Synthesis, Structure and Biological Activities of a Novel Anionic Organotin (IV) Complex {[(p- ClC6H4CH2)Sn(H2O)(Cl)2OCOCH(O)CH(O) CO2Sn(H2O)(Cl)2(p-ClC6H4CH2)]·2(HNEt3)}①
HE Tang-Feng ZHANG Fu-Xing YAO Shu-Fen ZHU Xiao-Ming SHENG Liang-Bing KUANG Dai-Zhi FENG Yong-Lan YU Jiang-Xi JIANG Wu-Jiu
Synthesis, Structure and Biological Activities of a Novel Anionic Organotin (IV) Complex {[(- ClC6H4CH2)Sn(H2O)(Cl)2OCOCH(O)CH(O) CO2Sn(H2O)(Cl)2(-ClC6H4CH2)]·2(HNEt3)}①
HE Tang-Feng ZHANG Fu-Xing②YAO Shu-Fen ZHU Xiao-Ming SHENG Liang-Bing KUANG Dai-Zhi FENG Yong-Lan YU Jiang-Xi JIANG Wu-Jiu
(421008)

anionic organotin(IV) complex, D-tartaric acid, synthesis, structure, vitro anticancer activity;
1 INTRODUCTION
Organotin compounds have attracted considerable attention due to their diverse structures, rich reactiva- tes and wide applications[1-8]. Ionic organotin com- pound is an important type of organotin compounds, where the central tin atoms carry the charges. According to the characteristics of charge, they can be divided into cationic and anionic organotin com- pounds, among which anionic organotin compounds are more common[9-11]. Recently, more and more attention has been paid on the ionic organotin com- pounds due to their high coordination, unique structures, good biological activities and applications in organic synthesis[12-16]. Studies have shown that ionic organotin compounds have high anticancer activity[11, 17], which may be attributed to their better solubility in water, the improved transport ability and the enhanced biological activity in the body.
In this work, we employed the di(-chloro- benzy)tin dichloride, D-tartaric acid and triethyl- amine to obtain a novel type of anionic organotin compounds {[(-ClC6H4CH2)Sn(H2O)(Cl)2OCO- CH(O)CH(O)CO2Sn(H2O)(Cl)2(-ClC6H4CH2)]·2(HNEt3)} (1), which has been characterized by ele- mental analysis, infrared spectrum, XRD and X-ray single-crystal diffraction. We have carried out the quantum chemicalinitio calculation for the stabi- lity, energy of molecular orbital and the composition of some frontier molecular orbitals.In addition, the thermal stability and in vitro anticancer activity of 1 were investigated.
2 EXPERIMENTAL
2. 1 Materials and chemicals
All reagents were chemically pure and used directly without further purification. Mds-15 micro- wave synthesis station was used, and IR spectra were recorded with a Japan Shimadzu IR Prestige-21infrared spectrometer in 4000~400 cm–1region using KBr pellets. The elemental analysis was determined by PE-2400 (II) elemental analyzer. Crystallographic data of the complex were collected on a Bruker SMART APEX ⅡCCD diffractometer. Melting points were measured using an X4 digital microsco- pic melting point apparatus without correction. Thermogravimetric analyses were executed on a TG209F3 thermogravimetric analyzer.
2. 2 Synthesis of complex 1
A mixture of di(-chlorobenzy)tin dichloride (0.441 g, 1.0 mmol), D-tartaric acid (0.075 g, 0.5 mmol), Et3N (2.0 mmol) and methol (30 mL) was sealed in a Teflon microwave reaction tank, heated to 120 °C for 2 h, and then the mixture was cooled and filtered. The resulting insoluble solids were removed. After that some solvents were removed by rotating evaporation of the filtrate and placed at room temperature, obtaining white solids which were recrystallized with acetone-ethanol (1:1, v/v). Colorless crystals (0.342 g) were obtained in 67.27% yield based on di(-chlorobenzy)tin dichloride. m.p.: 97~99 ℃.Anal. Calcd. (%) for 1 (C30H50Cl6N2O8Sn2): C, 35.44; H, 4.96; N, 2.76. Found (%): C, 35.32; H, 4.94; N, 2.77.IR (KBr, cm–1): 3246(m), 3080(m), 2983.88(m), 1631.78(s), 1600.92(s), 1487.12(m), 1388(m), 1355(m), 653(w), 551(w), 478(w).
