First report of Athelia bombacina causing postharvest fruit rot on pear
JIA Xiao-hui , FU Jun-fan WANG Wen-hui, CUI Jian-chao, DU Yan-min, ZHOU Ru-jun SUN Pingping
1Plant Protection College, Shenyang Agricultural University, Shenyang 110866, P.R.China
2Research Institute of Pomology, Chinese Academy of Agricultural Sciences, Xingcheng 125100, P.R.China
AbstractPear is an important fruit crop in the world. An uncharacterized disease has been observed on pear fruits during cold storage in Suning, Shenzhou, Xinji and other locations in Hebei Province, China. The incidence rate of the disease has reached 10%, and sometimes up to 20%. A particular fungus was consistently isolated from the infected pear fruit and cultured. Based on its morphology, molecular characteristics, pathogenicity and ITS sequence, the fungus was identified as Athelia bombacina. To our knowledge, this is the first report of Athelia bombacina causing postharvest fruit rot on pear.
Keywords:pear, pathogenicity, postharvest, Athelia bombacina, fruit rot
1. Introduction
Pear is an important fruit crop in the world. By 2016 in China, the total area of pear cultivation reached 1 113 thousand hectares, the output was 18.704 million tons, and the annual cold storage capacity for pear fruit was more than 5 million tons. Huangguan (Pyrus bretschneideri×Pyrus pyrifolia)pear fruit is a well-known cultivar in Hebei Province, which is the central area of storage and export of pear fruit in China. Unfortunately, an uncharacterized disease has been observed on Huangguan pears during cold storage in as early as 2016, when the incidence of fruit rot was low among stored fruits. By 2018, the incidence rate of the disease on Huangguan pears increased to as high as 10%,and sometimes up to 20% during cold storage in Suning,Shenzhou, Xinji and other locations in Hebei Province.Consequently, the disease has caused severe economic losses to storage enterprises.
The purpose of this study was to describe this new fruit rot disease and identify its pathogen in stored pear fruits.To this end, we isolated and identified the alleged pathogen,and determined the pathogenicity of the isolated strain. Our results can inform future studies on methods to control the disease.
2. Materials and methods
2.1. Observation of disease symptoms
In April, 2017, we collected pear fruits with typical symptoms of rot from the storage facilities within the main production areas of Huangguan pear in Suning and Shenzhou of Hebei Province. Then we observed and recorded the symptoms of the disease.
2.2. Separation, purification and preservation of fungal strains
A routine tissue separation method was used to isolate the pathogenic agent from diseased fruit samples, and purified fungal strains were obtained by the single spore separation technique and preserved at 4°C (Fang 2007).
2.3. Pathogenicity test
A pathogenicity test was performed according to the Koch’s postulates modified by Fang (2007). Healthy Huangguan pears (30 fruits) were gently washed by surface sterilizing with 70% ethanol for 2 s and then 1% sodium hypochlorite for 2 min; and lastly, fruits were washed thoroughly with sterilized distilled water. The healthy pears were then treated with three treatments, artificial unwounded inoculation, artificial wounded inoculation or the control. The artificial wounded inoculation was performed by stabbing a small hole about 0.5 cm deep on the equatorial surface of every fruit with a sterile inoculation needle. Then the fruits were inoculated at the hole with a 5-mm-diam mycelium from the 7-day-old culture of the purified fungal strain or sterile potato dextrose agar medium (PDA) that was also incubated for 7 days in three replicates. The artificial unwounded inoculation was performed by inoculating the equatorial surface of unwounded, healthy fruit with a 5-mm-diam mycelium.Sterile PDA was used to inoculate unwounded fruits as controls. After inoculation, pears of the three treatment groups were incubated in plastic containers on a layer of moist filter paper at 25°C for 10 days. The pathogenicity tests were repeated twice. After the infection developed on treated fruits, the pathogenic fungi were sampled from the lesion, isolated and cultured in order to compare them with the original strain.
2.4. Morphological identification
The purified strains (No. HG-AB20170418), by means of a single spore isolation, were inoculated on plates of PDA,and were cultivated in a biochemical incubator for 20 days at 25°C. After the 5th day, we observed the positive and negative culture traits of the colonies. The morphological characteristics of the cultures were observed under a light microscope after sporulation, and mycelium roughness and conidial size were measured. These characteristics were used to morphologically identify purified strains according to Lu (2004).
2.5. Molecular identification
The original strain was inoculated into a 100-mL PD liquid medium and cultured by shaking at 150 r min-1at 25°C for 7 days. The mycelium were filtered and collected.After freezing and drying, mycelium were preserved at-20°C. The genomic DNA was extracted and purified by the CTAB method. Amplification of DNA by PCR was carried out using fungal rDNA-ITS universal primers ITS1(5´-TCCGTAGGTGAACCTGCGG-3´) and ITS4 (5´-TCC TCCGCTTATTGATATGC-3´), that were synthesized by Shanghai Bioengineering Technology Services Co., Ltd. The PCR reaction conditions were: 94°C hot start for 3 min and 35 cycles of 95°C denaturation for 30 s, 55°C annealing for 30 s, and 72°C extension for 1 min. After detection by 1%agarose gel electrophoresis, the PCR product was purified and sequenced by Shanghai Bioengineering Biotechnology Services Co., Ltd. The sequence was compared with homologous sequences in the GenBank nucleic acid sequence library of NCBI. The phylogenetic tree was constructed using the Neighbor-Joining method of MEGA 7.0.
