Quantum Chemistry Study on Benzimidazoledithi Derivatives’ Selective Pre-enrichment of Cadmium Cation①
LI Sho-Yun② GENG Cho YANG Zi-Heng YANG Xi CHEN Xiu-Min CHEN Xiu-Hu MA Wen-Hui②
Quantum Chemistry Study on Benzimidazoledithi Derivatives’ Selective Pre-enrichment of Cadmium Cation①
LI Shao-Yuana② GENG ChaoaYANG Zi-HengbYANG XicCHEN Xiu-MinaCHEN Xiu-HuabMA Wen-Huia②
a(650093)b(650091)c(650093)
This work reports the mechanism of benzimidazoledithi (BDT) derivatives’ selec- tive pre-enrichment of Cd2+under the stimulation of glutathione (GSH).The geometric and electronic properties of five BDT-M2+complexes (M = Cd, Cu, Hg, Pb, Co) havebeen investigated using density functional theory (DFT) at the B3LYP/6-311G (d,p) level with the GAUSSIAN 09 package program. The results show that BDT ligand exhibits alternative behaviors to different metal ions with the binding affinity in the order of Cu2+> Cd2+> Pb2+> Hg2+> Co2+. After adding the BDT-M2+complex into the GSH solution, the new S–S bonds can be formed, resulting with benzimidazole-metal ions (MBI-M2+) falling off into the GSH solution. Furthermore, the weak interaction between the new glutathione derivative (GSHD) and MBI-M2+were found. However, the strong chelation was detected between GSHD and MBI-M2+(M = Cu, Pb, Hg, Co) to hinder the adsorbed Cu2+, Pb2+and Hg2+, Co2+completely falling into the GSH solution, which suggests porous silicon composite modified by BDT has a certain selective pre-enrichment of Cd2+ion.
benzimidazoledithi (BDT) derivatives, selective pre-enrichment, glutathione, cadmium ion, density functional theory;
1 INTRODUCTION
Cadmium ion, a kind of serious environmental pollution, limits the biggest content national stan- dards (China) for heavy metal residues in food, such as 0.03 mg/kg, fruit; 0.05 mg/kg, vegetables and 0.005 mg/L, drinking water. Therefore, the trace detection of Cd2+in the environmental samples is paid close attention[1, 2]. However, most conventional instrument analysis techniques are not enough sensitive such as emission spectrometry, spectro- photometry, and polarography because the content of cadmium ion in the samples is low. Its composition is complex and the components may cause a dis- turbance in different test methods. For these reasons, it is preliminarily essential to pre-enrich and separate the trace cadmium ions from matrix in water[3, 4]. Generally speaking, the high efficient and high selective separation of metal ions depends on the solid phase carrier and chelating group of the enriched material. It is important to obtain the new enri- chment materials with high selectivity in the trace of heavy metal cadmium field.
Pyridine disulfide can react with mercapto drugs, target ligands and other functional molecules and is broken off to form the by-products with new disul- fide bonds under mild conditions[5, 6]due to the chemical sensitivity of disulfide bonds, which can be reduced and split by the specific reductant such as GSH and dithiothreonol (DTT)[7, 8]. In addition, the reductant with the concentration of millimoles can split the disulfide bond[9]. This feature is referred to as stimuli response, which has been widely con- cerned and become one of hot researches in the targeted drug delivery and biological separation fields[10, 11]. 2-Mercapto benzimidazole and its derivatives, a highly efficient chelating agent of metal ion, are frequently used to pre-enrich the precious metal ions[12],or detect the toxic heavy metal ions[13]. As shown in Fig. 1, with the organic covalent coupling technology, we “grafted” the benzimidazoledithi derivatives (BDT) with the stimulate response to the nano-porous silicon surface of the huge specific surface area, and obtained a composite functional material, which can selectively enrich the cadmium ion[14].