2. 3 Crystal structure determination
The suitable sample (0.20mm × 0.21mm × 0.23mm) for 1 was chosen for the crystallographic study and then mounted at 296(2) K on a Bruker SMART APEX Ⅱ CCD diffractometer equipped with graphite-monochromated Moradiation (= 0.071073 nm) with ascan mode in the range of 0.91°≤≤25.35°. A total of 16562 (13607 inde- pendent,int= 0.0218) reflections were mea- sured, of which 7203were observed (> 2()). All the data were corrected byfactors and empirical absor- bance. The structure was solved by direct methods. All non-hydrogen atoms were determined in success- sive difference Fourier synthesis, and all hydrogen atoms were added according to theoretical models. All hydrogen and non-hydrogen atoms were refined by their isotropic and anisotropic thermal parameters through full-matrix least-squares techniques. All calculations were completed by the SHELXTL-97 program. The final= 0.0394,= 0.1092, (Δ)max= 1224 and (Δ)min= –840 e/nm3. The selected bond lengths, bond anglesand H-bonds for 1 are listed in Tables 1 and 2, respectively.
2. 4 Anti-tumor activity of 1
The proliferation inhibition activities of 1 against NCI-H460, A549 and MCF-7 were measured by means of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-di- phenyltetrazolium bromide) assay.The experiments were divided into drug group (with different concentrations of 1), control group (without complex 1) and blank group (without the complex and cells). The tumor cells in logarithmic phase were chosen and digested by the suitable amount of Trypsin to make the adherent cell fall off, and then the cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and grown at 310 K in a humidified atmosphere in the presence of 5% CO2. Harvested cells were seeded into 96-well plate with various concentrations of 1 (0.1~10 μmol·L–1) and incubated for 72 h. Each concentra- tion of 1 was set in parallel for six wells. Four hours to the end of incubations, 40 μL of MTT solution (4 mg·mL–1in D-Hanks) was added to each well containing fresh and cultured media. At the end, the insoluble formazan produced was completely dissolved in DMSO (150L) by incubating for 5 min on a shaker and optical density (OD) was read against the reagent blank with automatic immune enzymatic analytic system (Ap22 Speedy) at a wavelength of 570 nm. The cell proliferation inhibi- tion activities of the positive control (carbonplatin) were determined by the above similar assay. Experimental data were analyzed with nonlinear regression of the survival percentage to the drug concentration (curve fitting) using Graph Pad Prism 5.0 and the IC50values were determined using sigmoidal dose response equation (variable).

Table 1. Selected Bond Distances (nm) and Bond Angles (°) for Complex 1
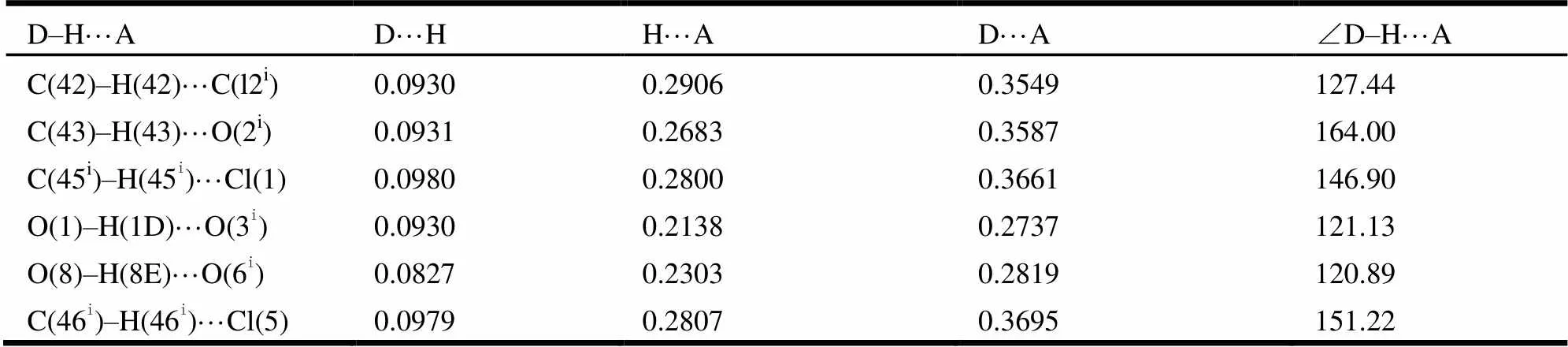
Table 2. Hydrogen Bonding Geometry (nm, °) for Complex 1
Symmetry code: i: 1 +,, z
3 RESULTS AND DISCUSSION
3. 1 Synthesis
We did not obtain the expectant product [(-ClC6H4CH2)2SnOCOCHO]2with di(-chloro- benzy)tin dichloride and D-tartaric acid at a molar ratio of 2:1 in the presence of trimethylamine but the title complex {[(-ClC6H4CH2)Sn(H2O)(Cl)2OCO-CH(O)CH(O)CO2Sn(H2O)(Cl)2(-ClC6H4CH2)]·2(HNEt3)}. Moreover, the debenzylation phenol- menon occurred during the reaction, which is explained with different views. One holds that it is caused by the self-catalysis of organic halogenated tin[18], the other considers the catalysis of organic ammonium salt generated leads to the debenzyla- tion[10, 19]. However, in the work, we find that the dehydroxylation of organotin compounds may be mainly related to the structures and properties of the ligands[20].
