QM/MM and MD Studies of the First Proton Transfer for O2 Activation in the Catalytic Cycle of Cytochrome P450cin①
LIU Feng-Jio SONG Jin-Shui LU Qin-Qin WEI Jing ZHANG Min-Yi HUANG Jing LI Chun-Sen
QM/MM and MD Studies of the First Proton Transfer for O2Activation in the Catalytic Cycle of Cytochrome P450cin①
LIU Feng-Jiaoa, bSONG Jin-Shuaia, cLU Qian-Qiana, cWEI JingaZHANG Min-Yia, cHUANG JingaLI Chun-Sena, c②
a(350002)b(100049)c(361005)
P450cin (CYP176A1) isolated fromis a biodegradation enzyme that catalyzes the enantiospecific conversion of 1,8-cineole to (1R)-6-hydroxycineole. In many P450 family members the mechanism of proton delivery for O2activation is proposed to require a conserved acid-alcohol dyad in the active area, while P450cin has no such residue with alcohol but asparagine instead. In the present work, the mechanism of the first proton transfer of O2activation in P450cin has been investigated by molecular dynamics (MD) and hybrid quantum mechanics/molecular mechanics (QM/MM) techniques. The MD simulation suggests there are two hydrogen bonding networks around the active site, one involving Asp241 and the other involving Glu356. According to our MD and QM/MM calculations, this Asp241 channel is proposed to be the energy accessible. MD results show that the hydrogen bonds around the substrate may contribute to regio- and stereo-oxidation of the substrate.
P450cin, CYP176A1, QM/MM, proton transfer;
1 INTRODUCTION
The cytochromes P450 constitute a superfamily of heme-containing monooxygenases which perform various biochemical transformations, such as C–H bond hydroxylation, C=C bond epoxidation, sulfoxi- dation, N-dealkylation and O-dealkylation[1-4]. These transformations are important for a vast array of vital processes including biosynthesis of hormones, biodegradation of xenobiotics, and drug metabo- lism[4]. It is commonly accepted that the high-valent iron(IV)-oxo porphyrin-radical cation species known as Compound I (Cpd I, in Scheme 1) is responsible for these oxygenation reactions[5]. In the consensus P450 catalytic cycle[1], the formation of Cpd I involves two subsequent proton transfers: the ferric peroxo complex (shown in Scheme 1) is first protonated to yield the hydroperoxo compound, so called Compound 0, (Cpd 0 in Scheme 1), which then accepts a second proton followed by heterolytic O–O bond cleavage to generate Cpd I and water. It has been suggested that along with the water network at active site the conserved aspartate and threonine (or serine) residues forming acid-alcohol pair in most P450s play a crucial role in proton delivery mechanism[6, 7]. However, there are also some special cases in P450s. For example, P450eryF which lacks the conserved theronine could transfer proton from the 5-OH group of its substrate DEB (6-deoxyerythronolide B)[8]. Intriguingly, P450cin (CYP176A1)[9]lacks the conserved threonine or serine residue and the substrate cineole has no OH group to assist the proton delivery, indicating that a distinct mecha- nism is responsible for the formation of Cpd I.
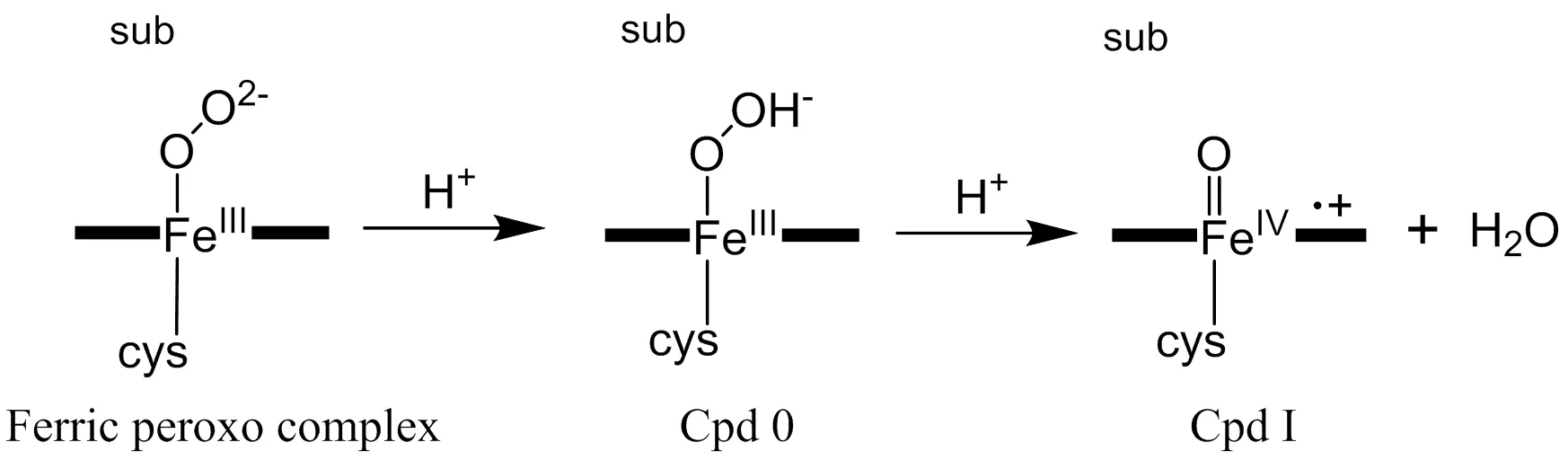
Scheme 1. Formation of Cpd I from ferric peroxo complexCpd 0
Up to now, two possible proton channels invol- ving Asp241 and Glu356 (see Fig. 1) were proposed for O2activation in P450cin[10, 11]. Asp241 is believed to play a similar role as Asp251 of the well-studied P450cam[12, 13], since the Asp241 mutant of P450cin has an influence on the reaction rate. Three vital groups may be involved in the proton delivery from Asp241, including Asn242, Gly238 and substrate cineole[14-16]. However, the mutant of Asn242Ala for P450cin gave a higher coupling than P450cam, and the coupling of Asn242Thr was not very efficient compared with the wild type. These results indicate that the asparagine seems not so important in proton delivery[14]. Compared with Asp241, Gly238 could form a hydrogen bond with a water through its carbonyl group, and thus it is more likely to facilitate the proton delivery[15]. Besides, the substrate cineole was also proposed to contribute to proton delivery[16]. However, another experiment showed that mass coupling is still obtained in the absence of the ethereal oxygen atom, suggesting this ethereal oxygen does not play a significant role in proton transfer[14]. In addition, for Glu356 though lacking of both mutant experimental results and hydrogen bond networks connecting to the protein surface, the channel involving this residue has also to be considered.