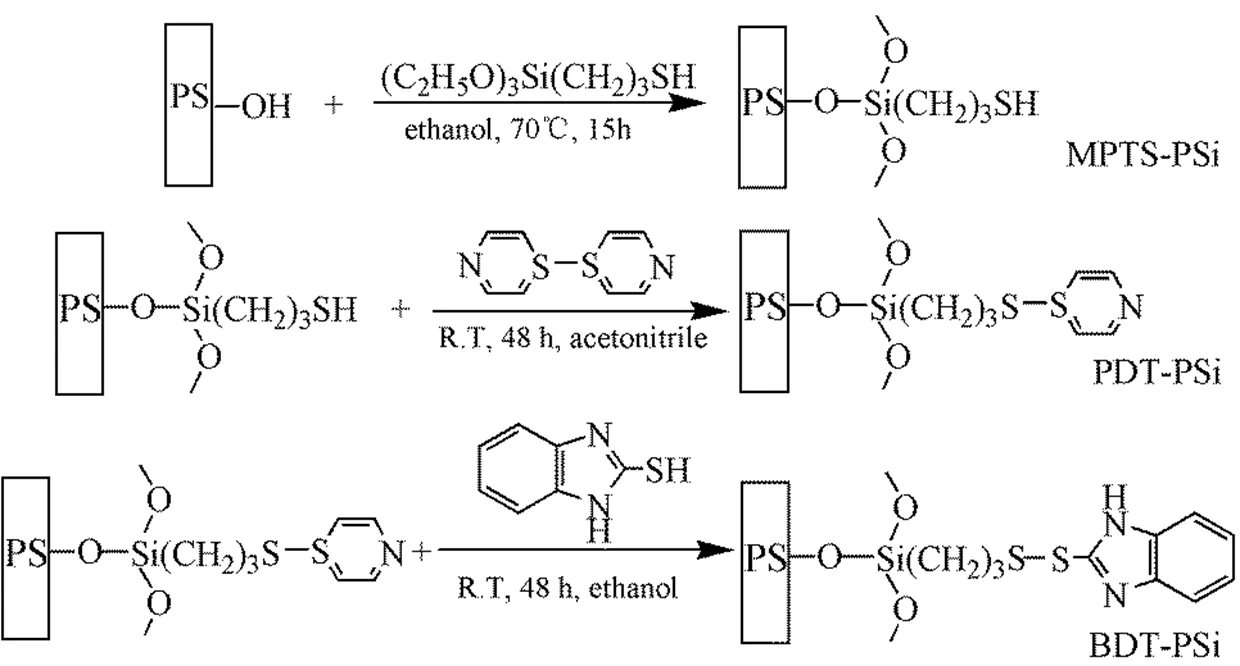
Fig. 1. Scheme for the preparation of BDT-PS
The electron density has replaced the wave func- tion as the research of basic amount in the density functional theory (DFT). DFT calculation methods have been successfully applied in various chemical issues such as the molecular structure and property, spectrum, energy spectrum, catalyst, reaction mecha- nism, reaction kinetics and thermodynamics, struc- tures of the transition state, the activation barrier and so on[15-20]. Among them, a number of studies have proven that the computational values with B3LYP method are consistent with the experimental ones. In the work, the chelating adsorption capacity, as well as the desorption property under the GSH stimulus response, of five BDT-M2+complexes (M = Cd, Cu, Hg, Pb, Co) havebeen investigated using DFT at the B3LYP/6-311G (d,p) level with the GAUSSIAN 09 package program. In essence, we revealed the mechanism of BDT to pre-enrich selectively the heavy metal cadmium ions.
2 EXPERIMENTAL AND COMPUTATIONAL METHODS
2. 1 Pre-enrichment metal ions of BDT-PS
The process of enrichment of trace metals adopted the vibration absorption method. The BDT-PS composite was immersed in the M2+(M = Cd, Cu, Hg, Pb, Co) solution (50 mL beaker) with con- centrations of 0.05 ppm at room temperature, respec- tively. After five hours, the BDT-PS was transferred into the GSH solution (3 mL). The soak time lasted up to 12 hours to ensure the complete reaction between GSH and the disulfide bonds of BDT- PS-M2+. The concentration of metal ions in the GSH solution was analyzed with the atomic absorption spectrometer.
2. 2 Computational methods
In order to ascertain the selective pre-enrichment mechanism of BDT-PS to Cd2+cation, we employed the Gaussian view (Gaussian company) to set up the initial configuration of BDT molecules and five BDT-M2+complexes (M = Cd, Cu, Hg, Pb, Co). All optimized geometries and vibrational frequencies were performed using the DFT method at the B3LYP/6–311G (d,p) level with the Gaussian09 package[21]. By this way, some properties of com- plexes were obtained including the optimized geo- metries, vibration frequency of the frontier mole- cular orbital energy level, frontier molecular orbital, charge distribution, configurational energy (Δ), etc.
3 RESULTS AND DISCUSSION
3. 1 Geometrical structure of BDT
Fig. 3 shows the optimized geometry of BDT molecule at the DFT-B3LYP/6-311G (d, p) level, the computing convergence precision selected a default value in the program, and the geometrical structure was determined with minimum point of energy surface (no imaginary vibration frequency), sugges- ting that the structure is the most stable.