3. 2 Crystal structure of 1
Fig. 1 shows each component unit in 1 contains three independent ionic organotin compound molecu- les, where the dinuclear anionic unit, [(-ClC6H4CH2)Sn(H2O)(Cl)2OCOCH(O)CH(O)CO2-Sn(H2O)(Cl)2(-ClC6H4CH2)]2–, connects two (-ClC6H4CH2)Sn(H2O)(Cl)2with D-tartaric acid, as presented inFig. 2. Both Sn(IV) ions adopt the same distorted octahedral coordination geometry [SnO3Cl2C]. Two Cl atoms are from the di(-chloro- benzy)tin dichloride, one C atom is from the me- thylene of chlorobenzy, three O atoms are provided by the water molecule, hydroxyl and carboxyl groups of D-tartaric acid, respectively. Furthermore, one Cl atom and one O from water molecule atom comprise the axial plane, and the other atoms occupy the equatorial plane. The bond angles of the axial posi- tion (∠O(1)–Sn(2)–Cl(2) = 172.93°, ∠O(8)–Sn(2)– Cl(4) = 170.33°) are much smaller than the linear angle (180°), and the bond angles around Sn between atoms of the axial and those of the equatorial positions have larger deviation with 90°, thus Sn atoms exhibit seriously distorted octahedral confi- gura-tion, and the degree of distortion of Sn(2) is greater than that of Sn(1).
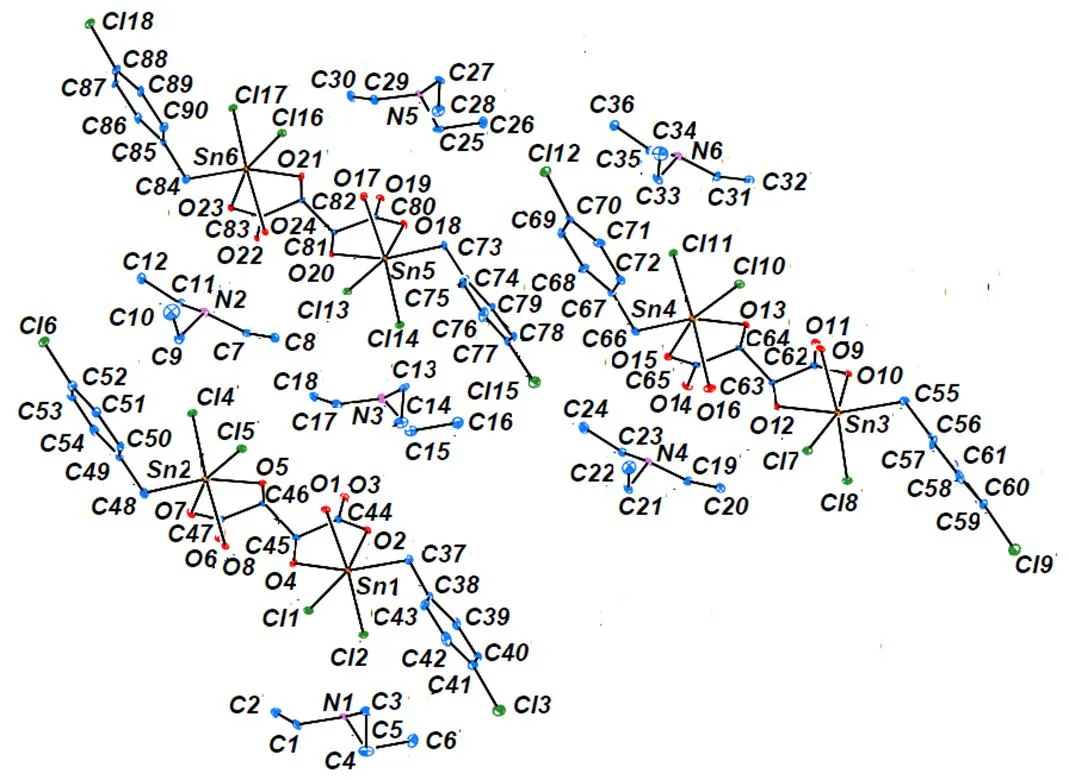
Fig. 1. Unit of the crystal structure
Fig. 2. Double negative ion of the complex
Adjacent dinuclear motifs are interlinked to form an infinite ribbon structure by the abundant hydrogen bonds (Fig. 3), among which the most powerful one is O–H···O, as shown in Table 2.