According to the previous work reported above, both Asp241 and Glu356 channels could be respon- sible for proton delivery leading to the formation of Cpd I in P450cin. In this work, we report combined MD and QM/MM studies for the first proton transfer to ferric peroxo complex that leads to the formation of Cpd 0 in the wild-type P450cin and related mutation. The possible proton transfer channels related to Asp241 and Glu356 will be presented. Our results provide a detailed mechanism of the first proton delivery and reveal the important roles of the active species or residues. These results can enrich the oxygen activation mechanism for P450s.
2 COMPUTATION METHODS AND DETAILS
The initial structure of the reduced hemecomplex was taken from the X-ray structure of P450cin-NO (PDB code 4FYZ)[15]. Chain B with definite occupancy was used in our model, from which the polyethylene glycol was removed and the axial NO ligand was replaced by O2. The protonation states of titratable residues (His, Glu, Asp) were designed on the basis of pKa values from PROPKA calcula- tions[17]as well as careful visual inspection of their local chemical environment. Histidine residues were dealt with three manners, including doubly proto- nated (52, 98, 267, 351, 391), or singly protonated at N(16, 342, 345), or protonated at N(18, 28, 176, 193, 337). Glutamic acid residues (32, 47, 85, 134, 178, 182, 225, 294, 356, 363, 370, 378, 404) and Aspartic acid residues (127, 241) were protonated. The generated neutral protein was immersed with a 16 Å layer of water molecules, yielding a total of ca. 8300 water molecules in the protein model. Afterwards, a classical MD run was performed for 2 ns using the CHARMM36 force fields[18-20]as implemented in the CHARMM program[21].
In addition to O2-coordinated simplified porphyrin and substrate cineole, some relevant amino acid side chains and water molecules were also included in the QM region. In details, Asp241, Asn242, Tyr81 and Wat612 were included in the Asp241 channel, while Glu356, Thr243 and Wat636 were contained in the Glu356 channel. During QM/MM geometry optimizations, atoms within 8 Å of the QM region were defined as the active region, and the rest was kept frozen.
The QM/MM calculations were performed with ChemShell[22, 23], combining Turbomole[24]for the QM part, DL_POLY[25]with CHARMM36 force field for the MM part, and HDLC optimizer[26]for geometry optimizations. The electronic embedding scheme[27]was adopted to explain the polarizing effect of the enzyme environment on the QM region. The QM/MM boundary was treated through hydro- gen link atoms with the charge shift model[22, 23]. In QM/MM geometry optimizations, the QM region was calculated by the hybrid UB3LYP[28]functional with two basis sets. For geometry optimization, a combined basis set including def2-TZVP for iron and def2-SVP for other atoms was used. The energies were further corrected with the large basis set def2-TZVP. All QM/MM geometry optimizations were carried out on the doublet state.
3 RESULTS AND DISCUSSION
PROPKA calculations show the pKa values of Asp241 and Glu356 are 6.84 and 8.15, implying that these two residues are likely to be protonated under physiological conditions. Thus, two proton transfer channels including these two residues are explored in our present study. In the Asp241 channel, proton could be transferred to distal oxygen atom of the bound O2of ferric peroxo complex via Asn242 and/or the crystal water Wat612, while the Glu356 may deliver proton through a network containing crystal water Wat636, Thr243 and the nearby residues (Fig. 1).