Fig. 2. Schematic diagram of the metal ions enrichment of BDT-PS under the stimuli of GSH
Fig. 3. Optimized geometry of BDT
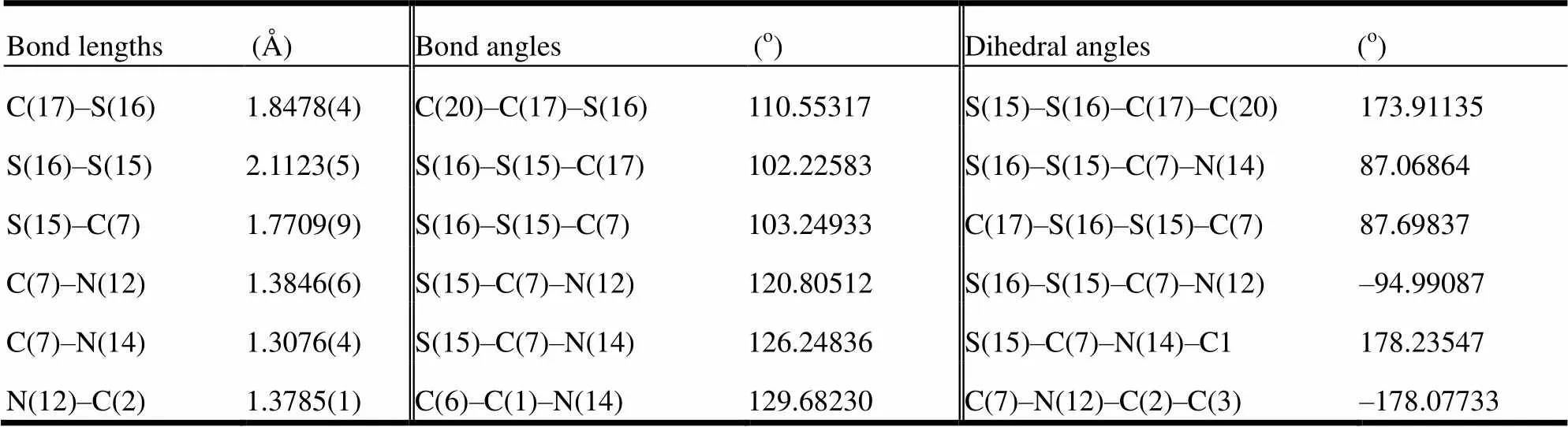
Table 1 lists the selected structural parameter of optimized BDT. M. Aida et. al[22] have drawn a conclusion that the disulfide bond length is closely related with its structure. When the dihedral angle is 90°, the disulfide bond length is around 2.14 Å. As can be seen from Table 2, in the BDT ligand, the bond length of S(16)–S(15) is 2.1123(5) Å and the dihedral angle of C(17)–S(16)–S(15)–C(7) is 90°, which agree well with M. Aida’s conclusion. In addition, the bond length of C(7)–N(14) is shorter than that of C(7)–N(12), showing C(7)–N(14) is a double bond. The shorter distance also presents the combination of C(7) and N(14) is obviously stronger than that of C(7) and N(12) atoms, which can be attributed to part of the electrons N(12) flowing to the H(13) atoms. Meanwhile, compared with S(15)–C(7)–N(12) (120°), the bond angle of S(15)– C(7)–N(14) is increased to 126° under the strong action of N(14). As can be seen from all dihedral angles, the benzimidazole group is coplanar. Further- more, bond lengths of C–N and C–C are shorter than the normal single bond[23] and the average bond lengths also suggest the participation of N atoms in the π conjugation.
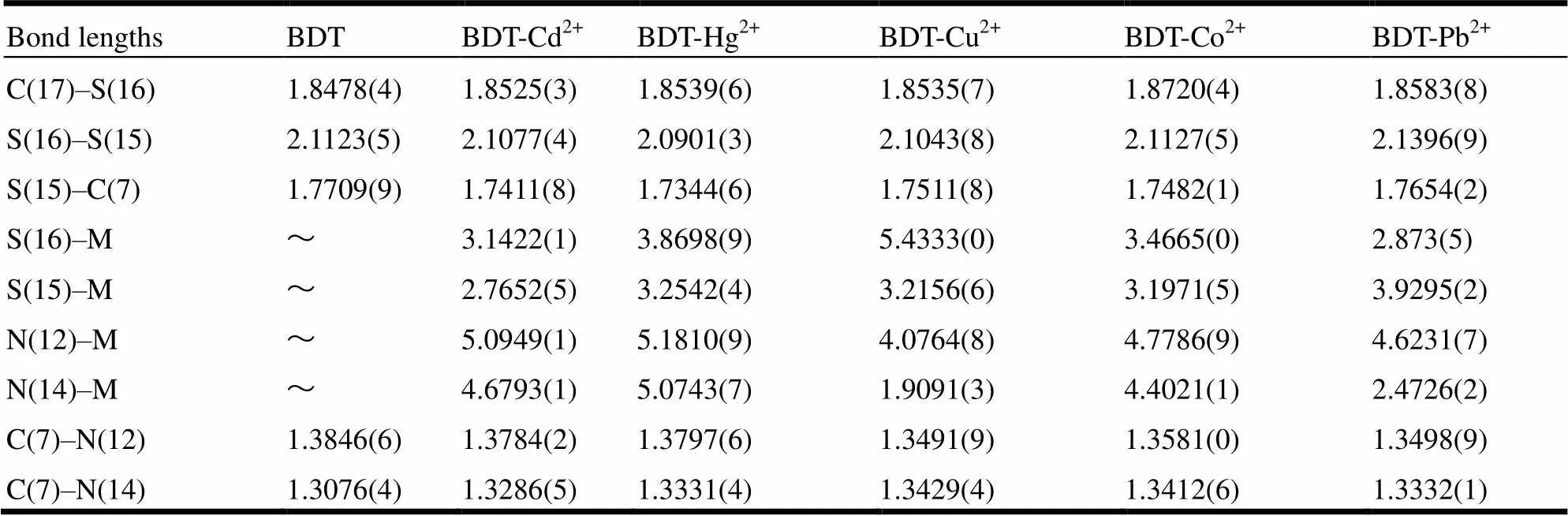
Table 2. Selected Bond Lengths of BDT and BDT-M2+ (Å)
3. 2 Molecular orbital analysis
N, S and O atoms of BDT ligand as the base can chelate with metal ions (the acid) to obtain new complexes. It is well known that the HOMO and LUMO play an especially important role in explaining the chemical reactivity. HOMO energy corresponds organic molecules to donate electronic power[26], and its components correspond to the center atoms of chemical reaction. However, LUMO energy associates with the ability of ligand to accept electrons.
To investigate the chelating ability of BDT, we employed the Gaussian 09 software to calculate the highest occupied-lowest unoccupied molecular orbital (HOMO-LUMO) of BDT, as shown in Fig. 4. The results displayed HOMO and LUMO energies of BDT were –0.237 and –0.066 a.u., respectively. The higher HOMO energy indicates the elec- tron donor ability of BDT molecule is stronger, while the lower and negative LUMO energy exhibits BDT has strong ability to accept the feedback electrons. Furthermore, we find that HOMO of BDT is mainly composed ofpandorbitals from C, N(14) and S(16) of the imidazole group, and LUMO is made up by theorbital from N atoms, as well asandfrom S atoms of the imidazole group. Hence, it can be seen that N and S atoms are the active coordination sites of BDT ligand and the metal cation.
