Stable isotopes reveal varying water sources of Caragana microphylla in a desert-oasis ecotone near the Badain Jaran Desert
Hai Zhou , WenZhi Zhao *, ZhiBin He , Heng Ren ,2
1. Linze Inland River Basin Research Station, Key Laboratory of Ecohydrology of Inland River Basin, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
2. Lanzhou Information Center, Chinese Academy of sciences, Lanzhou, Gansu 730000, China
ABSTRACT Understanding the variation in a plant's water sources is critical to understanding hydrological processes in water-limited environments. Here, we measured the stable-isotope ratios (δ18O) of xylem water of Caragana microphylla, precipitation,soil water from different depths, and groundwater to quantitatively analyze the proportion of water sources for the shrub.We found that the water sources of C. microphylla differed with the plant's ages and the seasons. The main water source for young shrubs was upper-soil water, and it showed significant changes with seasonal precipitation inputs. In summer,the proportion contributed by shallow water was significantly increased with increased precipitation inputs. Then, the contribution from shallow-soil water decreased with the decline in precipitation input in spring and autumn. However, the adult shrubs resorted to deep-soil layers and groundwater as the main water sources during the whole growing season and showed much less seasonal variation. We conclude that the main water source of the young shrubs was upper-soil water and was controlled by precipitation inputs. However, once the shrub gradually grew up and the roots reached sufficient depth, the main water sources change from the upper-soil layer recharged by precipitation to deep-soil water and groundwater, which were relatively stable and abundant in the desert ecosystem. These results also suggest that desert shrubs may be able to switch their main water sources to deep and reliable water sources as their age increases, and this adjustment to water availability carries significant importance for their acclimation to the desert habitat.
Keywords: water-use pattern; oxygen stable isotope; water sources; Caragana microphylla
1 Introduction
In arid and semi-arid environments, water is the principal limiting resource that determines a plant's survival and growth, its carbon fixation, and vegetation distribution (Fay et al., 2003; Bunker et al.,2005). Precipitation and groundwater are the main water sources for plants (Ehleringer et al., 1991;Dawson et al., 2002). Plant activity is tightly coupled to water availability, and water-use strategies influence plant species' responses to fluctuating environmental conditions (Xu and Li, 2006). In arid and semiarid regions, the uneven distribution of precipitation brings about extreme changes in soil water over both space and time (Schwinning and Ehleringer,2001; Loik et al., 2004; Trogisch et al., 2016). In re-gions where evaporation demand exceeds precipitation and the upper-soil water tends to be unavailable,plants are likely to regulate their water-use patterns;and their roots have to acquire any remaining soil moisture (Lilley and Fukai, 1994; West et al., 2008;Grossiord et al., 2017). Therefore, patterns in the water use of desert plants play a crucial role in shaping plant adaptation in water-use strategies and in determining the composition of plant communities.
Plants in desert environments often rely on access to deep and moist soil layers to withstand seasonal heat waves and droughts (Eggemeyer et al., 2009;Rossatto et al., 2012; Grossiord et al., 2017). Water-use patterns can also vary following changes in precipitation source (Snyder and Williams, 2003; Barbeta et al., 2015). This ability to shift among main water sources based on available soil moisture depends on the depth and distribution of functional roots (Dawson and Pate, 1996). Desert plants exploit deep, dependable water sources, such as deep-soil water or groundwater, making it possible for some plants to survive long periods without rain or to overcome extreme drought conditions (Wu et al., 2014; Dai et al., 2015).Some plants also have a dimorphic rooting system,with roots distributed in shallow and deep soils, which can enable the uptake of seasonal rain and simultaneously allow the extraction of water from deeper soils that have been charged during previous seasons(Ehleringer and Dawson, 1992; Dawson and Pate,1996). Therefore, species adapted to recurrent droughts by maintaining these characteristic rooting systems may have a competitive advantage in the arid ecosystem (Zhou et al., 2015; Grossiord et al., 2017).However, changes in water-use patterns by plants might be expected due to the different above- and belowground microenvironmental characteristics experienced by plants of different ages and sizes in arid and semiarid regions (Donovan and Ehleringer, 1991;Matzner et al., 2003; Song et al., 2016). Adult plants may have deeper roots and, therefore, greater access to deeper water sources than young plants (Drake et al., 2011; Kerhoulas et al., 2013), thereby avoiding or minimizing the effects of drought in arid and semiarid regions.
