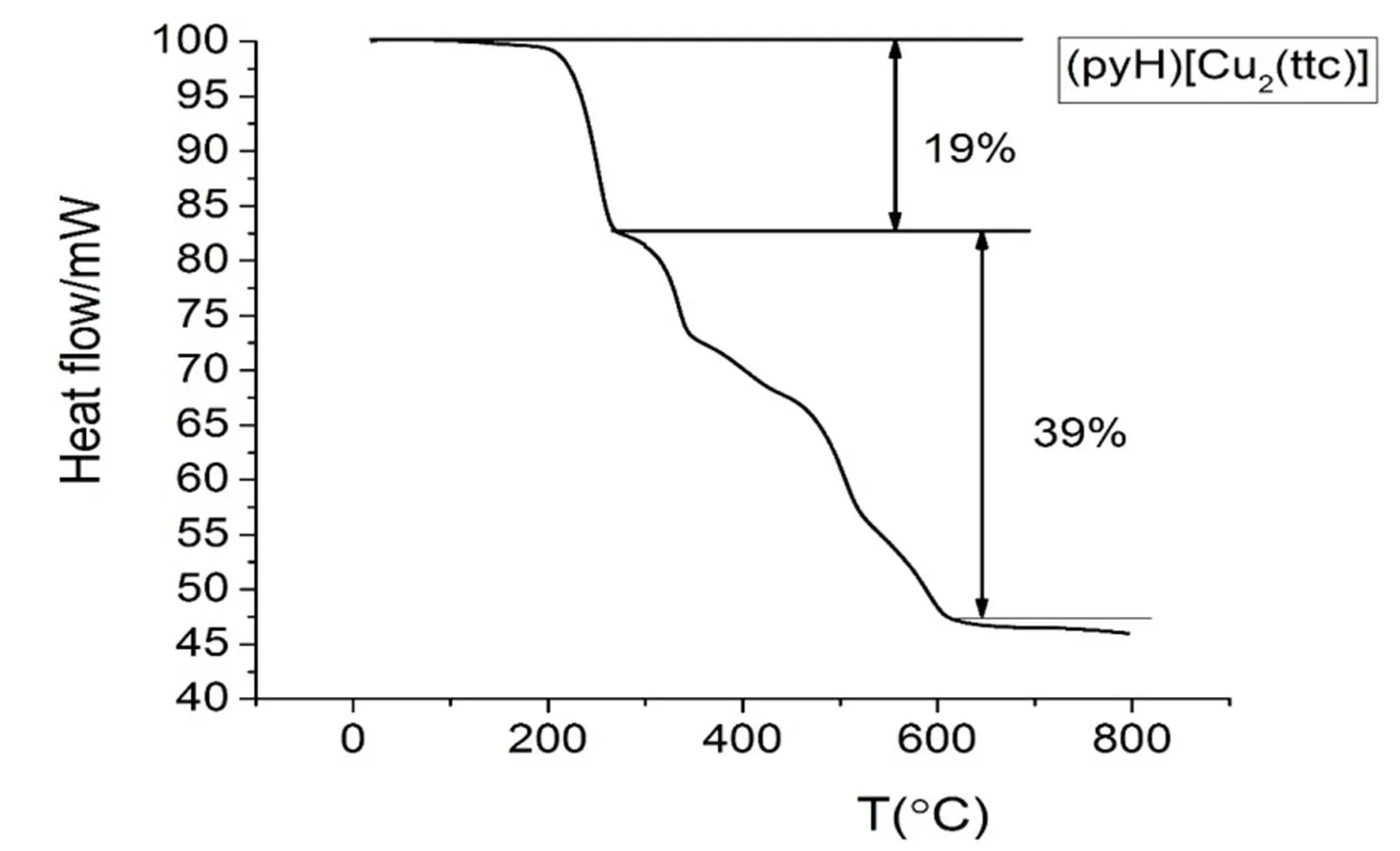
Fig. 8. TG curve for the compound (PyH)[Cu2(ttc)]
3. 2. 5 Uv-visible diffuse reflectance spectroscopy The Uv-vis reflectance spectrum was measured by the JASCO V-550UV/Vis with an integrating sphere at 296 K, and a BaSO4plate was used as reference. The reflectance data collected were converted to the adsorption data using the Kubelka-Munk functions. Fig. 9 shows the solid-state optical absorption spec- trum of (PyH)[Cu2(ttc)]. The curve suggests the band gap of compound (PyH)[Cu2(ttc)] is 1.93 eV, which indicates the compound is a semiconductor absorbing light with wavelengths less than 642 nm[19].
