Synthesis and Characterization of Compounds Containing 1,2,3-Triazole via Click Reaction and Ag(I) Complex①
LI Wen-Si FENG Sheng-Yu
Synthesis and Characterization of Compounds Containing 1,2,3-TriazoleClick Reaction and Ag(I) Complex①
LI Wen-Si FENG Sheng-Yu②
(250100)
In this work, 1,4-bis(4-phenyl-1,2,3-triazole)benzene, 1,3-bis(4-phenyl-1,2,3-tria- zole)propane, bis(1-phenyl-1,2,3-triazole)-methylphenylsilane, and 1-ally-4-phenyl-1,2,3-triazole have been designed and synthesized via Click reaction. Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance spectroscopy (NMR) were used to confirm the compounds’ structures. The effect of silicon atom on the optical properties has also been studied. The UV-vis absorption wavelength of silicon-containing compound is about. 10 nm red-shifted when compared with that of other three compounds. The fluorescence emission bands of the compounds in CHCl3solutions were observed around440 nm. And the luminescent coordina- tion compound, namely [AgL1·NO3·3H2O]n, based on the ligand 1-allyl-4-phenyl-1,2,3-triazole has been prepared. In addition, this complex exhibits a 1D chain structure. The crystal structure has been determined by single-crystal X-ray diffraction, and the optical properties have been investigated byfluorescence spectrum. In summary, our work may provide new materials with luminescent property which is potentially useful in material fields.
Click reaction, silicon-containing 1,2,3-triazole compounds, crystal structure;
1 INTRODUCTION
In recent years, the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction has been widely acknowledged for different purposes, varying from traditional organic synthesis to excellent materials[1, 2]. In 2001, Sharpless reported the reaction of azido compounds and high active alkyne, which is fast, reliable and steady[3, 4].Then Sharpless[5]also found the catalyzed effect of Cu(I) on the reaction of inactive alkyne and azido compounds, and came up with the conception of "Click chemistry". The Click reaction possesses characteristics as follows: mild reaction conditions, simple operations, fast reactive rate, high yield, single configuration products and immune from the effects of oxygen, water and other factors[4].And now, the Click reaction has appealed to more and more researchers because of its 1,2,3- triazolyl unit. Different kinds of ligands containing silicon atoms or 1,2,3-triazolyl units have received much attention owing to their specific and selective properties. With the development of technology, it has been demonstrated that CuAAC method can be used to generate a functionalized 1,2,3-triazole Click ring in excellent yields due to its wide applications in both material and pharmaceutical chemical industry.
Nowadays, luminescent metal-organic frameworks have continued to engender great interest due to their potential applications in molecular recognition, selective gas adsorption and gas storage[6-9]. In the meantime, designed synthesis containing 1,2,3-tria- zolyl ring organic ligands and relevant coordination compounds has also attracted researchers’ interest. Previously, most researchers have focused on the coordination of N3 located in 1,2,3-triazolyl ring and metal ions[10-14]. However, people now have moved on to the coordination of N2 located in the 1,2,3- triazolyl ring and metal ions[15-17]. The 1,2,3-triazole prepared by CuAAC Click reaction has provided us an effective method to construct more novel com- plexes and supramolecular functional systems.
It’s known to all that Ag+is a good candidate especially in the purposeful construction of lumine- scent metal-organic frameworks owing to its fantastic coordination ability. Ag(І) complexes with their metal cations adopting10configuration have drawn more and more attention because of their stronger fluorescence properties and potential appli- cations as functional luminescent materials[18]. For instance, according to literatures some kinds of Ag(І) complexes can be employed as organic light- emitting diodes (OLEDs) and “turn-on” fluorescent sensors[19].Recently, a great number of novel ligands have been explored and then constructed into metal-organic frameworks via coordination bonds or supramolecular interactions such as hydrogen bonds and-stacking.
This paper reports a series of compounds con- taining 1,2,3-triazolyl units prepared via Click reac- tion and their optical properties have been investi- gated by means of ultraviolet and fluorescence spec- tra. The absorption maximum of phenyl units of C3 is around 285 nm, and there is 5 to 10 nm red-shift compared to other compounds prepared in this work. This result could be ascribed to the silicon atom’s influence on the electrondelocalization.