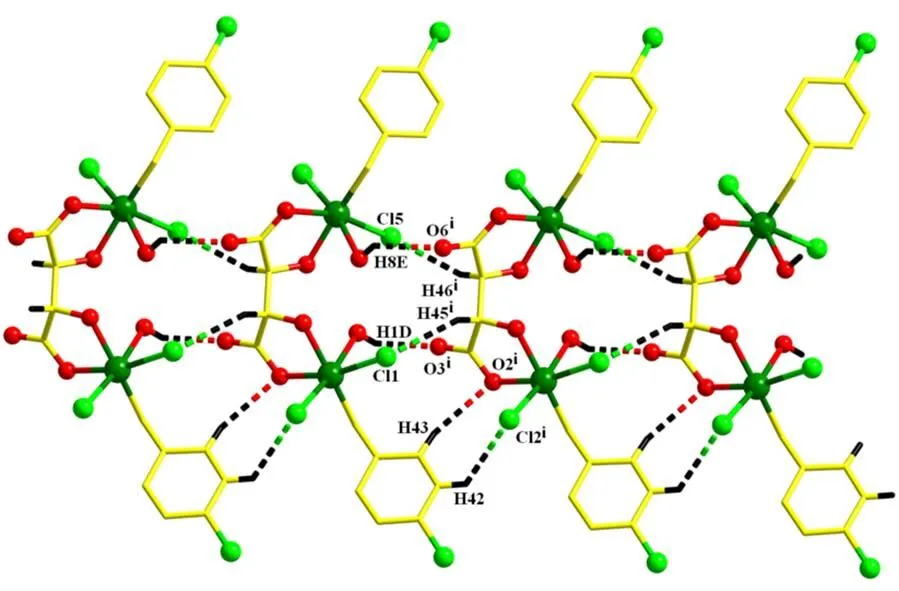
Fig. 3. Banded structure of the complex by O–H···O interactions
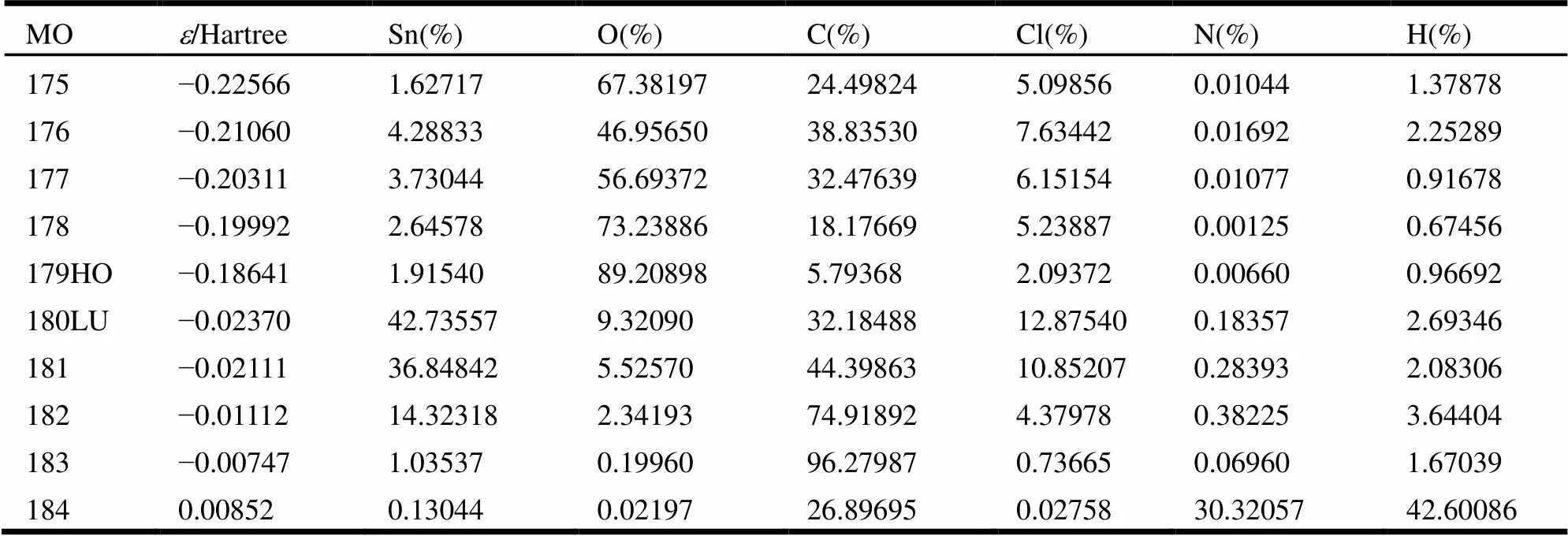
Table 3. Calculation on Some Frontier Molecular Orbitals Composition for Complex 1
3. 3 Analysis of the energy and frontier molecular orbital composition
3. 3. 1 Total and frontier molecular energy
According to the coordinate site of each atom in the crystal structure as well as symmetric geometry, a structural unit was applied to Gaussian 03W program to perform quantum chemistry single-point calcula- tion at the B3ylp/lanl2dz level. After calculation, the total energy of 1 is –1980.1906649 a.u, the highest occupied and lowest empty molecular orbital (HOMO and LUMO) were –0.18641 and 0.02370 a.u., respectively, and the energy gap (Δ) ΔLUMO-HOMO= 0.16271 a.u.. Based on the system energy and frontier molecular orbitals energy, both the total energy and the energy of HOMO are lower, suggesting that 1 is stable. However, the energy of HOMO is also lower and the energy gap is smaller. From the point of oxidation-reduction and charge transfer, 1 is easy to lose electrons to oxidize.