Fig. 1. Two possible proton channels of P450cin. The Asp241, Glu356 and nearby residues are highlighted
3. 1 Asp241 channel
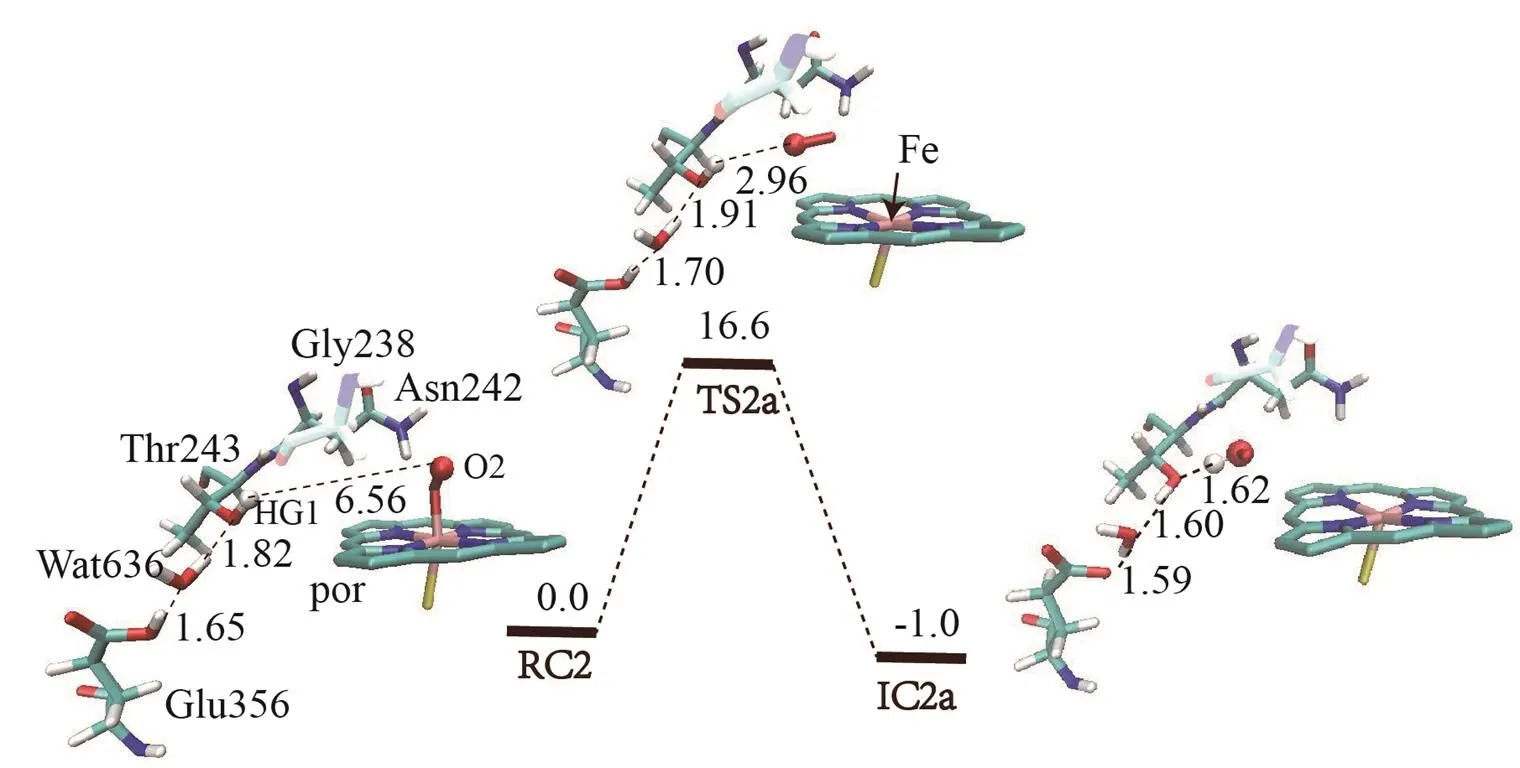
Proton transfer in the Asp241 channel involves three steps: protonating distal oxygen atom of ferric peroxo by Asn242, protonating the deprotonated Asn242 by Asp241, and reprotonating Asp241 via water channels from bulk solvent as suggested by Yarrow Madrona[10]. As there are many possibilities for reprotonating Asp241 from bulk solvent, we here only explore the previous two steps involved in Asp241 channel. The calculated potential energy surface and the optimized geometries of key species for the protonation involving Asp241 channel are shown in Figs. 2 and 3, respectively. As discussed above, the first step in Asp241 channel is the proto- nation of ferric peroxo center with the proton coming from NH2group of Asn242. Before this proton transfer occurs, the Fe(III)O2-group of RC undergoes an internal rotation along the Fe–Opbond to make the distal oxygen Odof Fe(III)O2-group getting close to the HD22 of Asn242. The rotation transition state TSa1 only has a tiny barrier of 0.4 kcal/mol, mainly due to the large space for rotation and the electrostatic interaction between Odand HD22. The resulted species ICa1 thus has a short Op–HD22 distance of 1.49 Å, which is similar to the geometry character of the conserved Thr252 and ferric peroxo in the P450cam crystal structure[6].Subsequently, the proton of HD22 of Asn242 transfers to the distal oxygen Odyielding interme- diate ICb1, of which Cpd 0 has been generated. This step is exothermic by 11.5 kcal/mol without any barrier. The barrierless process may be owing to the strong electrostatic interaction produced by the short distance of HD22 and distal oxygen. This process accords with the proton transfer of Thr252 in P450cam[29]and the proton transfer of DEB 5-OH group in P450eryF[8]. The high activity of RC explains why the super oxo species of P450cin is difficult to trap experimentally[15].