.
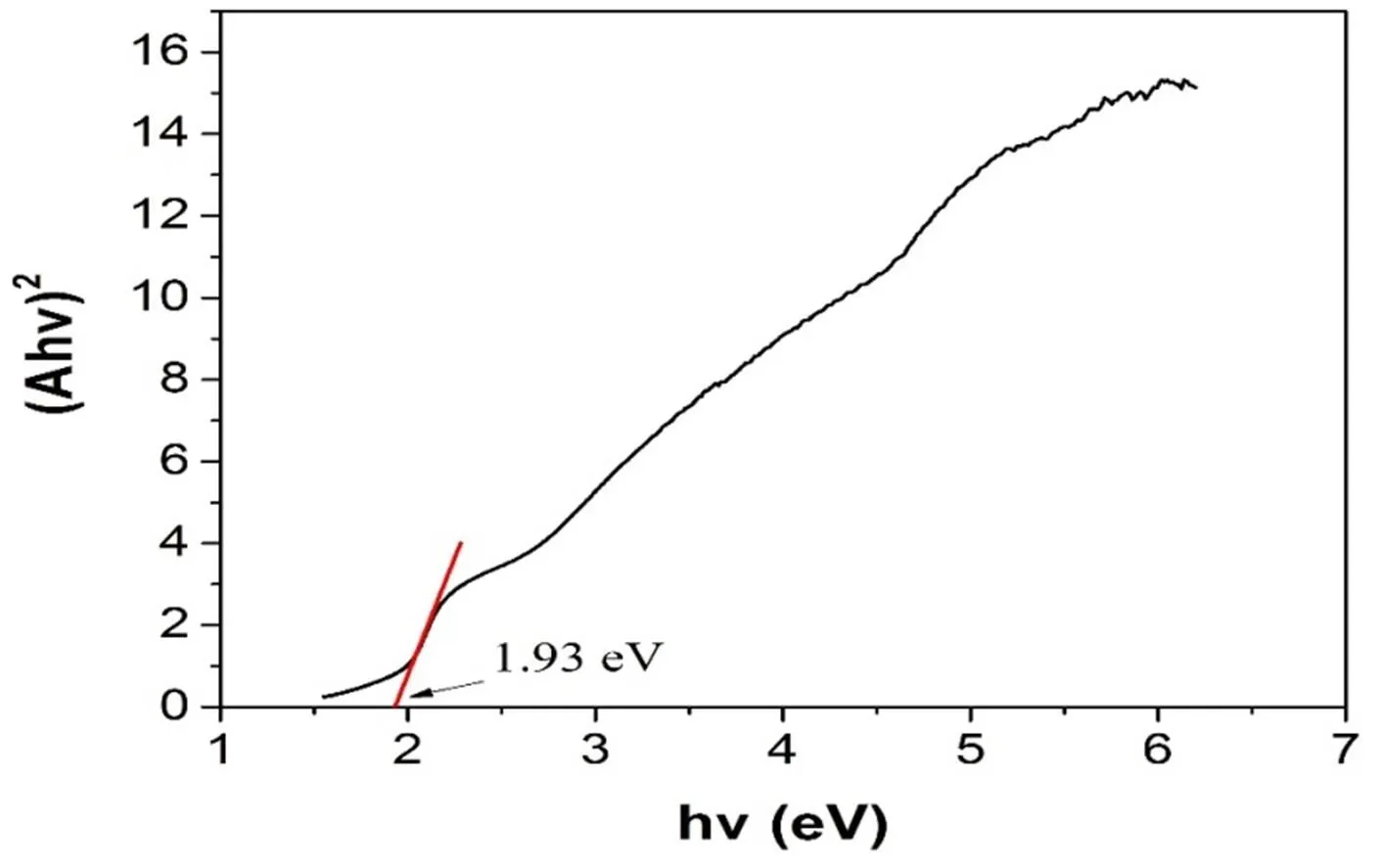
Fig. 9. Absorption spectrum of compound (PyH)[Cu2(ttc)]
3. 2. 6 Fluorescent analyses
The luminescent behaviors of compound (PyH)[Cu2(ttc)] as well as free ligand ttcH3have been measured in the solid state at room temperature, as shown in Fig. 10.The ligand ttcH3shows fluore- scent emission at 542 nm (ex= 400 nm) and the similar emission at 537 nm (ex= 400 nm) of (PyH)[Cu2(ttc)] also be seen, which may be caused by→*of the triazine rings. Another obvious peak at 623 nm is contributed to the ligand-to-metal (ttc3-→ Cu) charge transfer in nature[7].
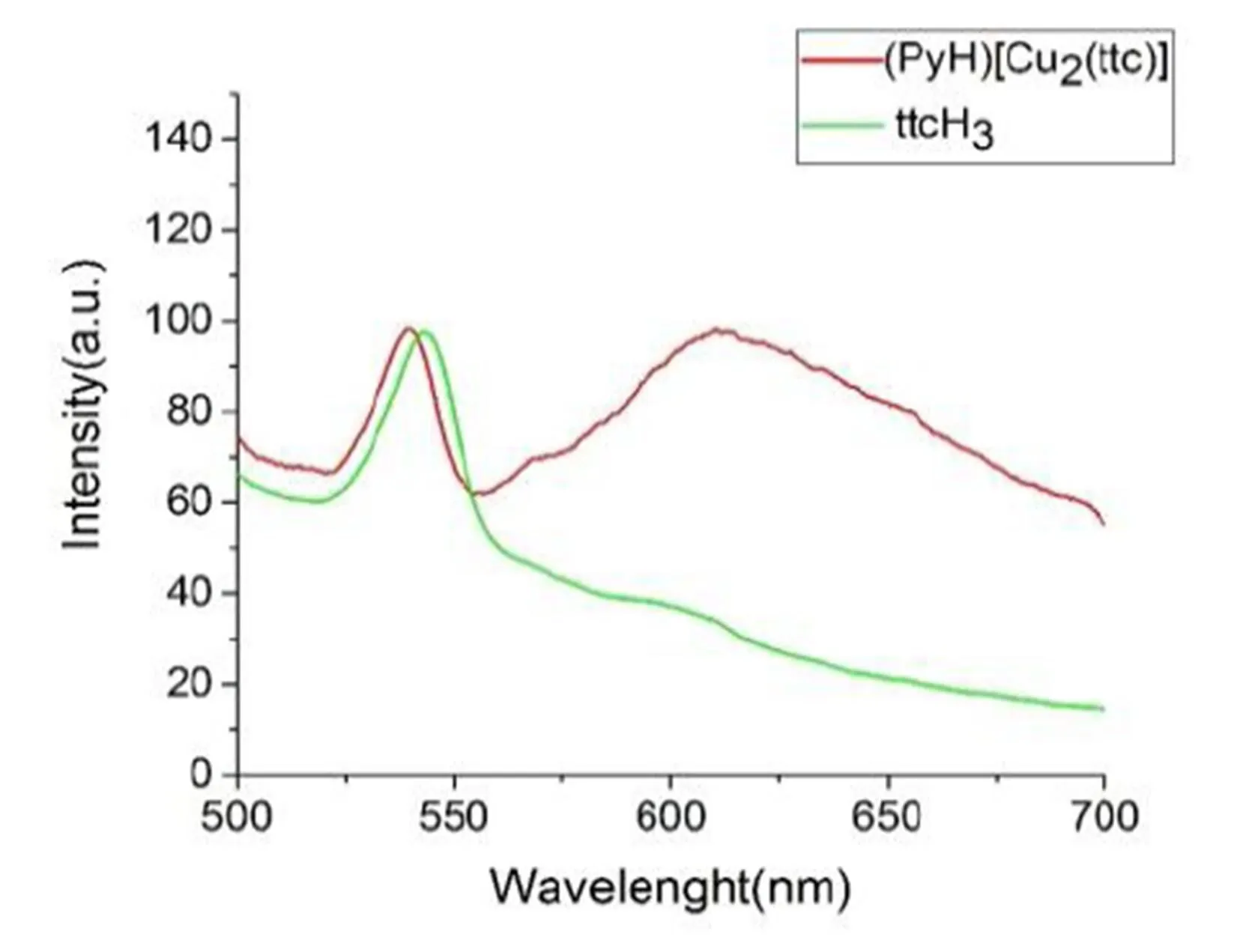
Fig. 10. Solid state fluorescence emission spectra of ttcH3and compound (PyH)[Cu2(ttc)]
4 CONCLUSION
In conclusion, a new 2compound (PyH)[Cu2(ttc)] was solvothermally synthesized in the presence ofmineralizer HSPh under a mild condition, and the pure (PyH)[Cu2(ttc)] shows a semiconductor pro- perty with a band gap of 1.93 eV, which provides potential applications in catalysis. (PyH)[Cu2(ttc)] also showed two types of intense fluorescent emis- sions, which enriches the possibility of exploring for potential luminescent materials.
(1) Matlock, M. M.; Henke, K. R.; Atwood, D. A. Aqueous leaching properties and environmental implications of cadmium, lead and zinc trimercaptotriazine (TMT) compounds.. 2001, 35, 3649-3655.
(2) Henke, K.; Atwood, D. A. Group 2 complexes of 2,4,6-trimercaptotriazine (TMT).. 1998, 37, 224-227.
(3) Henke, K. R.; Robertson, D.; Krepps, M. K. Chemistry and stability of precipitates from aqueous solutions of 2,4,6-trimercaptotriazine, trisodium salt, nonahydrate (TMT-55) and mercury(II) chloride.. 2000, 34, 3005-3013.
(4) Ogawa, K.; Nakata, K.; Ichkawa, K. Molecular structures of zinc complexes with bisbenzimidazole ligands in crystals and the kinetics of ligand-exchange reactions in their solutions.. 1997, 70, 2925-2933.
(5) Lobana, T. S.; Sharma, R.; Sharma, R. Versatility of heterocyclic thioamides in the construction of a trinuclear cluster [Cu3I3(dppe)3(SC5H4NH)] and CuI linear polymers {Cu6(SC5H4NH)6I6}·2CH3CN and {CuI6(SC3H6N2)6X6}(X = Br, I).. 2005, 44, 1914-1921.
(6) Li, D.; Shi, W. J.; Hou, L. Coordination polymers of copper(I) halides and neutral heterocyclic thiones with new coordination modes.. 2005, 44, 3907-3913.
(7) Chan, C. K.; Cheung, K. K. Structure and spectroscopic properties of a luminescent inorganic cyclophane from self-assembly of copper(I) and two ligand components.. 1996, 2, 227-228.
(8) Hu, X. D.; He, X. X.; Guo, Y. M. Two Zn(II) coordination complexes assembled by trithiocyanuric acid and two different N-donor auxiliary ligands.2014, 70, 764-769.