An analysis of the stable-isotope composition of water provides a powerful tool for expounding the movement of water through ecosystems (Kendall and McDonnell, 1998), and a plant's main water sources can be determined by comparing the stable-isotope ratios of all potential water sources (such as precipitation, soil water from varying depths, groundwater)with those of water extracted from the plant xylem(Ehleringer and Dawson, 1992; Dawson et al., 2002;Eggemeyer et al., 2009; Zhou et al., 2017). The stable isotope can be applied to water-acquisition studies because no fractionation occurs during water absorption by the roots of terrestrial plants (Mensforth et al.,1994; Dawson et al., 2002). If the hydrology and oxygen isotopic composition of potential water sources are analyzed before the water has been exposed to evaporative processes, this isotopic composition is an integrated measure of overall water uptake, reflecting the various zones and depths from which the plant is currently extracting water (Ehleringer and Dawson,1992; Dawson et al., 2002). As such, stable-isotope analyses of a plant's water sources provide a powerful tool to improve our understanding of the plant's active rooting zone and water-uptake processes, and also provide insight into the role of water in influencing the ecological and physiological processes (Ehleringer and Dawson, 1992). This technique has been widely applied in the field of ecohydrology, especially in arid and semiarid regions (Dawson et al., 2002; Eggemeyer et al., 2009; Wu et al., 2014; Garcia-Forner et al., 2016).
C. microphylla, as a typical xerophyte, has physiological and morphological traits that allow it to survive in the frequent aridity, torridity, and other environmental stresses in arid regions (Su and Zhao,2003; Guan et al., 2015). But the influence of variability in water sources on C. microphylla growth is not well understood. Lack of quantitative research on plants' water sources is mainly due to the unpredictable nature of precipitation and the complexity of its function. Based on the stable-isotope technology, the objectives of the current study were to understand how the shrub uses precipitation inputs, which vary strongly with the season, and the groundwater, and how this use changes with as the shrub ages.
2 Materials and methods
2.1 Study site
This study was conducted at the Linze Inland River Basin Research Station, Chinese Academy of Sciences (39°21'N, 100°07'E, 1,374 m a.s.l.), which is located at the Linze oasis fringe and near the Badain Jaran Desert (Figure 1). This region experiences an extremely arid climate. The mean annual precipitation is 116.8 mm (mean for the period 1965-2000),and more than 80% of the precipitation occurs between May and September. But the mean annual open-water evaporation is 2,390 mm, exceeding the mean annual precipitation. The prevailing wind direction is northwest, with a 3.2 m/s mean annual wind velocity. Soils are characterized by a coarse texture and loose structure, and are very susceptible to wind erosion. The groundwater table depth is about 5.0 m,and its seasonal change scale is small. The landscape includes fixed, semifixed, semimobile, and mobile dunes. Native plants on the fixed and semifixed dunes include shrubs (e.g., C. microphylla, Haloxylon ammodendron, Tamarix ramosissima, and Nitraria sphaerocarpa) and herbaceous species (e.g., Bassia dasyphylla, Halogeton arachnoideus, Suaeda glauca,and Agriophyllum squarrosum).
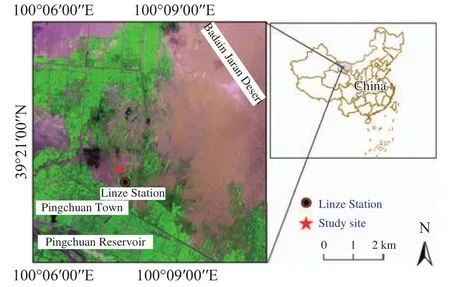
Figure 1 Location of the study site
2.2 Sample collection
Precipitation samples were collected by a simple rain isotopic-sample collector during almost all precipitation events. On each sampling date, we collected groundwater samples as well. Both precipitation and groundwater samples were filtered using a 0.22-μm filter and immediately enclosed in glass vials, wrapped in Parafilm, then refrigerated at 2 °C.Precipitation amounts and temperatures in the study area were recorded by a weather station at the Linze Inland River Basin Research Station. Plant and soil sampling was conducted simultaneously to characterize seasonal water-isotope signatures from April to October 2014. At each site, three healthy shrubs with mean crown breadth of 250 cm were selected; and five small twigs (diameter 0.2-0.5 cm, length 4-5 cm)were collected at the base of the live crown of each study plant at monthly intervals. Phloem from the twigs was immediately removed and placed in glass vials and sealed with a screw-lid and Parafilm wrap,then placed in a frozen box for transportation to the laboratory. For the soil samples, three replicates at soil depths of 0 to 300 cm were obtained by hand auger under the canopy of the study plants on each sampling date; from depths as 0 to 100 cm, collected at 10-cm intervals, and from depths as over 100 to 300 cm at 20-cm intervals. Soil samples were separated into two parts, for stable-isotope analyses and water-content determination. One subsample was immediately double-bagged in glass vials, refrigerated,and transported to the laboratory for stable-isotope analyses; the other subsample was sealed in tin cups for measuring soil-water content (SWC) by the ovendrying method.