The deprotonated residue Asn242 could abstract a proton from nearby residue Asp241 or, alternatively, from the crystal water Wat612 in the vicinity. As shown in Fig. 3, in ICb1 the distance between the terminal proton HD2 of Asp241 and ND2 of Asn242 is 6.40 Å, which is too far for direct proton transfer. Therefore, the side chain rotation of Asp242 and Asp241 is required. In this transformation, the acid side chain of Asp241 rotates via transition state TSc1 with a barrier of 11.8 kcal/mol. The generation of ICc1 is endothermic by 4.4 kcal/mol. It is noteworthy that the orientation of the side chain of Asp241 in ICc1 is quite similar to the Asp251 in P450cam[29, 30]. Subsequently, the side chain of Asn242 in ICc1 also undergoes an internal rotation along the CB–CG bond toward the proton source Asp241 to shorten the distance of HD2–ND2. As a result, the proton transfer from Asp241 to Asn242 then occurs via transition state TSd1 to generate ICd1. This step is exothermic by 24.0 kcal/mol with a small barrier of 6.8 kcal/mol. As such, from ICc1 to ICd1, Asn242 directly accepts the proton from Asp241 without crystal water Wat612 in the proton shuttle network[11].
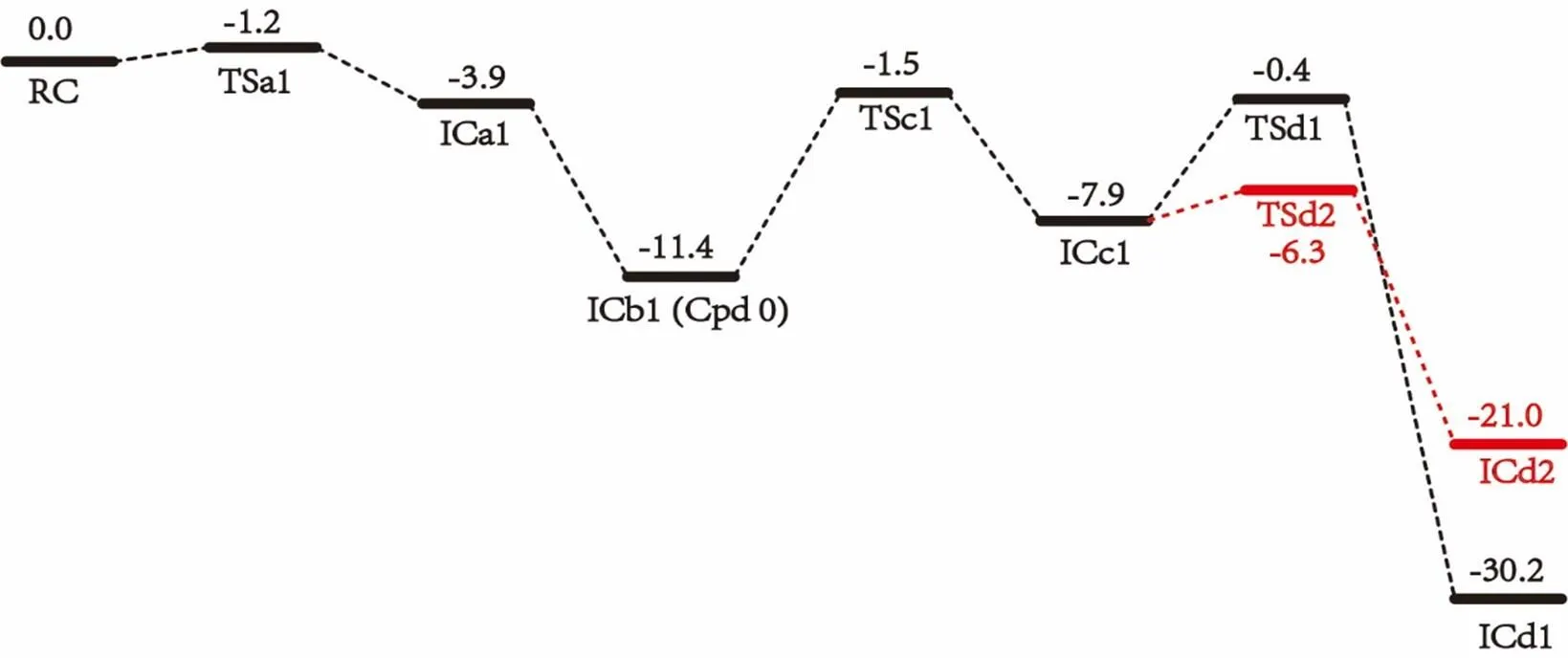
Fig. 2. QM/MM relative energies (kcal/mol) for the first proton transfer via Asp241 channel
Fig. 3. Geometries and bond distances (Å) of key residues
Another possible pathway to deliver the proton from Asp241 to the deprotonated Asn242 is with the assistance of Wat612. All attempts to find energy feasible pathway involving proton transfer from Wat612 to the deprotonated NH group of Asn242 failed as the calculated barriers were quite high, ca. 26.1 kcal/mol. However, interestingly, as shown in Fig. 2, we found an alternative pathway in which the hydrogen HD2 of Asp241 is transferred to OD1 rather than the ND2 of Asn242 via Wat612. The corresponding transition state TSd1 only has a much small energy barrier of 1.6 kcal/mol. Although the resulting species ICd2 is less stable than IC1d by 9.4 kcal/mol, it is still possible to be involved in the second proton delivery as suggested by Kim et al[31]. The classical MD simulation results in Fig. 4 show that Wat612 is held by hydrogen bonds constituted by Asp241, Asn242, Tyr81 and substrate, and does not escape from the protein pocket during the 2ns simulation. As such, the crystal water Wat612 is possible to serve as a node in the hydrogen bond network by transferring proton from Asp241 to Asn242.