(9) Cecconi, F.; Ghilardi, C. A.; Midollini, S. Organomercury derivatives of the 2,4,6-trimercaptotriazine (H3TMT). X-ray crystal structure of (HgMe)3(TMT).. 2002, 645, 101-104.
(10) Bailey, J. R.; Hatfield, M. J.; Henke, K. R. Transition metal complexes of 2,4,6-trimercapto-1,3,5-triazine (TMT): potential precursors to nanoparticulate metal sulfides.. 2001, 623, 185-190.
(11) Mahon, M. F.; Molloy, K. C.; Venter, M. M. Unsymmetrically-substituted 2,4,6-trimercaptotriazine: supramolecular self-assembly through C–S⋯ H–N hydrogen bonds in the crystal structures of C3N3S3H2Na·3H2O and C3N3S3H2Cu(PPh3)2.2003, 348, 75-81.
(12) Kopel, P.; Cermakova, S.; Dolezal, K. Synthesis and properties of a trinuclear copper(II) complex with trithiocyanurate bridge.. 2007, 81, 327-328.
(13) Zhang, C. Mild solvothermal syntheses of thioargentates A–Ag–S (A = K, Rb, Cs) and A−Ag−Ge−S (A = Na, Rb): crucial role of excess sulfur.. 2013, 52, 12367-12371.
(14) Zhang, R. C.; Yao, H. G.; Ji, S. H. (H2en)2Cu8Sn3S12: a trigonal CuS3-based open-framework sulfide with interesting ion-exchange properties..2010, 46, 4550-4552.
(15) Yao, H. G.; Zhou, P.; Ji, S. H. Syntheses and characterization of a series of silver-thioantimonates(III) and thioarsenates(III) containing two types of silver-sulfur chains..2009, 49, 1186-1190.
(16) Sheldrick, G. M.. University of Göttingen, Germany1997.
(17) Sheldrick, G. M.. University of Göttingen, Germany1997.
(18) Sheldrick, G. M. Crystal structure refinement with SHELXL.2015, 71, 3-8.
(19) Li, Z. H.; Du, S. W. A new layer W/Cu/S polymer with heterobimetallic nodes and tridentate trithiocyanuric acid.. 2010, 1, 1874-1878.
(20) Li, D.; Wu, T.; Zhou, X. P. Twelve-connected net with face-centered cubic topology: a coordination polymer based on [Cu12(4-SCH3)6]6+clusters and CN−linkers.. 2005, 44, 4175-4178.
(21) Kitagawa, S.; Kawata, S.; Nozaka, Y. Synthesis and crystal structures of novel copper(I) co-ordination polymers and a hexa copper(I) cluster of quinoline-2-thione.. 1993, 9, 1399-1404.
(22) Youinou, M. T.; Rahmouni, N.; Fischer, J. Self-assembly of a Cu4complex with coplanar copper(I) ions: synthesis, structure, and electrochemical properties.. 1992, 31, 733-735.
(23) Clark, R. J. H.; Williams, C. S. The far-infrared spectra of metal-halide complexes of pyridine and related ligands.. 1965, 4, 350-357.
(24) Kucharski, M.; Chmiel, S. E. Reactions of trithiocyanuric acid with oxiranes. I. Synthesis of polyetherols.. 2000, 76, 439-445.
(25) Kopel, P.; rávníček, Z.; Kvítek, L. Coordination compounds of nickel with trithiocyanuric acid. Part IV. Structure of [Ni(pmdien)(ttcH)] (pmdien = N,N,N΄,N΄,N΄΄-pentamethyldiethylenetriamine, ttcH3= trithiocyanuric acid).. 2001, 26, 282-286.
20 March 2018;
16 May 2018(CCDC 1825628)
① This research is supported by National Natural Science Foundation of China (21770643)
.E-mail: ylan@dlut.edu.cn
10.14102/j.cnki.0254-5861.2011-2011
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