As is well-known, Ag+is readily able to coor- dinate to two or six ligands. For further study, the author has constructed a novel complex, namely, [AgL1·NO3·3H2O]n, which has not been reported yet. Two ligands bridge each other through the Ag(I) ions center and lead to a 1D chain structure. In order to obtain more information about optical properties of this complex, luminescent property has been inves- tigated, which indicates strong fluorescent emissions.
2 EXPERIMENTAL
2. 1 General
Anhydrous tetrahydrofuran (THF) and diethyl ether (Et2O) were freshly distilled over sodium and benzophenone before use. 1,4-Diazidobenzene was prepared according to the reported procedure[16].1,3-Diazidopropane, 1-azidobenzene and 3-azidopro- pene were prepared by modifying the reported procedure[17]. Diethynylmethylphenylsilane was prepared from reported papers elsewhere[18, 19]. Trimethylsilylacetylene and other chemicals were purchased from Aldrich and used as received unless otherwise noted[20].
FT-IR spectra were recorded with a Bruker Tensor27 spectrophotometer.1H NMR and13C NMR spectra were measured using CDCl3and DMSO-d6as solvents on a Bruker AVANCE-300 NMR Spec- trometer. UV-vis absorption and fluorescence spectra were analyzed with UV-7502PC and ISS K2-Digital spectrophotometers, respectively. Fluorescence quantum yields were measured using quinine sulfate in 0.1 N H2SO4(Ф = 54.6%) as standard. TGA was carried out under nitrogen flow at a heating rate of 10 ℃/min on a MettlerToledo SDTA-854 TGA system.
2. 2 Preparation of compounds containing 1,2,3-triazolyl units Preparation of C1
C1 was prepared according to the reported pro- cedure[18]. Data for C1: pale yellow solid (Yield = 48%);1H NMR (in DMSO-d6) 9.47 (s, 2H), 7.97(d, 4H), 7.53 (d, 4H), 7.3 (m, 6H); FT-IR (KBr plate) 3126(=CH-N), 3058, 1518, 845, 821cm-1. Anal. Calcd. (%) for C22H16N6: C, 72.53; H, 4.40; N, 23.07. Found (%): C, 72.61; H, 4.41; N, 22.98.
Preparation of C2
1,3-Diazidopropane (1 mmol, 0.126 g), phenyl- acetylene (2 mmol, 0.204 g), CuSO4(2mol%, 0.0032 g), sodium ascorbate (10 mol%, 0.0176 g) and water/TBA were stirred for 24 h at 70 ℃. Then the mixture was poured into EtOAc and washed with H2O, and dried over MgSO4, filtered and distilled under reduced pressure. Then the crude product was purified and gave a pale yellow solid (Yield: 35%).1H NMR (in DMSO-d6) 7.9 (s, 2H, C=CH), 7.49 (d, 4H, ArH), 7.34 (d, 4H, ArH), 7.2 (s, 4H, ArH), 3.76(s, 2H, CH2), 2.3(d, 2H, CH2); FT-IR (KBr plate) 3128(=CH-N), 1538, 1268, 768 cm-1. Anal. Calcd. (%) for C19H18N6: C, 69.09; H, 5.45; N, 25.45. Found (%): C, 69.22; H, 5.51; N, 24.27%.
Preparation of C3
The mixture of diethynylmethylphenylsilane (2 mmol, 0.34 g), 1-azidobenzene (2 mmol, 0.244 g), CuI (0.0191 g, 5 mol%), pyridine (0.5 mL) and DMF (5 mL) was stirred for 24 h at 60 ℃ in the dark. Then the mixture obtained was extracted by EtOAc and washed with H2O, then dried over MgSO4, filtered and distilled under reduced pressure. The crude product was purified by a silica gel column (PE:EtOAc = 3:2) to give C3 as a pale yellow solid (Yield: 31%).1H NMR (in DMSO-d6) 8.49 (s, 2H, C=CH), 7.58 (d, 4H, ArH), 7.46 (d, 4H, ArH), 7.2 (s, 10H, ArH), 0.46(s, 6H, CH3); FT-IR (KBr plate) 3125(=CH-N), 3061, 2961, 1260(Si-CH3), 758 cm-1. Anal. Calcd. (%) for C30H22N6: C, 77.25; H, 4.72; N, 18.03. Found (%): C, 77.06; H, 4.81; N, 18.13%.