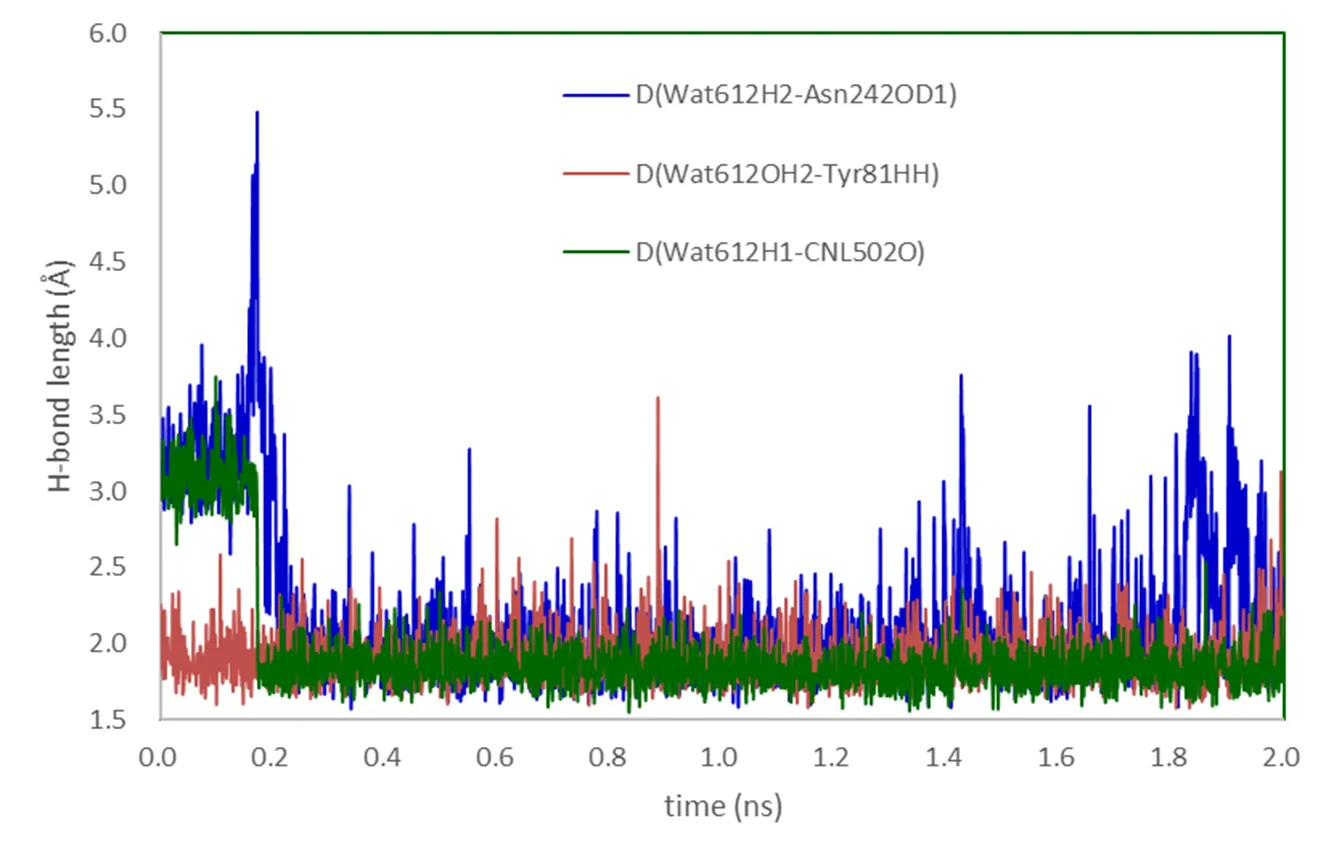
Fig. 4. H-bonds of Wat612 of MD simulations
3. 2 Glu356 channel
The proton transfer from Glu356 was also investigated because similar channel has been reported in P450cam[30]and P450eryF[8]. As shown in Fig. 5, the Glu356 could deliver proton through the hydrogen bond network formed by Wat636 and Thr243. No additional water was found to enter the space between Thr243 and ferric peroxo group during the 2 ns MD simulation. As such, the distance between HG1 of Thr243 and Odis 6.6 Å, which is much longer than the distances between the distal oxygen atom and the Thr252 in P450cam[30](1.923 Å) or the WatA in P450eryF[8](3.35 Å). To locate the proton transfer from the HG1 of Thr243 to the distal oxygen Od, we performed the potential energy surface scan by shortening the distance between Fe(III)O2-and Thr243. The calculated results lead to the breaking of Fe–Op bond and the dissociation of O2(Fig. 5a). Therefore, proton transfers direct from Thr243 to ferric peroxo group is impossible. Alternatively, we attempted to find the pathway in which the proton transfer from Thr243 to ferric peroxo group is mediated by the carboxyl group of the backbone of Gly238 in vicinity. However, even the barrier for HG1 transferring from Thr243 to carboxyl group of Gly238 is 16.1 kcal/mol (Fig. 5b), the distance between protonated Gly238 and Odof ferric peroxo group is about 4.7 Å, which is too far to accomplish the subsequent proton transfer. To sum up, the Glu356 channel is failed to deliver proton to ferric peroxo group, because Glu356 is far away from the distal oxygen atom of Fe(III)O2-and there are neither appropriate residues nor additional waters that could cooperate with Glu356 to deliver proton. These findings are consistent with the results from P450cin mutation experiments in which replacing Glu356 with other residues leads to the coupling efficiency unchanged.
(a)
(b)
Fig. 5. (a) Potential energy surface scan of HG1-Odbond, (b) Proton transfer of HG1 to Gly238. The distances are given in Å and the energies in kcal/mol
3. 3 Discussion