3. 3. 2 Molecular orbital composition
To explore the electronic structure and bonding characteristics of 1, the molecular orbitals were systematically investigated.The contribution of one atom to the molecular orbital is denoted as the sum of square of orbital coefficient and normalization.Complex 1 was divided into six parts: (a) Sn atom, (b) O atom, (c) C atom, (d) Cl atom, (e) N atom and (f) H atom. Five frontier molecular orbitals and unoccupied molecular orbital are studied, as shown in Table 3 and Fig. 4.
The results suggest the largest contribution for the energy is O atom toreach89.21%; the second one is C and Cl atoms which occupy 5.79% and 2.09%, respectively; Sn atoms significantly contribute to the molecular orbitals up to 1.92%, indicting the bonds, including Sn–O, Sn–C and Sn–Cl, are stronger and the structure is stable in the ground state. Furthermore, compared to the HOMO and LUMO orbital composition, the electrons are excited from HOMO to LUMO. Primarily, the electrons of O atom transfer to the chloride atoms and the dichlorinated- benzyl groups of the ligand through tin atoms. Hence, Sn atoms are not only the bridge for electron transfer, but also the main donor for electron transfer.
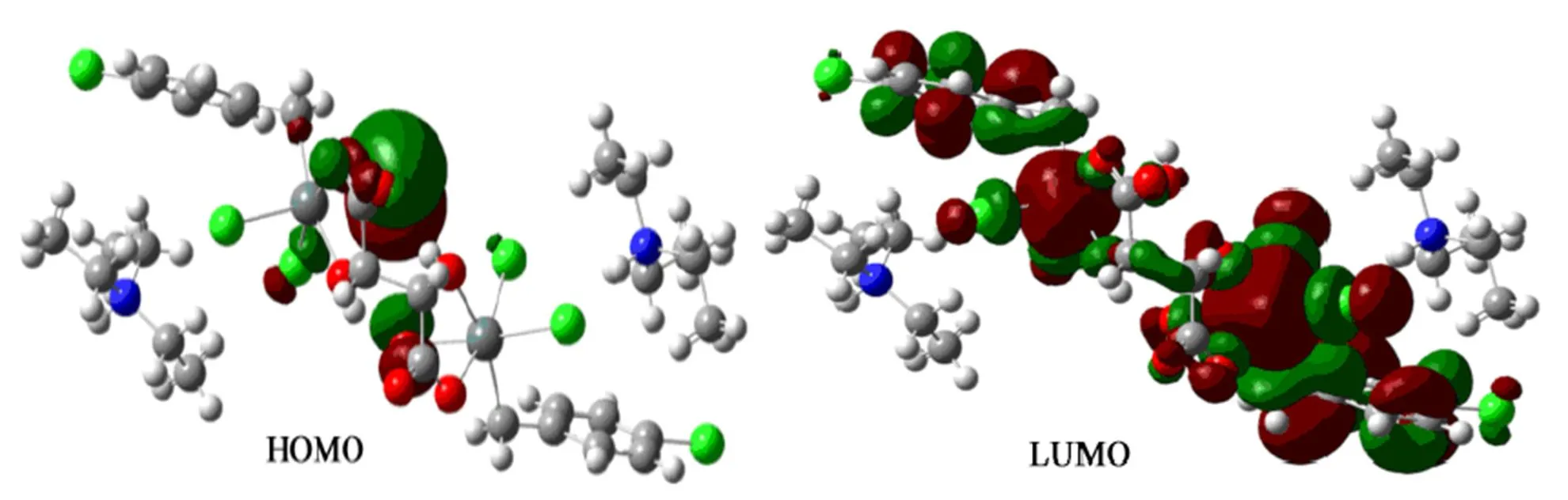
Fig. 4. Schematic diagram of frontier MO for complex
3. 4 XRD and thermal stability (TG)
As shown in Fig. 5, the experimental XRD pattern () of 1 is in agreement with the simulated one from the single-crystal X-ray data (), suggesting complex 1 is pure.