2.3 Isotopic analyses
Using cryogenic vacuum distillation, the water of the plant xylem and soil was extracted (Ehleringer et al., 2000) and stored in sealed glass vials at 2 °C before being measured. All water samples were measured by infrared spectroscopy (IRIS) analyzer—the Liquid Water Isotope Analyzer (LWIA, 912-0008-1001,Los Gatos Research Inc., Mountain View, CA, USA).IRIS-extracted stem water of some species may contain compounds that interfere with accurate isotope ratio measurements; these potential contaminants were identified and quantified with the spectral contamination identifier (LWIA) post-processing software, and the isotope values of contaminated water samples were corrected (Schultz et al., 2011). For details of the procedure, refer to Wu et al. (2014). Because hydrogen isotopic fractionation has been observed, but oxygen isotopic fractionation is negligible during water uptake by certain halophytic or xerophytic plants (Ellsworth and Williams, 2007), we used only oxygen isotopes to determine plant-water sources. All stable isotope values are reported in"delta" notation, which expresses the isotopic composition of a material relative to that of an accepted standard (Vienna Standard Mean Ocean Water, VSMOW) on a per mil (‰) basis:
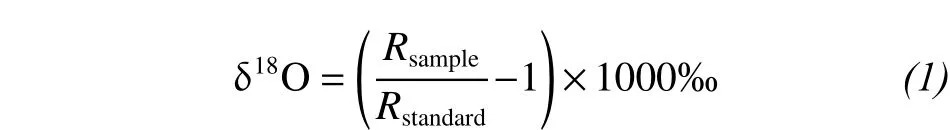
where Rsampleand Rstandardare the oxygen stableisotopic compositions (18O/16O molar ratio) of the sample and standard water (Standard Mean Ocean Water, SMOW).
2.4 Data analyses
Isotopic composition of xylem water was compared with that of potential water sources, with five potential water sources being tested in our research:precipitation, shallow-soil water (0-50 cm), middepth-soil water (50-150 cm), deep-soil water(150-300 cm), and groundwater. The contribution of each source to the plant was calculated by the Iso-Source model (Phillips and Gregg, 2003). Independent-sample t-tests were used to test the differences in δ18O values of xylem water between the young and adult C. microphylla. Significance was determined at the 95% confidence level (a = 0.05). All statistical analyses were performed with SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA).
3 Result
3.1 Stable-isotope variations in precipitation and soil water
In the study area, the total precipitation in 2014 was 102.8 mm, 11.7% lower than the long-term mean annual precipitation. During the measurement period(from April to October 2014), total precipitation was 99.6 mm, accounting for 96.8% of that year's precipitation. There was no precipitation between January and March. Precipitation events less than or equal to 5 mm accounted for 57.8% of the total precipitation and 87.8% of the events, and precipitation events of 5-10 mm accounted for 34.9% of the total precipitation and 14.6% of the events. On July 22, the precipitation event (18 mm) in 2014 happened and was the only event greater than 10 mm (Figure 2). The stableisotope ratios (δ18O) of precipitation changed significantly during the precipitation events, ranging from-7.03‰ to 8.85‰ (Figure 2). The stable isotope of summer precipitation in δ18O values was negatively correlated with precipitation amount (r = -0.732, p =0.039, n = 15), exhibiting a significant effect of precipitation amount (Dansgaard, 1964). The stable-isotope ratios of precipitation in spring (April to May)and autumn (September to October) were lower than those in summer (June to August).