Preparation of C4
3-Azidopropene (0.166 g, 2 mmol), phenylacety- lene (2 mmol, 0.204 g), CuSO4(2 mol%, 0.0064 g), sodium ascorbate (10 mol%, 0.0352 g), and water/TBA were stirred for 24 h at 65 ℃. Then the mixture was poured into EtOAc and washed with H2O, and dried over MgSO4, filtered and distilled under reduced pressure. Then the crude product was purified by a silica gel column (PE:EtOAc = 3:2) and gave a pale yellow solid (Yield: 51%).1H NMR (in DMSO-d6) 8.65(s, 1H), 7.87 (d, 2H), 7.45(d, 2H), 7.35 (s, 1H), 6.12 (s, 1H), 5.275 (d, 2H), 5.07(d, 2H); FT-IR (KBr plate) 3132(=CH-N), 3100, 2414 cm-1. Anal. Calcd. (%) for C11H11N3: C, 71.35; H, 5.95; N, 22.7. Found (%): C, 71.20; H, 5.765; N, 21.43.
2. 3 Preparation of [AgL1·NO3·3H2O]n
C4 (0.04 mmol, 7.4 mg) dissolved in CH2Cl2(4 mL) was spread in the under layer of colorimetric tube. Then CH2Cl2(2 mL) and CH3OH (2 mL) were added in order as buffer layer in the middle of the tube. At last, AgNO3(0.08 mmol, 13.6 mg) dissolved in CH3OH (4 mL) was spread above the buffer layer. The tube was placed in the dark place and colorless crystal was produced in the tube after two weeks (Yield = 67.5%). Anal. Calcd. (%) for C11H17AgN5O6: C, 33.1; H, 4.26; N, 17.5. Found (%): C, 33.2; H, 4.27; N, 17.3.
2. 4 X-ray crystallography
Diffraction intensities for the complex were collected on a Bruker SMART 1000 CCD diffrac- tometer with graphite-monochromatic Mo-radia- tion (= 0.7103 Å) by using the-2scan technique. The structure was solved by direct methods using the program SHELXS-97 and refined with full-matrix least-squares techniques using the program SHELXL-97. Continuously different Fourier trans- forms and corrections were assigned to all non-hy- drogen atoms. The organic hydrogen atoms of benzene rings were generated geometrically. The selected hydrogen bond lengths and bond angles for the complexes are listed in Table 1.
3 RESULTS AND DISCUSSION
3. 1 Synthesis of compounds containing 1,2,3-triazolyl units via Click reaction
It is known to all that the Cu(I) catalyst is very important in the Click reaction, and for clarity, we divide the obtaining methods of Cu(I) catalyst into A and B. As shown in Scheme 1, group of CuI, polar solvents, such as DMF and DMSO, and pyridine is one approach called method A; group of CuSO4, sodium ascorbate and water/TBA is the other approach called method B.