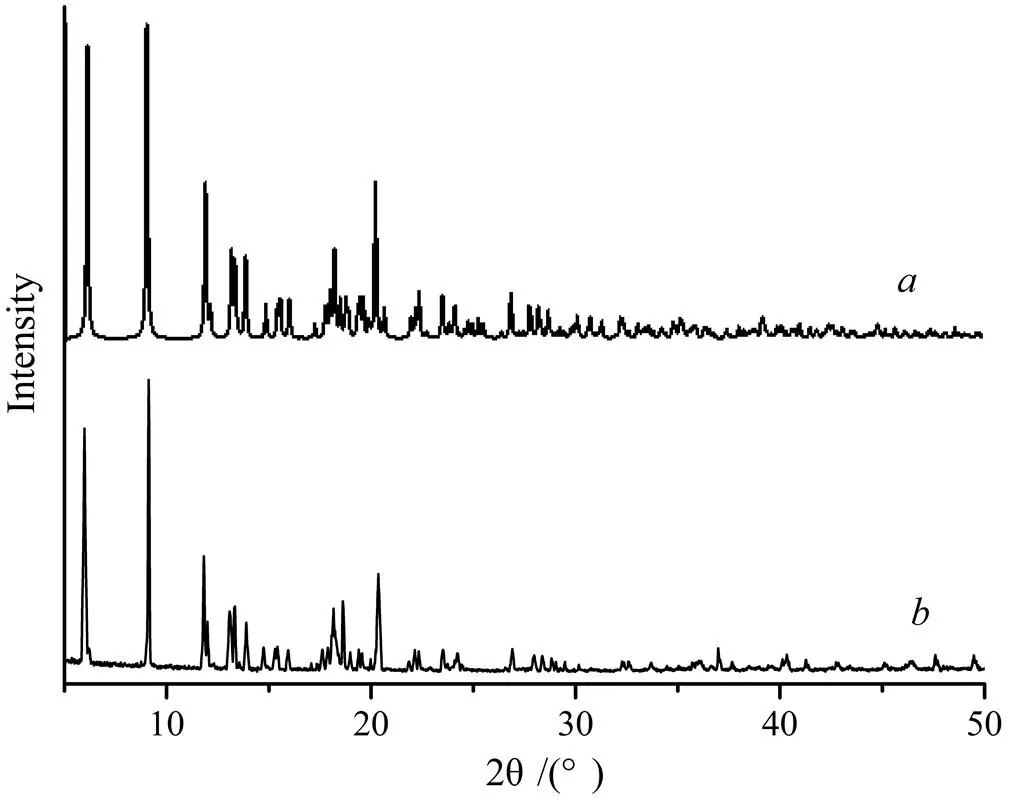
Fig. 5. PXRD patterns of 1
TG analysis was carried out in air atmosphere from 40 to 900 °C at a heating rate of 20 °C·min–1and gas flowing velocity was 20 mL·min–1. As shown in Fig. 6, complex 1 had no loss at40~110 °C. The first weight loss corresponding to the removal of two water molecules is from 110 to 185 °C (obsd. 3.68 %); the second weight loss is rapider, which occurs in the range of 185~270 °Cdue to the departure of four coordinated Cl atoms and two Et3N molecules (obsd. 25.35%); the third step in 270~430 °Cis slower because of the decomposition of two dichlorinated-benzyl groups (obsd. 10.14%); the last loss becomes so slow, which occurs in the range of 430~670 °Cresulting from the removal of D-tartaric acid, and the loss stops until 670 °C. The amorphous power, which is calculated to be SnO2, is left as the final product for 1 (obsd. 27.86%, calcd. 29.64%).
3. 5 Anti-tumor activityof complex 1
Theantitumor activity test results show that 1 exhibits different IC50values of 1.74 ± 0.2, 12.4 ± 1.6 and 0.94 ± 2.4mol·L–1to NCI-H460, A549 and MCF7, exhibiting that complex 1had strong inhibitory activity on human cancer cells. In addition, the inhibitory activity was relatively better for H460 and MCF7, whereas that was weak for A549. The detailed biological activity of 1 will be further studied.