In the whole process, the hydrogen bond network plays a significant role in proton transfer. A strong hydrogen bond between Wat612 and oxygen of cineole was also found as the hydrogen bond length ranging from 1.72 to 2.19Å. As shown in Figs. 3 and 4, Wat612 is sustained by hydrogen bond networks interacted by Tyr81, Asp241 and Asn242. In addition, in most intermediates there is an hydrogen bond between HD21 of Asn242 and oxygen of substrate ranging from 2.17 to 2.70 Å, which is in consistence with crystal structure[32].Moreover, it should be noted that the conformation of the substrate maintained by the hydrogen bond network may contribute to the regio- and stereo- selectivity in the subsequent oxidation reactions catalyzed by Cpd I generated after O2activation.

Fig. 6. Hydrogen bond networks of (a) Asn242Ala and (b) Asn242Thr
According to the above results, we found that residue Asn242 has two significant roles in the proton delivery. Firstly, Asn242 is the nearest residue with respect to the Fe porphyrin. The distances of HD22Asn242and the distal oxygen of ferric peroxo complex are in the range of 2.5~3.5 Å (MD results), which leads to a low barrier of proton transfer as discussed before (Fig. 2). Secondly, Asn242 could form strong hydrogen bonds with cineole (CNL) and Wat612 as the distances of ND2Asn242···OCNL, Wat612···OD1Asn242and Wat612···OCNLare about 2.42, 1.67 and 1.92 Å, respectively. The hydrogen bond networks support a stable geometry and include additional water for proton transfer. Thus, Asn242 is indispensable for the proton transfer. The oxygen atom of cineole is fixed by Asn242 and Wat612, and thus cineole loses the activity of proton transfer. This is consistent with experimental results that replacement of the cineole with no ethereal oxygen has no obvious changes in reaction rate and coupling efficient[14]. Therefore, cineole does not play a key role in proton delivery process. The crystal structure of substrate-free P450cin indicated that Gly238 forms a hydrogen bond chain that lies close to the Fe center, thus Gly238 may contribute to the O2activation. However, the hydrogen bonding chain formed by Gly238 does not exist in the present study, because of the missing crystal waters around the active area. All attempts to locate the proton transfer from Gly238 for O2activation failed. Compared with wide type of P450cin, the mutants Asn242Thr and Asn24Ala have a larger space around the substrate and flexible hydrogen bond networks (Fig. 6). Thus, the increased freedom of the substrate would decrease the regio-selectivity of C–H bond activa- tion, which is also in consistence with experimental findings[14].