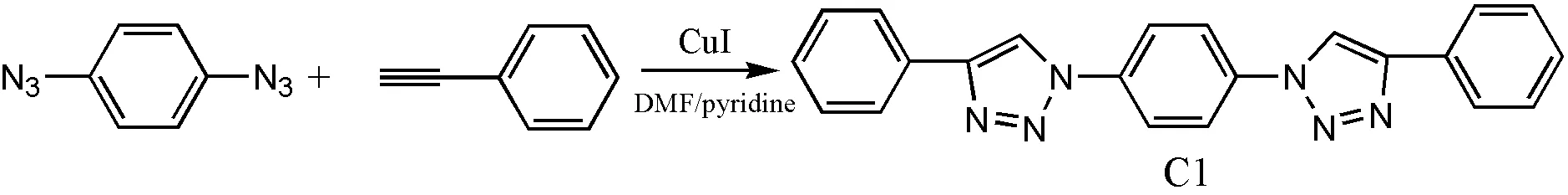
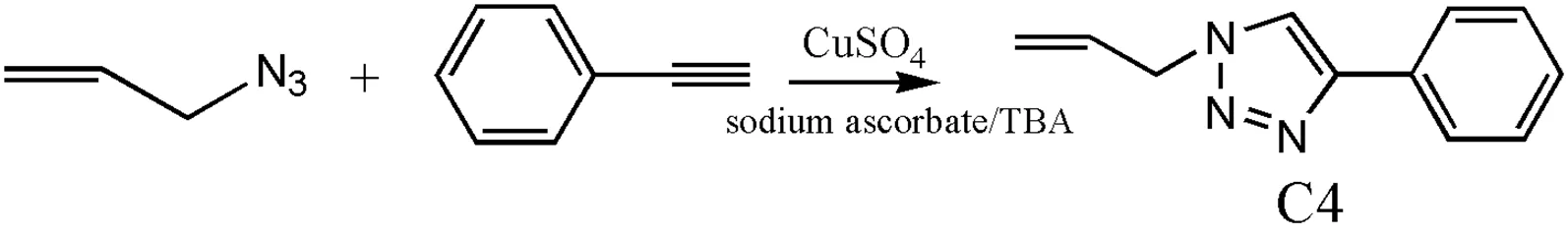
Scheme 1. Syntheses of compounds containing 1,2,3-triazolyl units
3. 2 Optical properties
In Fig. S1, the absorption maximum wavelengths of all compounds show around 255 nm, attributed to-*electron transition of triazolyl units[18]. In addition, the absorption maximum of the phenyl unit of C3 is around 285 nm, and there is about 5 to 10 nm red-shift compared to those of other compounds. This result illuminates vacant 3-orbitals of silicon atoms have certain effects on the conjugation with adjacent π-conjugated systems. Thereby, the red-shift can be assigned to the electron delocalization of theelectron segments[19]. However, differences in the absorption of phenyl units between the silicon-con- taining compound and the compounds without silicon atoms are not obvious. This indicates that the silicon atom can influence the electron delocalization, but unfortunately, electronic transitions are not strong enough to connect the adjacent units.
In Fig. S2, the fluorescence maxima of compounds are observed at the similar peaks440 nm when excited at 350 nm[18]. Owing to its more phenyl and 1,2,3-triazolyl units, the fluorescence intensity of C3 is stronger than that of other compounds. The author thinks this result should be assigned to two factors: one is that the silicon atom contributes an electronic communication, and the other is that-conjugation can effectively enhance the emission efficiency. The emission wavelength of C3 is about 5 nm blue-shift in comparison with those of other compounds, and this result indicates electron transitions between silicon atom and aromatic segments[18].
In summary, the above results show that the silicon atoms have certain effects on the electronic com- munication with the adjacent-conjugated segments, nevertheless the influence is limited. Meanwhile, the fluorescence intensity was enhanced when there are more aromatic and 1,2,3-triazolyl units in the compounds due to the-conjugation.
As shown in Fig. S3, at room temperature, upon excitation of solid samples of the ligand C4 and the Ag+complex (ex= 265 nm), the emission band maximum of C4 is at410 nm and that of the Ag+complex at400 nm, so there is blue-shift of about 10 nm for the Ag+complex. This cluster-based Ag(I) coordination complex increases the ligand conforma- tional rigidity, thereby reducing the non-radiative decay of the intraligand (-*) excited state. The preparation of this novel Ag(I) complex with 1,2,3- triazolyl unit can be an excellent method for obtai- ning more functional types of luminescent materials.