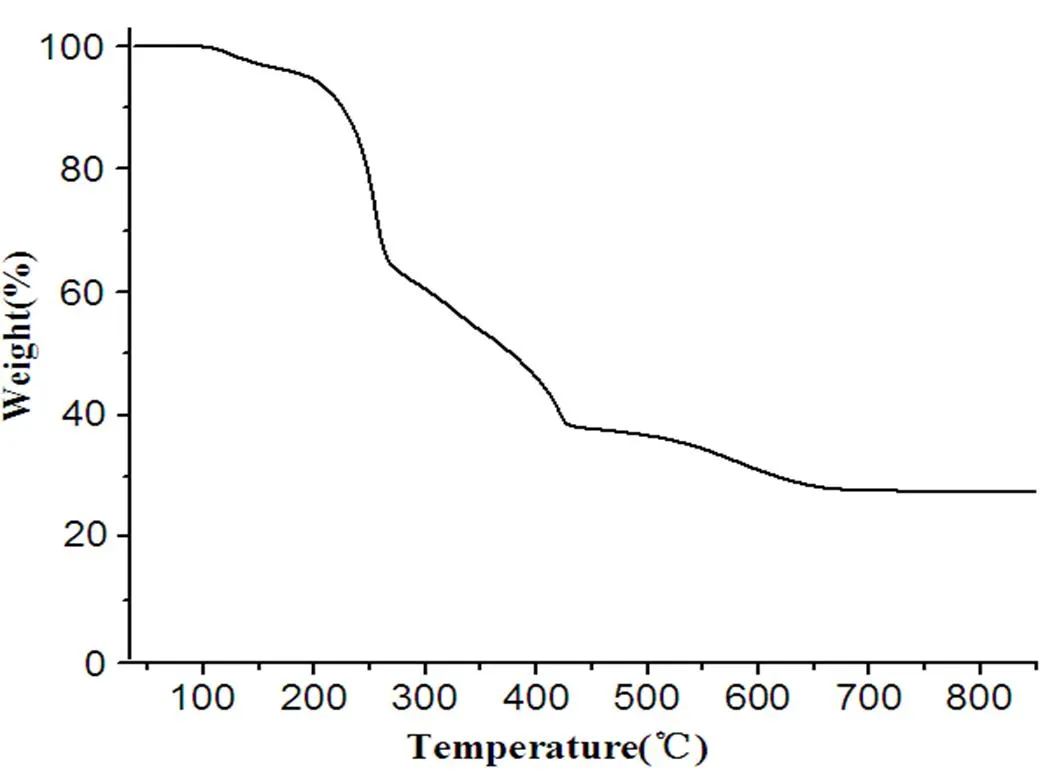
Fig. 6. Thermogravimetric curves of 1
4 CONCLUSION
In summary, synthesis, structure and anti-tumor activity of a novel anionic organotin (IV) complex{[(-ClC6H4CH2)Sn(H2O)(Cl)2OCOCH(O)CH(O)CO2-Sn(H2O)(Cl)2(-ClC6H4CH2)]·2(HNEt3)} was repor- ted. In the title dinuclear complex, both Sn atoms are in the distorted octahedral [SnO3Cl2C] coordination environment. TG analysis shows complex 1 is stable until 110 °C. Theantitumor activity confirms that 1had strong inhibitory activity on H460, MCF7 and A549. Especially, the inhibitory activity is relatively better for H460 and MCF7.
(1) Vieira, F. T.; Lima, G. M. D.; Maia, J. R. D. S.; Speziali, N. L.; Ardisson, J. D.; Rodrigues, L.; Junior, A. C.; Romero, O. B.Synthesis, characterization and biocidal activity of new organotin complexes of 2-(3-oxocyclohex-1-enyl)benzoic acid.2010, 45, 883–889.
(2) Yin, H. D.; Wang, H. Y.; Wang, D. Q. Synthesis, characterization and crystal structure of a novel 3D network triorganotin(IV) polymer containing two types of macrocycles.2008, 693, 585–589.
(3) Xiao, X.; Liang, J. G.; Xie, J. Y. Organotin(IV) carboxylates based on 2-(1,3-dioxo-1H-benzo[de]-isoquinolin-2(3H)-yl)acetic acid: syntheses, crystal structures, luminescent properties and antitumor activities.2017, 1146, 233–241.
(4) Munoz-Flores, B. M.; Santillán, R.; Farfán, N. Synthesis, X-ray diffraction analysis and nonlinear optical properties of hexacoordinated organotin compounds derived from Schiff bases2014, 769, 64–71.
(5) Tiekink, E. R. T. Tin dithiocarbamates: applications and structures.2008, 22, 533–550.