4 CONCLUSION
P450cin has two distinct hydrogen bond networks involving Asp251 and Glu356 that are capable of shuttling protons for O2activation. In the Asp241 channel, the Asp241 residue has a direct access to bulky water and thus can be easily reprotonated. The first proton transfer from Asp241 to distal oxygen proceeds via Asn242 to produce the Cpd 0 species. It was found that Asn242 is indispensable among proton delivery process, since this residue is an important proton bridge in the first proton delivery from Asn241 to ferric peroxo center. In the proton transfer from Asp241 to Asn242, two possible pathways are found. One involves direct proton transfer to form species ICd1, whereas the other forms ICd2 via Wat612. MD results show Wat612 plays a significant role for the latter process. In comparison, the Glu356 channel lacks the connectivity with bulky water, which casts doubt on the feasibility of the requisite reprotonation of Glu356. Moreover, our calculation shows that it is difficult to transmit proton from Glu356 to the ferric peroxo center. Our work presents a detailed mecha- nism of the first proton transfer for the O2activation of P450cin, which has shown distinct characters as compared with P450cam. This new mechanism may provide new insights for understanding the proton transfer for similar P450 enzymes. The second proton-transfer step is under investigation.
(1) Ortiz de Montellano, P. R.3rd ed. Kluwer Academic/Plenum Publisher: New York 2005, p1-24.
(2) Meunier, B.; de Visser, S. P.; Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes.2004, 104, 3947-3980.
(3) Shaik, S.; Kumar, D.; de Visser, S. P.; Altun, A.; Thiel, W. Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes.2005, 105, 2279-2328.
(4) Shaik, S.; Cohen, S.; Wang, Y.; Chen, H.; Kumar, D.; Thiel, W. P450 enzymes: their structure, reactivity, and selectivity-modeled by QM/MM calculations.2010, 110, 949-1017.
(5) Yamazaki, H. Springer Japan: Tokyo2014, p132.
(6) Schlichting, I.; Berendzen, J.; Chu, K.; Stock, A. M.; Maves, S. A.; Benson, D. E.; Sweet, B. M.; Ringe, D.; Petsko, G. A.; Sligar, S. G. The catalytic pathway of cytochrome P450cam at atomic resolution.2000, 287, 1615-1622.
(7) Nebert, D. W.; Nelson, D. R.; Coon, M. J.; Estabrook, R. W.; Feyereisen, R.; Fujiikuriyama, Y.; Gonzalez, F. J.; Guengerich, F. P.; Gunsalus, I. C.; Johnson, E. F.; Loper, J. C.; Sato, R.; Waterman, M. R.; Waxman, D. J. The P450 superfamily-update on newsequences, gene-mapping, and recommended nomenclatrue.1991, 10, 1-14.
(8) Sen, K.; Thiel, W. Role of two alternate water networks in compound I formation in P450eryF.2014, 118, 2810-2820.
(9) Hawkes, D. B.; Adams, G. W.; Burlingame, A. L.; de Montellano, P. R. O.; De Voss, J. J. Cytochrome P450cin(CYP176A), isolation, expression, and characterization.2002, 277, 27725-27732.
(10) Madrona, Y.; Hollingsworth, S. A.; Khan, B.; Poulos, T. L. P450cin active site water: implications for substrate binding and solvent accessibility.2013, 52, 5039-5050.
(11) Stok, J. E.; Yamada, S.; Farlow, A. J.; Slessor, K. E.; De Voss, J. J. Cytochrome P450cin(CYP176A1) D241N: investigating the role of the conserved acid in the active site of cytochrome P450s.2013, 1834, 688-696.
(12) Gerber, N. C.; Sligar, S. G. Catalytic mechanism of cytochrome-P-450-evidence for a distal charge relay.1992, 114, 8742-8743.
(13) Gerber, N. C.; Sligar, S. G. A role for Asp-251 in cytochrome P-450cam oxygen activation.1994, 269, 4260-4266.
(14) Slessor, K. E.; Farlow, A. J.; Cavaignac, S. M.; Stok, J. E.; De Voss, J. J. Oxygen activation by P450cin: protein and substrate mutagenesis.2011, 507, 154-162.
(15) Madrona, Y.; Tripathi, S.; Li, H. Y.; Poulos, T. L. Crystal structures of substrate-free and nitrosyl cytochrome P450cin: implications for O2activation.2012, 51, 6623-6631.
(16) Meharenna, Y. T.; Li, H. Y.; Hawkes, D. B.; Pearson, A. G.; De Voss, J.; Poulos, T. L. Crystal structure of P450cin in a complex with its substrate, 1,8-cineole, a close structural homologue to D-camphor, the substrate for P450cam.2004, 43, 9487-9494.