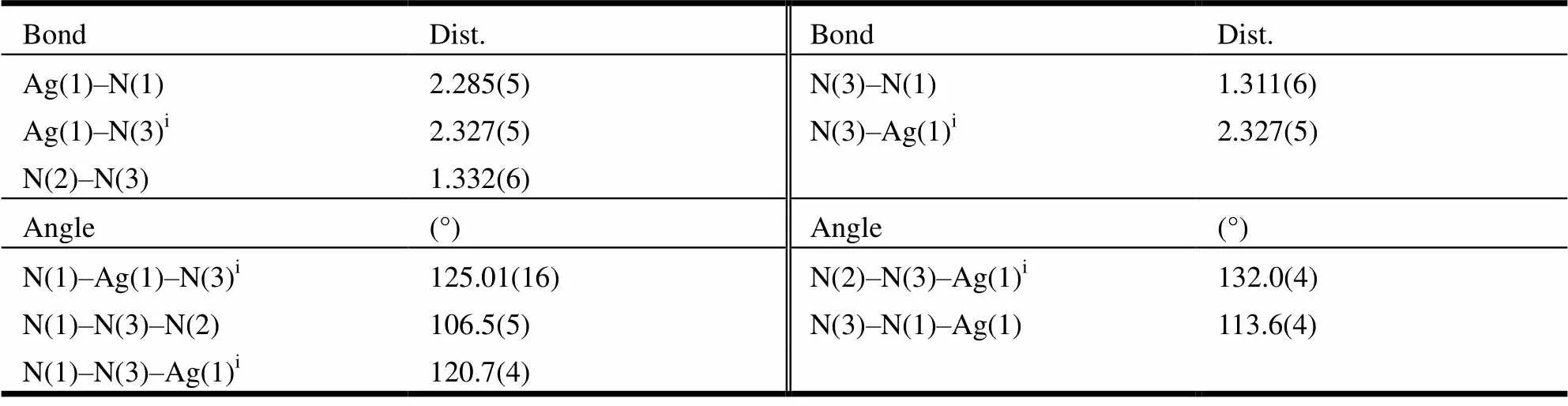
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Complex 1
Symmetry code: (i) −x, −y+1, −z+2
3. 3 Structure description
As shown in Fig. 1, the crystal structure of the complex forms a six-membered ring which is con- nected with two silver ions and four nitrogen atoms. The N(1)–N(3) and N(2)–N(3) bond lengths are 1.311 and 1.332 Å, respectively. The neighboring 1,2,3-triazolyl units are coordinated to Ag(I) through more electron-rich nitrogen atoms. And centered on Ag(I), hexahedron spatial configuration was cons- tructed by two nitrogen and three oxygen atoms. The C–N bond lengths in triazole moiety are typical for triazole rings. For instance, the N(1)–C(8) and N(2)–C(12) both indicate a typical double bond character in 1.3424 and 1.3394 Å, respectively. In addition, the other C–C bond lengths fall in the normal range and it can be concluded that there are two kinds of hydrogen atoms from the bound water and hydrogen atoms at the carbon atoms.
According to Tables 1 and 2, the combination ability of Ag(I) with N3 is stronger due to the bond length ranging from 2.28 to 2.29 Å, with the average to be 2.285 Å. In comparison, the combination ability of Ag(I) with N2 is weaker owing to the bond length varying from 2.3216 to 2.3332 Å, averaged by 2.3274 Å. Therefore, the coordination ability of N3 is much better than that of N2. In addition, according to the data, there is a deviation of 15.54obetween the angle of N×××Ag×××N and the angle of barycenter and two vertexes of standard regular tetrahedron. In Fig. 2, two ligands combined with two Ag atoms have constructed an Ag ion dimer. It is indicated that the nearest Ag×××Ag distance is 5.2900 Å, which is longer than the sum of the van der Waals radii of two Ag atoms (3.56 Å)[1-5]. Therefore, no stabilizing interac- tions exist between Ag···Ag.