3. 3 Geometries of BDT-M2+
In order to study the coordination process of BDT and the heavy metal ions (M2+), the total optimized stable geometries of BDT-M2+were obtained. After chelating different metal cations, the disulfide group of BDT will generate significant transformations. Table 3 lists the selected bond lengths of BDT and BDT-M2+. As we can see, N and S atoms of the BDT ligand are most likely atoms that can mainly bond with the metal ions, which is basically consistent with the result of previous frontier orbital analysis. The shorter bond lengths of N(14)–Cu (1.9091(3) Å) and N(14)–Pb (2.4726(2) Å) indicate that stable covalent bonds exist between Cu2+or Pb2+and N(14) atoms. In addition, Pb2+cation can also form the covalent bond with the S(16) atom, which con- tributes to the stability of the BDT-M2+complexes. Expect Cu2+and Pb2+, metal cations Cd2+, Co2+and Hg2+mainly bond with the S(15) atoms and their combining abilities are not equal to that of Pb2+and Cu2+cations by comparison to the bond length. Thus, five kinds of metal cations combine the BDT by chelating order where Pb2+, Cu2+ions occupy the first place best, Cd2+cation comes second, and Hg2+and Co2+cations take third place.
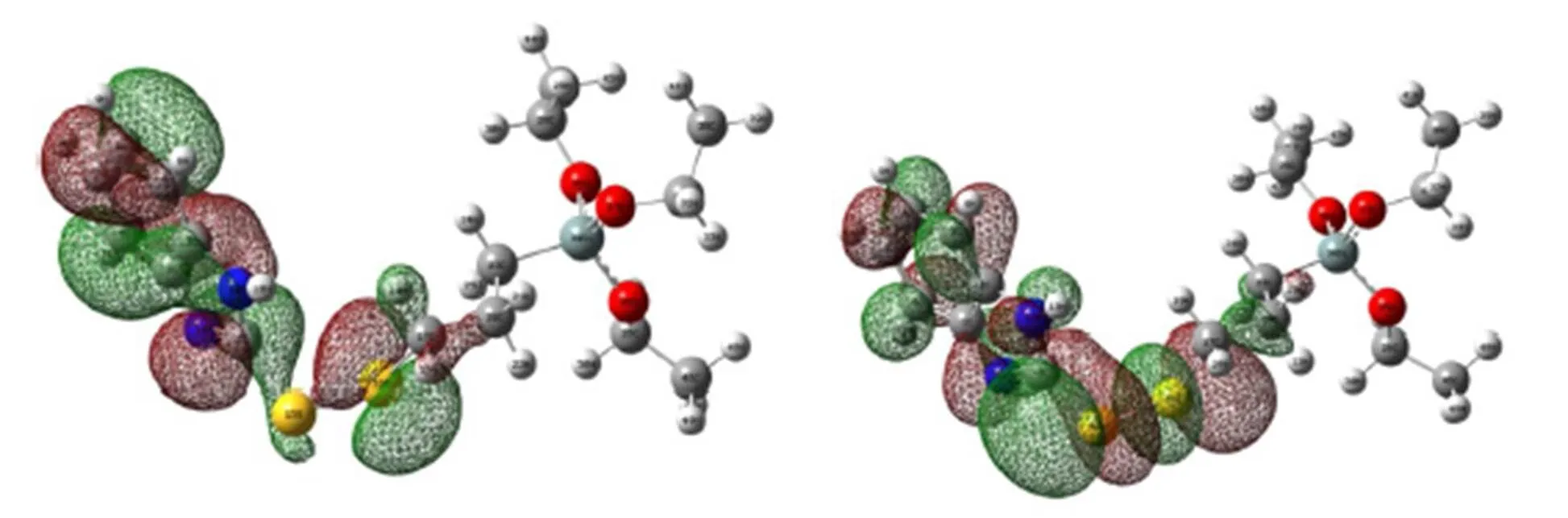
Fig. 4. HOMO and LUMO orbitals of BDT
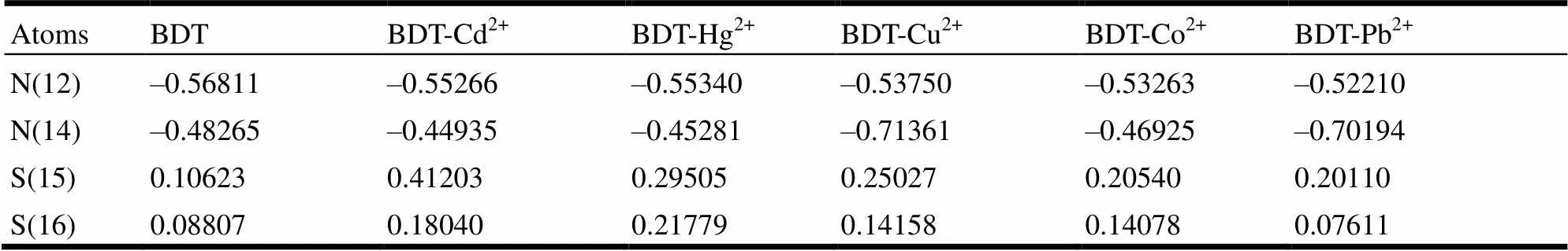
Table 3. Natural Population Analysis (NPA) of Selected Atoms in BDT and BDT-M2+
In order to in-depth study the contribution of charge distribution and charge transfer of BDT-M2+to their coordination structures and geometry energies, we probed into the natural population analysis (NPA) of BDT-M2+(M = Cd, Cu, Hg, Pb, Co), and list the natural charges of selected atoms in Table 4. Compared with the BDT ligand, the charge distributions of N(14), S(15) and S(16) atoms have greatly changed in the BDT-M2+complexes, indica- ting that the charge transfer occurred between the ligand and metal ions due to the redistribution of charge. To sum up, the order of charge transfer between the metal cations and N, S atoms of BDT is as follows: Cu2+>Pb2+>Cd2+>Hg2+>Co2+, which is basically in good accordance with the bonds of BDT-M2+.