(17) Olsson, M. H. M.; Sondergaard, C. R.; Rostkowski, M.; Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pK(a) predictions.2011, 7, 525-537.
(18) Best, R. B.; Zhu, X.; Shim, J.; Lopes, P. E. M.; Mittal, J.; Feig, M.; MacKerell, A. D. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles.2012, 8, 3257-3273.
(19) MacKerell, A. D.; Bashford, D.; Bellott, M.; Dunbrack, R. L.; Evanseck, J. D.; Field, M. J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; Joseph-McCarthy, D.; Kuchnir, L.; Kuczera, K.; Lau, F. T. K.; Mattos, C.; Michnick, S.; Ngo, T.; Nguyen, D. T.; Prodhom, B.; Reiher, W. E.; Roux, B.; Schlenkrich, M.; Smith, J. C.; Stote, R.; Straub, J.; Watanabe, M.; Wiorkiewicz-Kuczera, J.; Yin, D.; Karplus, M. All-atom empirical potential for molecular modeling and dynamics studies of proteins.1998, 102, 3586-3616.
(20) Mackerell, A. D.; Feig, M.; Brooks, C. L. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations.2004, 25, 1400-1415.
(21) Brooks, B. R.; Brooks III, C. L.; Mackerell, A. D. Jr.; Nilsson, L.; Petrella, R. J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; Caflisch, A.; Caves, L.; Cui, Q.; Dinner, A. R.; Feig, M.; Fischer, S.; Gao, J.; Hodoscek, M.; Im, W.; Kuczera, K.; Lazaridis, T.; Ma, J.; Ovchinnikov, V.; Paci, E.; Pastor, R. W.; Post, C. B.; Pu, J. Z.; Schaefer, M.; Tidor, B.; Venable, R. M.; Woodcock, H. L.; Wu, X.; Yang, W.; York, D. M.; Karplus, M. CHARMM: the biomolecular simulation program.2009, 30, 1545-1614.
(22) Sherwood, P.; de Vries, A. H.; Guest, M. F.; Schreckenbach, G.; Catlow, C. R. A.; French, S. A.; Sokol, A. A.; Bromley, S. T.; Thiel, W.; Turner, A. J.; Billeter, S.; Terstegen, F.; Thiel, S.; Kendrick, J.; Rogers, S. C.; Casci, J.; Watson, M.; King, F.; Karlsen, E.; Sjovoll, M.; Fahmi, A.; Schafer, A.; Lennartz, C. QUASI: a general purpose implementation of the QM/MM approach and its application to problems in catalysis.2003, 632, 1-28.
(23) Metz, S.; Kästner, J.; Sokol, A. A.; Keal, T. W.; Sherwood, P. ChemShell-a modular software package for QM/MM simulations.2014, 4, 101-110.
(24) Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic-structure calculations on workstation computers - the program system turbomole.1989, 162, 165-169.
(25) Smith, W.; Forester, T. R. DL_POLY_2.0: a general-purpose parallel molecular dynamics simulation package.1996, 14, 136-141.
(26) Billeter, S. R.; Turner, A. J.; Thiel, W. Linear scaling geometry optimisation and transition state search in hybrid delocalised internal coordinates.2000, 2, 2177-2186.
(27) Bakowies, D.; Thiel, W. Hybrid models for combined quantum mechanical and molecular mechanical approaches.1996, 100, 10580-10594.
(28) Becke, A. D. Density-functional thermichemistry. 3. The role of exact exchange.1993, 98, 5648-5652.
(29) Wang, D. Q.; Zheng, J. J.; Shaik, S.; Thiel, W. Quantum and molecular mechanical study of the first proton transfer in the catalytic cycle of cytochrome P450cam and its mutant D251N.2008, 112, 5126-5138.
(30) Zheng, J. J.; Wang, D. Q.; Thiel, W.; Shaik, S. QM/MM study of mechanisms for compound I formation in the catalytic cycle of cytochrome P450cam.2006, 128, 13204-13215.
(31) Kim, D.; Heo, Y. S.; Ortiz de Montellano, P. R. Efficient catalytic turnover of cytochrome P450camis supported by a T252N mutation.2008, 474, 150-156.
(32) Meharenna, Y. T.; Slessor, K. E.; Cavaignac, S. M.; Poulos, T. L.; De Voss, J. J. The critical role of substrate-protein hydrogen bonding in the control of regioselective hydroxylation in P450cin.2008, 283, 10804-10812.
28 February 2018;
11 June 2018
①This project was supported by the National Natural Science Foundation of China (No. 21573237, 21603227, 21403242, 21703246) and the Natural Science Foundation of Fujian Province (2017J05032)
. Male, born in 1978, professor, majoring in theoretical chemistry. E-mail: chunsen.li@fjirsm.ac.cn
10.14102/j.cnki.0254-5861.2011-2029
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