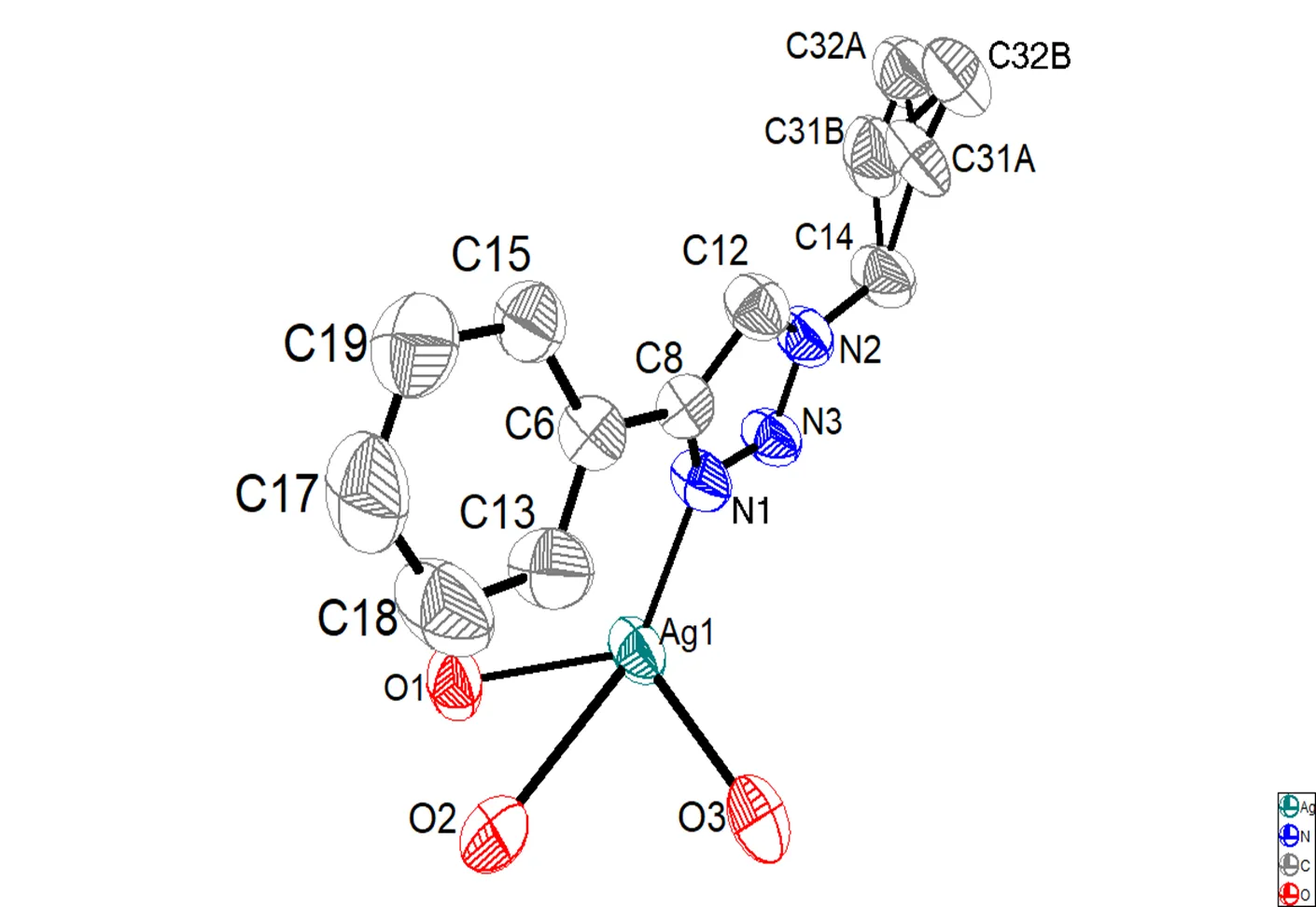
Fig. 1. Molecular structure of the complex
Fig. 2. Packing diagram performs a 1D structure, with water molecules omitted for clarity
3. 4 Thermal properties
Fig. S4 shows thermal stability of the Ag+complex at a heating rate of 10 ℃/min under N2atmosphere. The Ag+complex exhibits normal heat-resistant pro- perties. There is a weight loss of about 6.9% ascribed to the removal of H2O from 27 to 100 ℃ compared to the theoretical value of 5.3%because the complex has some solvents. Then the product keeps the weight constant until 198 ℃ and then a fast weight loss occurs, which is obviously attributed to the break of the complex framework. Comparing the theoretical (27.1%) and actual (27.4%)[18]values indicates that the residue is elemental silver.
4 CONCLUSION
The author has synthesized a series of compounds containing 1,2,3-triazole and prepared the Ag(I) complex based on the ligand C4. Then optical pro- perties of the compounds and the complex have been investigated through ultraviolet and fluorescence spectra. By contrast of the optical properties of com- pound containing silicon atom and compounds without silicon atoms, the effect of silicon atom on the electronic communication has been displayed. Studies of the Ag(I) complex have also been explo- red by different measurements. Other compounds containing silicon atoms and 1,2,3-triazolyl units prepared via Click reaction are currently explored in progress for the potential applications in materials fields.