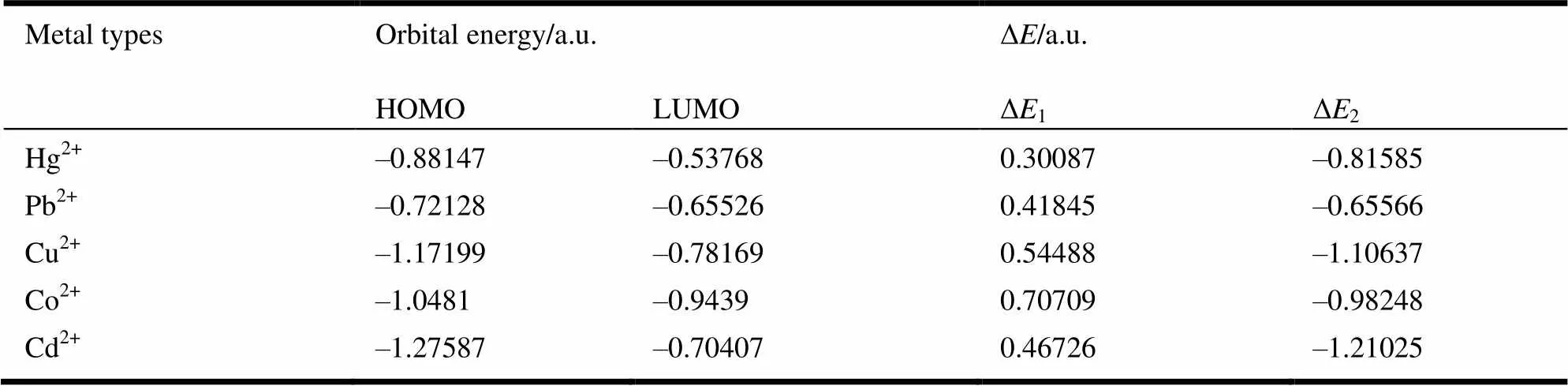
Table 4. Energies of HOMO, HULO and the Energy Gaps of Different Metal Ions
3. 4 Chelation ability of BDT-M2+ complexes
In order to determine the match degree of binding energy between ligand BDT and different metal ions, B3LYP/6-31+G (d, p) was employed to calculate the HOMO and LUMO of five metal ions, and the results are shown in Fig. 5. Wang et al.[27]have reported that the energy matching degree can been proved by the energy difference (Δl) of HOMO of BDT and LUMO of metal ions, as well as the energy difference (Δ2) of HOMO of metal ions and LUMO of BDT[28]. Table 5 presents theenergies of HOMO, LUMO and the energy gap of different metal ions. Owing to the small difference of Δl, binding of BDT with metal ions can be identified as the "soft is close to soft" process. Hence, for metal ions, the softer the acidity is, namely, the higher the LUMO energy is, the more energy matching with BDT ligands, that is, the order of BDT energy matching with metal ions follows by Hg2+>Pb2+>Cd2+>Cu2+>Co2+. Compared with the above order of charge transfer, Hg2+and Cu2+cations are found to interchange in the order of energy matching. The reason may be that besides energy matching, the symmetry of spatial structure is the other key factor in the coordination process of metal ion and BDT ligand. Based on the outermost electronHg (51062) and Cu atoms (31041), when they lose two electrons to form ions, the outermost electrons layer turned into 510and 39. Due to the full electron state of outermost orbital of Hg2+, when chelated, its outermost electrons are so stable that they are difficult to interact with the BDT ligand, resulting in the less charge transfer between Hg2+and the BDT ligand. On the contrary, the outermost electron of Cu2+cations is not full state, and vacant orbitals are very good to accept electrons from the ligand. Therefore, the charge transfer is more between them to form stable chemical bonds, and BDT shows strong adsorption capacity for Cu2+cation.