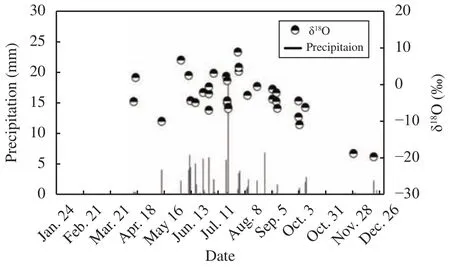
Figure 2 δ18O values for rainwater and the corresponding precipitation events, with the average daily temperature between April and October 2014
3.2 Stable-isotope variations in soil water
During the measurement period in 2014, soil waters (at depths of 0-100 cm) had highly variable stable-isotope ratios (δ18O) due to inputs from rain with variable isotope signatures and from the evaporative enrichment effect. Especially for the upper 40 cm of the soil layer, the stable-isotope ratio (δ18O)for soil water was more affected by enrichment in summer and autumn than in spring, and was depleted as the depth increased (Figure 3a). However, the stable-isotope ratio (δ18O) of the soil water at depths of 40-60 cm and 60-100 cm was significantly more affected by enrichment in spring and summer than in autumn. Perhaps the summer precipitation infiltrated into the deeper soil profile and changed the stable isotope. For the SWC, the upper-soil layer (0-40 cm)changed more extensively than that of the deeper-soil layers, due to the effect of precipitation and soil evapotranspiration. The SWC in the 0-20 cm soil profile was significantly lower than that at 20-40 cm and 40-60 cm in May and June, but it gradually increased from July to September due to the increase in precipitation. At the depth of 50-100 cm, SWC exhibited mild seasonal fluctuations and significantly increased with depth at the site (Figure 3).
3.3 Stable-isotope variations in shrub xylem water
The stable-isotope ratios (δ18O) of C. microphylla xylem water exhibited high variability within seasons and across ages (Figure 4). During the measurement period (April to October 2014), young shrubs had stable-isotope ratios (δ18O) of xylem water ranging from -1.39‰ to 5.66‰. Seasonal change in the stable-isotopic ratios was nearly synchronous with that in precipitation (Figure 4), showing precipitation influenced water sources of the young shrubs. For the adult shrubs, the stable-isotope ratios (δ18O) were lowest, and fluctuated mildly with seasons, and were similar to the ratios of deep-soil water and groundwater. The stable-isotope ratios (δ18O) between the young and adult shrubs showed significant differences (P <0.01), probably indicating that the shrub's water sources changed from shallow-soil water recharged by precipitation, to deep water (including deep-soil water and groundwater) as the plant grew older.
The stable-isotope ratios (δ18O) of soil water at the study site changed with the seasons and exhibited a monotonic decline along the depth of the soil profile (Figure 5). The upper-soil layers were consistently more enriched with the heavier isotope than the lower-soil horizons (Figures 3a, 5). The stable-isotope ratios (δ18O) of soil water at 40-150 cm showed relatively mild fluctuations with the seasons (P <0.05), while those below 150 cm had no significant seasonal variation (P >0.05). Deep soil was usually more depleted in isotope ratios than upper soils but was more enriched than groundwater. Compared with the soil water, the groundwater exhibited relatively steady isotope values (-8.17‰ to -8.91‰ for δ18O)during the measurement period. The stable-isotope ratios (δ18O) of the xylem water of the young shrubs matched that of the soil water at a depth of 20-40 cm and 20-60 cm in April and May, 0-40 cm from June to August (Figure 4). For the adult shrubs, the stable-isotope ratios (δ18O) of the xylem water were similar to those of the soil water at a depth of 150-300 cm and even to the groundwater table during the measurement period (Figure 5).