(6) Yin, H. D.; Chen, S. W.; Li, L. W. Synthesis, characterization and crystal structures of the organotin(IV) compounds with the Schiff base ligands of pyruvic acid thiophene-2-carboxylic hydrazone and salicylaldehyde thiophene-2-carboxylic hydrazone.2007, 360, 2215–2223.
(7) Fard, Z. H.; Müller, C.; Dehnen, S. Syntheses and structures of organotin(IV) thioesters of N-phthaloylamino acids.2008, 634, 1851–1856.
(8) Yang, Y. G.; Hong, M.; Xu, L. D. Organotin(IV) complexesderived from Schiff base N′-[(1E)-(2-hydroxy-3-methoxyphenyl) methylidene]pyridine-3-carbohydrazone: synthesis, in vitro cytotoxicities and DNA/BSA interaction.2016, 804, 48–58.
(9) Yin, H, D.; Wang, Q. B.; Xue, C. S. Synthesis and characterization of ionic organotin compounds [R2SnCl2(2-quin)]-(HNEt3)+and crystal structures of [(2,4-Cl2-C6H3CH2)2SnCl2(2-quin)]-(HNEt3)+.2005, 690, 831–836.
(10) Sun, L. J.; Song, X. Q.; Chen, J. X.; Zhong, G. Y.; Xie, Q. L. Studies on ionic organotin compounds(II)-the reaction of trialkyltin chloride and triphenyltin hydroxide with mercaptoacetic acid in the presence of organic bases2004, 25, 646–650.
(11) Zhong, G. Y.; Liu, Q. F.; Sun, L. J.; Xie, Q. L. Reaction of Ph2CySnI and MeCy2SnCl with HSCH2COOH in the presence of organic amines and biological activity for the reaction products.. 2011, 31, 728–732.
(12) Willem, R.; Biesemans, M.; Boualam. M.; Delmotte, A.; Khloufia, A. E.; Gielen, M. Tetraethylammonium (diorgano)halogeno-(2,6-pyridinedicarboxylato)stannates: synthesis, characterization andantiturnour activity.... 1993, 7, 311–317.
(13) Ng, S. W.; Kumar Das, V. G.; Holecek, J.; Lycka, A.; Gielen, M.; Drew, M. G. B. Organostannate derivatives of dicyclohexylammonium hydrogen 2,6-pyridinedicarboxylate: solution/solid-state13C,119Sn NMR and in vitro antitumour activity of bis(dicyclohexylammonium)bis(2,6-pyridinedicarboxylato)dibutylstannate, and the crystal structure of its monohydrate..... 1997, 11, 39–45.
(14) Willem, R.; Biesemans, M.; Kayser, F.; BouSlam, M.; Gielen, M. Tetraethylammonium (diorgano)halogeno(thiosalicylato)stannates: synthesis, characterization and in vitro antitumor activity....1992, 197, 25–30.
(15) Durand, S.; Sakamoto, K.; Fukuyama, T.; Orita, A. Cationic organotin clusters for highly efficient alcohol acetylation catalysts... 2000, 19, 3220–3223.
(16) Ng, S. W.; Kumar Das, V. G. Dicyclohexylammonium (dicarboxylato) triorganostannates crystal structure of bis(dicyclohexylammonium) tris(malonato) tetrakis( tributylstannate).... 1992, 430, 139–148.
(17) Zhong, G. Y.; Sun, L. J. Synthesis and biological activity study of ionic organotin compounds.2011, 28, 287–393.
(18) Yin, H. D.; Wang, C. H.; Wang, Y.; Ma, C. L.; Shao, J. X.; Zhang, J. H. Synthesis and crystal structure of a novel ionic organotin {[Ph2Sn]2[2,6-Py(CO2)]3H2O}2−[HNEt3]2+.2002, 60, 143–149.
(19) Zhong, G. Y.; Sun, L. J.; Xie, Q. L. Synthesis of new tin(Ⅳ) complexes and crystal structure of [(i-Pr)2NH2[(PhCH2)3Sn(2-SCH2COO)].2010, 31, 2218–2221.
(20) Zhang, F. X.; Kuang, D. Z.; Wang, J. Q.; Feng, Y. L.; Chen, Z. M. Synthesis and crystal structur e of the di(-fluorobenzyl)tin bis(2-picolinate) and the tri(o-fluorobenzyl)tin 4-picolinate.. 2006, 22, 7–12.
1 April 2018;
10 September 2018
the Open Fund Project innovation platform of Key Laboratory of Higher Educational Institutions of Hunan Province (GN16K01), Scientific & Technological Projects of Hengyang (2016KL03), Aid programs for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province, the Key Discipline of Hunan Province, Project funding for research and innovation experiment of university students in Hunan Province
. E-mail: zfx8056@163.com
10.14102/j.cnki.0254-5861.2011-2027
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