Fig. 5. Energy of the HOMO, LUMO of BDT and different metal ions (M2+)
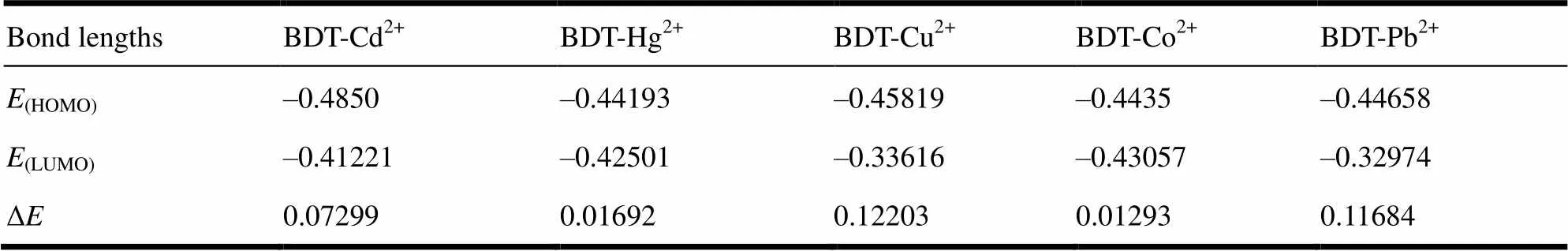
Table 5. Energies of HOMO, HULO and the Energy Gap of BDT-M2+
Fukui[29]suggested in the frontier orbital theory that energy difference Δ= (LUMO–HOMO) is very important to the stability index, that is, the greater Δvalue is, the more stable the molecule, the worse the activity in the chemical reaction; the smaller Δvalue is, the better the activity in the chemical reaction. The related theory has been confirmed by a large number of prior studies[30, 31]. So, it is speculated that the better the stability of BDT-M2+is, the more impossible the BDT and M2+bond will be. We can estimate the coordination ability of BDT- M2+with their stability. Table 6 shows the energies of HOMO (HOMO), HULO (LUMO) and the energy gap of BDT-M2+(Δ). As can be seen from Table 6, the higherHOMOvalues indicate electrons are easy to transfer from the highest occupied orbital of ligand to the central metal cation to form complexes. The lower and negativeHOMOvalues point out the complexes are stable and not easy to lose electrons but to accept electrons, which has also been verified by the lowerLUMOvalues. The order Δvalues of BDT-M2+follows by: [BDT-Cu2+]>[BDT-Pb2+]>[BDT-Cd2+]>[BDT-Hg2+] >[BDT-Co2+], suggesting the chelating ability of BDT to Cu2+, Pb2+, Cd2+, Hg2+and Co2+decreases in turn. The result agrees with those of the bond length and NPA.
3. 5 Stimulus response behavior of BDT
To calculate the disulfide bond fracture of BDT under the GSH action, Fig. 6 shows the optimized geometry of BDT-GSH at the DFT-B3LYP/6-311G (d,p) level. The computing convergence precision selected a default value in the program, and the geometrical structure was determined with minimum point of energy surface (no imaginary vibration frequency), suggesting that the structure is the most stable. As can be obviously seen, after adding the BDT ligand into the GSH solution, the thiol (-SH) of GSH can affect the disulfide bond (S–S) of BDT ligand and "split" S–S bond to make the benzimi- dazole group (MBI) fall off from the BDT molecules. The new S(51)–S(50) bond is formed between the thiol (-SH) of GSH and the BDT ligand, and compared with that of S(15)–S(16) in the BDT ligand (2.1123(5) Å), S(51)–S(50) bond length slightly reduces to 2.0929(0) Å, which indicates that the new disulfide bond is stronger. Meanwhile, the S(49) atoms from the detached MBI are far away from the S(50) and S(51) atoms, where the bond lengths are 4.0117(6) and 5.8106(3) Å, respectively. The conclusion also confirms that the benzimidazole group disconnects from the BDT ligand under the action of GSH.
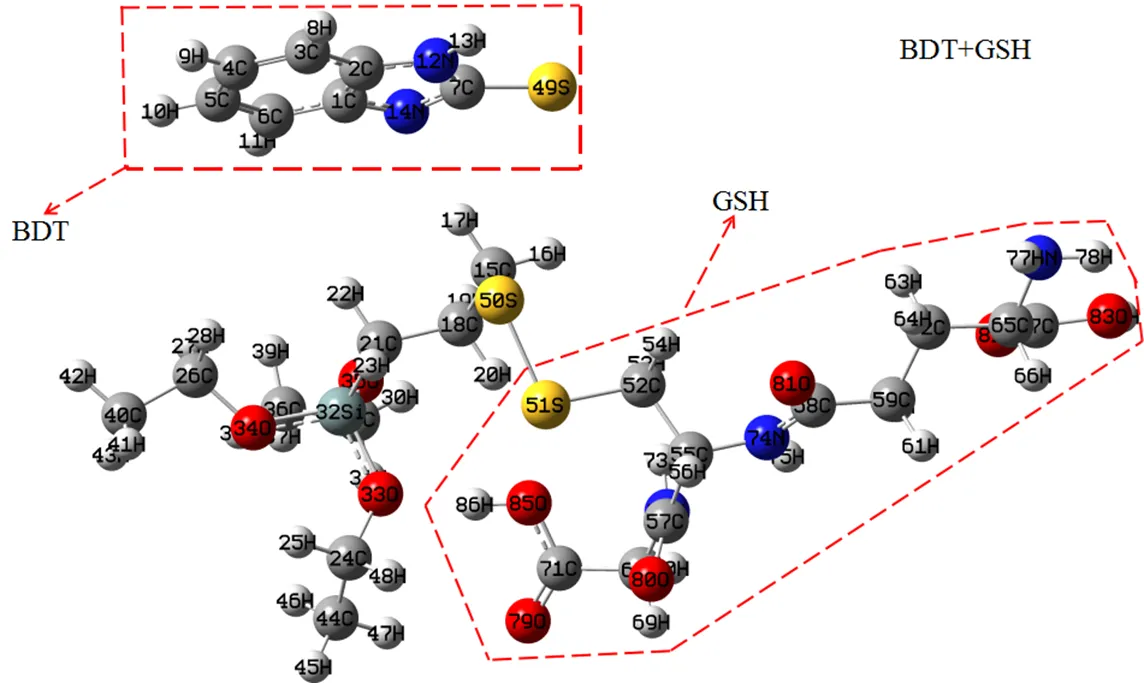
Fig. 6. Optimized geometries of 2-mercap to BDT after adding GSH
Fig. 7. Optimized geometries of five BDT-M2+complexes after adding GSH
In addition, we established the initial and optimi- zed geometries of BDT-M2+after adding the GSH, as shown in Fig. 7. Comparing with the optimized structures, we found, for the different BDT-M2+system, GSH has almost "attacked" the disulfide bond of the BDT ligand and the new disulfide bond linked GSH to BDT molecules to obtain the new GSH derivative (GSHD). However, after BDT–Cd2+reacts with GSH, the deciduous MBI–Cd2+molecu- lar fragments entered the GSH solution due to the weaker bond with SGHD. On the contrary, after the BDT-M2+(M = Hg, Pb, Cu, Co ) react with GSH, the loss of MBI-M2+will continue to chelate part of atoms of GSHD to prevent the fragments from entering into the solution. Therefore, we can draw a conclusion that the BDT ligand can pre-enrich selectively the Cd2+cation in response to GSH stimulation, which is basically consistent with the enrichment experiments.