Figure 3 Seasonal variations in the stable-isotope composition of soil water (a) and soil water content(b) at depths of 0-100 cm in the study site
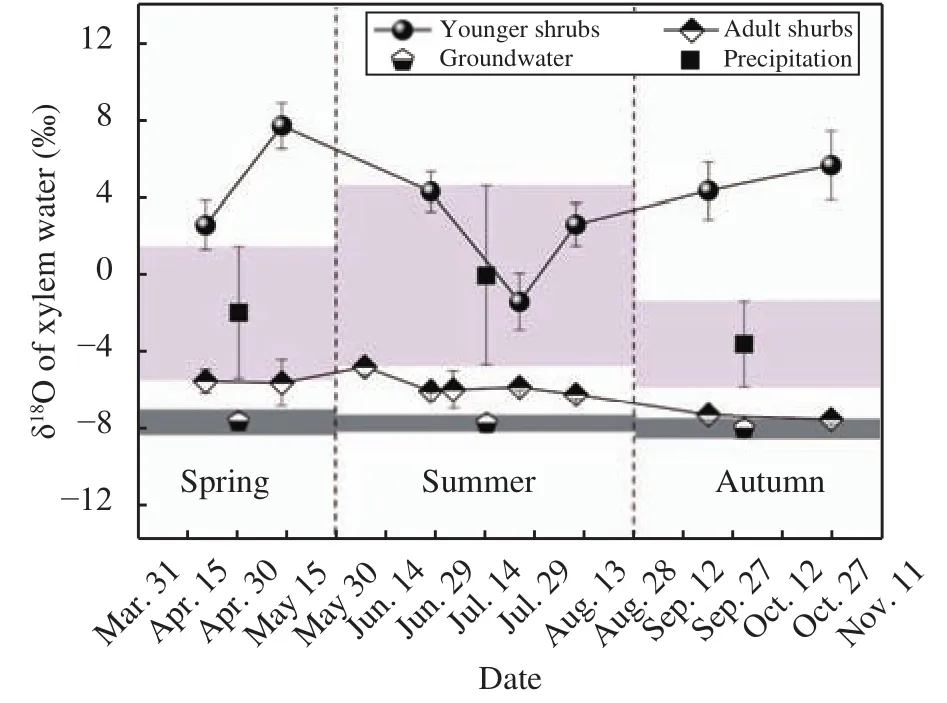
Figure 4 Stable-isotope ratios of precipitation, groundwater,and stem water of Caragana microphylla in the 2014 growing season. The light gray bars depict the average δ18O values for precipitation; the dark grey bars depict the average δ18O values for groundwater
3.4 Plant-water sources
During the measurement period, the main water source of the study species showed great season variation (Figure 5). Using the IsoSource mixing models indicated that shrubs were able to uptake water simultaneously from four potential water sources (shallow-,mid-, deep-soil water, and groundwater) but in varying relative amounts, depending on the seasonal variation of the soil moisture. For the young shrubs in spring (April and May), the contribution of shallow-soil water was in the range of 41.2%-68.3%;and the possible range of contribution by mid- and deep-soil water was 38.5%-55.9% and 0.0%-14.2%,respectively. However, the shallow-soil water contribution increased to 54.2%-78.9% in summer (June to August) as the precipitation increased, decreasing in autumn. Water sources for adult shrubs also fluctuated with the seasons. Shallow- and mid-soil water contributions were 0.0%-13.5% and 0.0%-23.8%, respectively; the deep-soil water and groundwater contributions were 32.0%-45.7% and 41.6%-69.8% in spring, respectively. During the summer, the contribution of shallow-soil water increased, while those of deep-soil water and groundwater decreased, slightly.In fact, the adult shrubs' main water sources were the deep-soil water and groundwater. So, the shrubs'water sources changed from shallow soil charged by precipitation to the more stable and abundant deep water, such as deep-soil water and groundwater as the shrub aged.
4 Discussion
4.1 Variation in stable-isotope of precipitation, soil water, and groundwater
Precipitation is a primary input for soil water and groundwater, and impacts a plant's acquisition of resources and its survivorship in arid regions (Weltzin et al., 2003; Schwinning et al., 2005; Tan et al.,2017). The stable-isotope ratio in precipitation is an integrated tracer of atmospheric processes worldwide and been used widely in understanding ecohydrological processes (Gat, 1996; Farquhar and Gan, 2003;Zhang and Wang, 2016). At the study site, the precipitation stable-isotope composition significantly varied with the seasons, perhaps caused by atmospheric flow paths of vapor trajectories, different water vapor origins, or temperature and the precipitation amounts(Johnson and Ingram, 2004; Yamanaka et al., 2007;Aggarwal et al., 2016). The mean stable-isotope ratio of precipitation was enriched in the hot summer and depleted in spring and autumn, showing a significant temperature effect in arid regions (Guo et al., 2015,Akers et al., 2017).