(1) Zhao, H.; Zhu, Y. Q.; Feng, C. One novel Mn(II) complex with 1-substituted-1H-1,2,3-triazole-4-carboxylic acid: crystal structure, fluorescence and Hirshfeld surface analysis.. 2017, 36, 66–72.
(2) Huo, L. N.; Chen, R.; Liao, Y. F.; Liu, H. G.; Li, P. Y.; Lu, R. M.; Zhong, Z. G. Synthesis, crystal structure and biological evaluation of acridine-1,2,3-triazole derivatives.2016, 35, 698–704.
(3) Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions.2001, 40, 2004–2021.
(4) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes.2002, 41, 2596–2599.
(5) Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radic, Z.; Carlier, P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks.2002, 41, 1053–1057.
(6) Cui, Y. J.; Yue, Y. F.; Qian, G. D.; Chen, B. L. New three-dimensional lanthanide-alkali-heterometallic frameworks constructed from isonicotinic acid: synthesis, structures and properties..2014, 25, 581–590.
(7) Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A. M. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions.2014, 3289–3306.
(8) Schulze, B.; Schubert, U. S. Beyond click chemistry-supramolecular interactions of 1,2,3-triazoles.2014, 43, 2522–2571.
(9) Huisgen, R. In 1,3-dipolar cycloaddition chemistry.1984, 1–176.
(10) Zheng, Z. B.; Wu, R. T.; Li, J. K.; Sun, Y. F. Hydrothermal syntheses and structural characterization of four complexes with in situ formation of 1,2,3-triazole-4-carboxylate ligand.2009, 928, 78–84.
(11) Kolb, H. C.; Sharpless, K. B. The growing impact of click chemistry on drug discovery.2003,24, 1128–1137.
(12) Kharb, R.; Sharma, P. C.; Yar, M. S. Pharmacological significance of triazole scaffold.2011,26, 1–21.
(13) Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Efficient synthesis and in vitro antitubercular activity of 1,2,3-triazoles as inhibitors of mycobacterium tuberculosis.2011,24, 7273–7276.
(14) Tornøe, C. W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides.2002,67, 3057–3064.
(15) Stefani, H. A.; Vasconcelos, S. N.; Manarin, F.; Leal, D. M.; Souza, F. B.; Madureira, L. S.; Zukerman-Schpector, J.; Eberlin, M. N.; Godoi, M. N.; de Souza Galaverna, R. Synthesis of 5-organotellanyl-1-1,2,3-triazoles: functionalization of the 5-position Scaffold by the sonogashira cross-coupling reaction.2013,18, 3780–3785.
(16) Ahmed, M. N.; Hameed, S.; Yasin, K. A.; Arshad, I.; Ihsan-ul-Haq.; Tahir, M. N. Synthesis, crystal structure and antimicrobial activity of ethyl 2-(1-cyclohexyl-4-phenyl-1-1,2,3-triazol-5-yl)-2-oxoacetate.2014,33, 1666–1672.
(17) Ye, L. N.; Wu, C. Z.; Guo, W.; Xie, Y. MoS2 hierarchical hollow cubic cages assembled by bilayers: one-step synthesis and their electrochemical hydrogen storage properties.. 2006, 45, 4738–4740.
(18) Wang, Y.; Wang, D.; Xu, C.; Wang, R.; Han, J.; Feng, S. Y. Click polymerization: synthesis of novel σ-π conjugated organosilicon polymers.2011, 696, 3000–3005.
(19) Fletcher, J. T.; Bumgarner, B. J.; Engels, N. D.; Skoglund, D. A. Multidentate 1,2,3-triazole-containing chelators from tandem deprotection/Click reactions of (trimethylsilyl)alkynes and comparison of their ruthenium(II) complexes.2008, 27, 5430–5433.
(20) Takagi, K.; Kakiuchi, H.; Yuki, Y.; Suzuki, M. Synthesis and optical properties of fluorene-based polymers incorporating organosilicon unit.():2007, 45, 4786–4794.
21 February 2018;
30 May 2018 (CCDC 1842462)
① This work was supported by the National Natural Science Foundation of China (No. 21274080)
. E-mail: fsy@sdu.edu.cn
10.14102/j.cnki.0254-5861.2011-1999
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