4 CONCLUSION
Density functional theory (DFT) at the B3LYP/6- 311G (d,p) level has been performed for five BDT-M2+complexes. The mechanism of BDT derivatives to pre-enrich selectively Cd2+cation under the stimulation of GSH was investigated, and the conclusions are as follows:
(1) The HOMO-LUMO and NPA of BDT indicate that N, S atoms of BDT as the active site can combine with metal ions to build the BDT-M2+complex; compared with the bond length and frontier orbital energy gaps of five BDT-M2+complexes, the BDT ligands show selectivity coordination characteristics with different metal ions with the coordination ability order as follows: Cu2+>Pb2+>Cd2+>Hg2+>Co2+.
(2) The computation results showed before chelating the heavy metal ions, the disulfide bond (S–S) of the BDT ligands will be “attacked” by the -SH of GSH, then the MBI group falls off, a new S–S bond is to build, and GSH will replace MBI to form a new GSH derivatives (GSHD); after chela- ting the heavy metal ions, the addition of GSH will also construct a new S–S bond between GSH and BDT-M2+to obtain the MBI-M2+group. It is worth mentioning that only in the MBI-Cd2+group, the deciduous MBI can not chelate GSHD, while the other four MBI-M2+can combine GSHD so as to prevent the metal ions from entering into the solution. Hence, we think porous silicon composite modified by BDT has a certain selective pre- enrichment of Cd2+cation.
(1) Xiao, M.; Fu, Q.; Shen, H.; Chen, Y.; Xiao, W.; Yan, D.; Tang, X.; Zhong, Z.; Tang, Y. A turn-on competitive immunochromatographic strips integrated with quantum dots and gold nano-stars for cadmium ion detection.2017,178, 644-649.
(2) Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J. A.; Ma, S. A metal-organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection.2017,5, 8385-8393.
(3) Deng, G. X.; Li, K. Z.; Cheng, X. M.; Gu, Z. H.; Lu, C. Q.; Zhu, X. Red mud as oxygen carrier for chemical looping combustion of methane: reactivity and cyclic performance.2018, 39, 327-336.
(4) Gao, C. Y.; Tong, J. H.; Bian, C.; Sun, J. Z.; Li, Y.; Wang, J. F.; Gong, S.; Hui, Y.; Xia, S. H. Electroanalytical sensing of trace Cd(Ⅱ) usingbismuth modified boron doped diamond electrode.2018, 39, 447-454.
(5) Oishi, M.; Hayama, T.; Akiyama, Y.; Takae, S.; Harada, A.; Yamasaki, Y.; Nagatsugi, F.; Sasaki, S.; Nagasaki, Y.; Kataoka, K. Supramolecular assemblies for the cytoplasmic delivery of antisense oligodeoxynucleotide: polyion complex (PIC) micelles based on poly (ethylene glycol)-SS-oligodeoxynucleotide conjugate.2005, 6, 2449-2454.
(6) Bulmus, V.; Woodward, M.; Lin, L; Murthy, N.; Stayton, P.; Hoffman, A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs.2003, 93, 105-120.
(7) Wang, Y.; Lu, J.; Tang, L.; Chang, H.; Li, J. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds.2009, 81, 9710-9715.
(8) Qiu, B.; Stefanos, S.; Ma, J.; Lalloo, A.; Perry, B. A.; Leibowitz, M. J.; Sinko P. J.; Stein S. A hydrogel prepared by in situ cross-linking of a thiol-containing poly(ethylene glycol)-based copolymer: a new biomaterial for protein drug delivery.2003, 24, 11-18.
(9) Wang, S.; Zhou, Y.; Guan, W.; Ding, B. Preparation and characterization of stimuli-responsive magnetic nanoparticles.2008,3, 289-294.
(10) Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery.2013, 12, 991-1003.