Under the influence of seasonal precipitation inputs and the evaporation effect, the stable-isotope ratios of upper-soil water shows significant seasonal changes; however, the range of variation of isotope values is obviously less than that of precipitation because soil water is generally a mixture generated by precipitation and the moisture already in the soil(Tang and Feng, 2001; Orlowski et al., 2016). Hydrologic processes—such as precipitation, evaporation,infiltration, and plant uptake and transpiration—regulated the soil-water condition (Piao et al., 2009), causing clear isotopic gradients in the soil profiles (Figure 4).In early spring, low temperature and some water from ice and snow melt recharged the soil water, bringing about low stable-isotope values (Wu et al., 2014;Zhou et al., 2015). As time went on, the isotope values of upper-soil water were changed by rainfall and strong evaporation-caused isotopic enrichment; and the values were over about 5‰ in summer and early autumn (Figure 3a). Due to water molecules that contain light oxygen evaporating slightly more efficiently than water molecules containing heavy oxygen, evaporation produces residual water enriched in the heavier isotopes, relative to the initial isotopic composition (Lee et al., 2007; Song et al., 2011). Because of the limited amount of summer rain that can infiltrate into deep soil, the groundwater capillary uplift is more likely to be recharged the deep soil; the SWC increases and the stable isotope of soil water decreases with depth (Figure 5). It was proposed by Zimmermann et al. (1967) that the effect of evaporation on stable-isotope composition of soil water is affected, with the heavy isotope enriched close to the upper layer and decreased exponentially with depth.In fact, soil water is a mixture from multiple precipitation events' infiltration, even the groundwater capillary recharge, which is the link between rainfall and groundwater (Tan et al., 2017; Yang and Fu, 2017).The stable isotope composition of groundwater showed mild changes with the seasons and was isotopically depleted compared with the summer precipitation. So, the local precipitation infiltration may not be the main source for groundwater. However, the similarity in isotopic compositions of groundwater at our site and river water indicated that the mountain river was a major contributor to recharge in the southern basin. The calculated recharge altitudes indicated that recharge water came from the Qilian Mountains(Zhou et al., 2017).
4.2 Variation in plant-water sources of C. microphylla
Our results showed that the shrub species C. microphylla exhibited strong differences in sources of water uptake between the young and adult shrubs(Figures 4, 5 and 6). This pattern was consistent with the findings of several other studies, which reported that plants could shift water sources from shallow-soil water to deep water as their age increased (Song et al., 2016). The stable-isotope ratio (δ18O) of the shrub xylem water and the IsoSource Model results showed that the young shrubs' main water sources was the upper soil water, but the adult shrubs' water utilized both soil water and groundwater, indicated that adaptive changes in water-use patterns exists in xerophilous plants. In fact, the trees are attributed to the different root distribution, defining the depth to or volume from which plants can potentially extract water (Ehleringer et al., 1991; Dawson and Pate, 1996). For the young shrubs, precipitation-derived upper-soil water is the main water source, with the limited root distribution determining the range of its water uptake. However,given the scant rain in the extremely arid region, precipitation can't meet the water requirement as the shrub's size increases. Thus the species develops not only the shallow, resource-acquiring lateral roots but also, with age, the deep-penetrating tap (sinker) roots,a common trait in desert ecosystems (Zhou et al.,2017). The adult shrubs uptake mostly deep water (including deep-soil water and groundwater), providing a relatively stable water source to avoid drought stress in the desert environment. Indeed, some desert shrubs'survival in desert ecosystems has been showed to totally depend on their ability to absorb deep water by tap roots (Pate et al., 1995; Canadell et al., 1996; Bordron et al., 2018).