(11) Wu, W.; Lin, Z. F.; Liu, Y. P.; Xu, X. Y.; Ding, C. M.; Li, J. S. Thermoresponsive hydrogels based on a phosphorylated star-shaped copolymer: mimicking the extracellular matrix for in situ bone repair.2017, 5, 425-434.
(12) Xue, G.; Lu, Y. Various adsorption states of 2-mercaptobenzimidazole on the surfaces of gold and silver studied by surface enhanced Raman scattering.1994, 10, 967-969.
(13) Pourreza, N.; Ghanemi, K. Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole.2009, 161, 982-987.
(14) Zhang, M. L.; Zhang, Z. H.; Luo, L. J.; Yang, X.; Liu, Y. N.; Nie, L. H. Preparation and adsorption properties of magnetic Fe3O4@SiO2@CS cadmium ion-imprinted polymer.2011, 32, 2763-2768.
(15) Xu, F.; Shi, X.; Zhang, Q.; Wang, W. Mechanism for the growth of polycyclic aromatic hydrocarbons from the reactions of naphthalene with cyclopentadienyl and indenyl.2016, 162, 345-354.
(16) Xiao, R.; Noerpel, M.; Luk, H. L.; Wei, Z.; Spinney, R. Thermodynamic and kinetic study of ibuprofen with hydroxyl radical: a density functional theory approach.2014,114, 74-83.
(17) Zhuang, S.; Wang, H.; Ding, K.; Wang, J.; Pan, L.; Lu, Y.; Liu, Q.; Zhang, C. Interactions of benzotriazole UV stabilizers with human serum albumin: atomic insights revealed by biosensors, spectroscopies and molecular dynamics simulations.2016, 144, 1050-1059.
(18) Qu, R.; Liu, H.; Feng, M.; Yang, X. Wang, Z. Investigation on intramolecular hydrogen bond and some thermodynamic properties of polyhydroxylated anthraquinones.2012, 57, 2442-2455.
(19) Zeng, X.; Qu, R.; Feng, M.; Chen, J.; Wang, L.; Wang, Z. Photodegradation of polyfluorinated dibenzo--dioxins (PFDDs) in organic solvents: experimental and theoretical Studies.2016, 50, 8128-8134.
(20) Chen, J.; Qu, R.; Pan, X.; Wang, Z. Oxidative degradation of triclosan by potassium permanganate: kinetics, degradation products, reaction mechanism and toxicity evaluation.2016, 103, 215-223.
(21) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery Jr., J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian, Inc., Petersson, G. A.2009.
(22) Aida, M.; Nagata, C. AnMO study on the disulfide bond: properties concerning the characteristic SS dihedral angle.1986, 70, 73-80.
(23) Yang, G.; Long, X. Y. A quantum chemistry study on the electronic structure of mercapto flotation reagents and its coordination with metal ions.2001, 22, 86-90.
(24) Pearson, R. G. Hard and soft acids and bases.1963, 85, 3533-3539.
(25) Pearson, R. G.; Songstad, J. Application of the principle of hard and soft acids and bases to organic chemistry.1967, 89, 1827-1836.
(26) Liu, G. Y.; Zhan, J. H.; Zhong, H.; Xia, L. Y.; Wang, S. Theory study on chemical reactivity of 2-mercaptobenzothiazole,2-mercaptobenzoxazole and 2-mercaptobenzimidazole in solution.2010, 20, 2248-2253.
(27) Zhong, H.; Wang, S.; Qiu, Y.; Wang, A.Synthesis of chelating resin PETU and its adsorption to Ag(I).2007, 7, 689-693.
(28) Qiu, Y.; Zhang, Q.; Wang, S. Preparation of felt-metal supported modified polyvinyl alcohol composite hydrophilic ultrafiltration membrane.2005,12, 448-452.
(29) Fukui, K. Formulation of the reaction coordinate.1970, 74, 4161-4163.
(30) Shi, J.; Qu, R.; Feng, M.; Wang, X.; Wang, L.; Yang, S.; Wang, Z. Oxidative degradation of decabromodiphenyl ether (BDE 209) by potassium permanganate: reaction pathways, kinetics, and mechanisms assisted by density functional theory calculations.2015, 49, 4209-4217.
(31) Qu, R.; Liu, J.; Li, C.; Wang, L.; Wang, Z.; Wu, J. Experimental and theoretical insights into the photochemical decomposition of environmentally persistent perfluorocarboxylic acids.2016, 104, 34-43.
27 March 2018;
25 August 2018
① This work was supported by the National Natural Science Foundation of China (No. 51504117, 61764009, 51762043), Yunnan Youth Fund Project (2016FD037), Talent Development Program of KUST (KKSY201563032) and the Program for Innovative Research Team in University of Ministry of Education of China (No. IRT_17R48)
E-mail: lsy415808550@163.com
10.14102/j.cnki.0254-5861.2011-2015
- 结构化学的其它文章
- Nanoclusters Au19Pd and Au19Pt Catalyzing CO Oxidation: a Density Functional Study①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Review on Li-insertion/extraction Mechanisms of LiFePO4 Cathode Materials①
- Synthesis of a NovelImidazole Ionic Liquid Modified Mesoporous Silica SBA15 for Selective Separation and Determination of Inorganic Arsenic in Rice①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①
- Studies on a Series of Coumarin Derivatives for Anticancer Activity against Breast Carcinoma Cell Line MCF-7 and Their Molecular Design