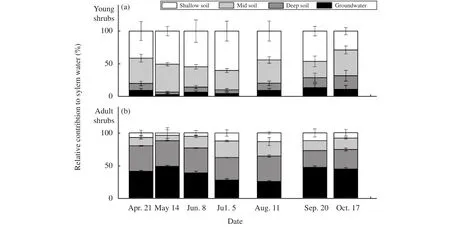
Figure 6 Seasonal changes in the relative mean contribution of soil water at different depths to Caragana microphylla. Data obtained from the IsoSource mixing model: shallow soil (0-50 cm), mid soil (50-150 cm), deep soil (150-300 cm),and groundwater (below 300 cm). Bars represent possible ranges of potential water sources
Isotopic values (δ18O) of shrub xylem water revealed that the young and adult C. microphylla shrub uptake of the water sources showed significantly seasonal variation (Figures 4, 5 and 6). When soil water was relatively plentiful in summer, the shrubs increased the proportion of upper-soil water absorbed,especially for young shrubs. However, when the upper-soil water became less available in spring and autumn, the shrubs increased the proportion of deepersoil water absorbed, especially for adult shrubs, for which the main water sources were the deep-soil water and groundwater. Many studies have demonstrated seasonal variations in utilization of water sources, with most of their transpiration water derived from shallow layers under wet conditions and more from deep-soil layers or groundwater under dry conditions (Dawson and Pate, 1996; Wu et al., 2014;Song et al., 2016). Especially in dry conditions, the shift of the main water sources from shallow layers to deep layers appears to be a very important survival strategy for shrubs in arid desert lands (Dawson and Pate, 1996).
C. microphylla, generally planted in sand dunes for decreasing wind speed and intercepting drift sand,had limited use of shallow-soil water once it grew into an adult shrub (Figure 6). Although plants could use reliable summer precipitation, as the upper-soil water condition had been improved by the precipitation input (Ehleringer and Dawson, 1992), the adult shrubs' main water sources were the deep-soil water and groundwater. A combination of deep-soil water and groundwater has been recognized as a stable and reliable water source, although more than 80% of the rainfall occurred during summer in the study area; the shallow-soil water maintains only more shallow and short-lived soil-moisture resources due to high evaporative demand (Dai et al., 2015; Zhou et al., 2017).The results revealed that upper-soil water was an unreliable water source, especially for some adult shrubs in an arid region. So, with age, shrubs exploit deeper water sources for long-term survival and sustainable development.
5 Conclusions
In this study, we used the stable-isotope ratio(δ18O) for detecting water sources of young and adult C. microphylla species. The results indicated that xylem water δ18O values exhibited a significant difference between the young and adult shrubs, suggesting that the shrub absorbed different water sources at different ages. According to the results of the IsoSource model, the young shrubs took up 41.2%-68.3% of their water from shallow-soil water, with the variation determined by the seasonality of precipitation inputs; but the adult shrubs took up 41.6%-69.8% of their water from groundwater, with very limited seasonal variation. Namely, the water-use patterns of C.microphylla exhibited significant variation with the age. For the young shrubs, upper-soil water recharged by precipitation was the main water source;and it has a significant seasonal variation with the seasonal variation in precipitation inputs. However, the main water sources for adult shrubs were the deepsoil layers and groundwater. Therefore, from a longterm perspective, this water-use strategy of C. microphylla could be an advantage in the competition for the scarce water resources in desert ecosystem.
Acknowledgments:
This study was supported by the National Science Foundation for Distinguished Young Scholars of China (Grant No. 41701035), the Key Program of National Natural Science Foundation of China (Grant No. 41630861), and the National Science Foundation for Post-doctoral Scientists of China (Grant No.2016M602902). The authors are very grateful to the anonymous reviewers and editors for their critical reviews and comments that helped to improve and clarify the manuscript.
 Sciences in Cold and Arid Regions2018年6期
Sciences in Cold and Arid Regions2018年6期
- Sciences in Cold and Arid Regions的其它文章
- The 2018 Academic annual meeting of China Society of Cryospheric Science was held successfully in Foshan on November 17-18, 2018
- Academic Workshop of China Society of Desert in the Geographical Society of China successfully held in Changsha, Hunan Province
- Fossil Taiwannia from the Lower Cretaceous Yixian Formation of western Liaoning, Northeast China and its phytogeography significance
- Local meteorology in a northern Himalayan valley near Mount Everest and its response to seasonal transitions
- Contrasting vegetation changes in dry and humid regions of the Tibetan Plateau over recent decades
- Simulation and prediction of monthly accumulated runoff,based on several neural network models under poor data availability