3. Results and discussion
3.1. Symptoms of infected pear fruit
Initially, the disease symptoms appeared on fruit surfaces as a scatter of light brown spots. The spots enlarged and eventually coalesced together, appearing as circular or elliptical lesions with sunken spots in the center. Over time, a hole developed in the fruit flesh of the infection site(Fig. 1-A). The boundary between diseased and healthy flesh was not obvious (Fig. 1-B). One fruit could have several lesions (Fig. 1-A). Ultimately, the entire surface of a fruit was covered white mycelia.
3.2. Pathogenicity test
Twenty-four hours after inoculation, the site of artificial wounded inoculation began to show symptoms and formed a visible diseased spot (Fig. 1-C). The average rate of expansion of disease spots was 1.21 mm d-1. Pears of the unwounded inoculation treatment showed their first symptoms 72 h after inoculation. They developed multiple small spots, and the average expansion rate of these disease spots was 1.01 mm d-1(Fig. 1-D). Similar symptoms were observed on fungus-inoculated fruits after 4 days.No decay occurred on the control positions of every pear during the experimental time period. Tissue isolation and cultures were carried out to obtain colonies to compare with the original strain. The isolated strain was identical to the original strain. According to the Koch’s postulates modified by Fang (2007), the original strain was a pathogen that caused an unknown disease on Huangguan pear.
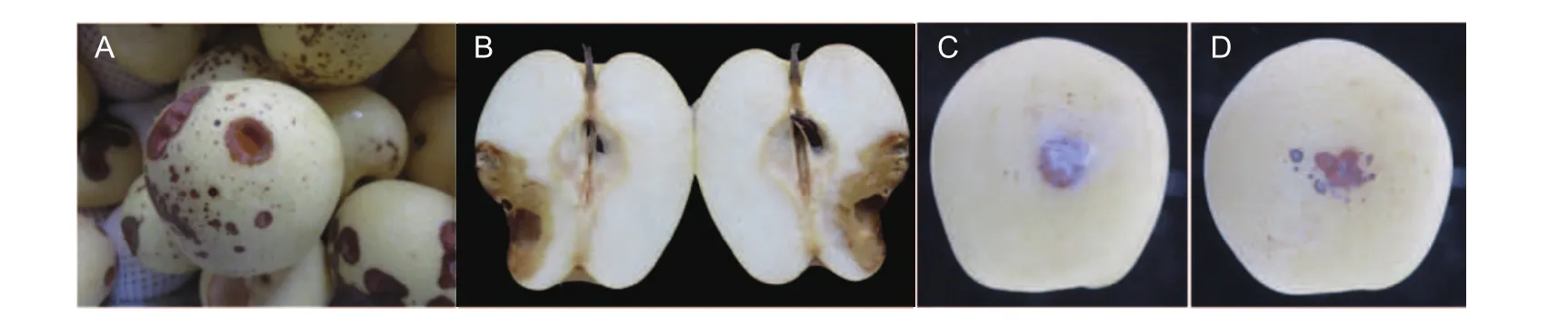
Fig. 1 Symptoms of postharvest fruit rot caused by Athelia bombacina on Huangguan pear. A, natural infection. B, internal symptoms of the spot. C, artificial wounded inoculation at the 10th day. D, artificial unwounded inoculation at the 10th day.
3.3. Morphological characteristics of the pathogen
Uniform colonies were isolated from the tissue of the artificially induced disease from each experimentally treated fruit(Fig. 2-A and B). Colonies consisted of masses of white aerial mycelia that radially expanded at an average rate of 12.77 mm d-1(Fig. 2-C). A mycelium radially expanded on the PDA plate appearing white, slender with a width of 2-3 μm and seperative (Fig. 2-D). It also had a large number of oil drops and clamp connections (Fig. 2-D). The hymenial were white and smooth. The conidia were pear-shaped and hyaline. The size of a spore was (5-8) μm×(3-5) μm(Fig. 2-E).
3.4. Molecular identification of the original strain
The internal transcribed spacer (ITS) region of the pathogen was amplified with primers ITS1/ITS4 and sequenced(White 1990). The resulting sequence was deposited in GenBank under the accession number MH201276 (680 bp).A BLAST search was conducted in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) with the ITS sequence that obtained 99% identity to Athelia bombacina (acc. no. DQ449026 and U85795). We constructed a phylogenetic tree to display the relationships of MH201276 and the group of sequences A. bombacina (KY440254, DQ449026, U85795, etc.) that were available in GenBank (Fig. 3).
By combining the morphological characteristics and molecular methods used to identify the pathogen, we determined that the pathogen causing postharvest fruit rot in our pear samples was A. bombacina. In 2016 and 2017, we isolated the same pathogen of A. bombacina from the stored fruit of two other cultivars of pear, Korla Xiang and Hongxiangsu accession number of ITS sequences are KX610836, MH201277 and MH201278 (Fig. 3). In 2017, Fibulorhizoctonia psychrophila was observed as causing lenticel spot on apple and pear in the Netherlands(Wenneker et al. 2017). According to MegaBLAST analysis,ITS sequences of F. psychrophila revealed 97.2 and 95.4%match to the ITS sequence of Athelia arachnoidea CBS:418.72 (GU187504) and A. epiphylla CFMR: FP-100564(GU187501), respectively.
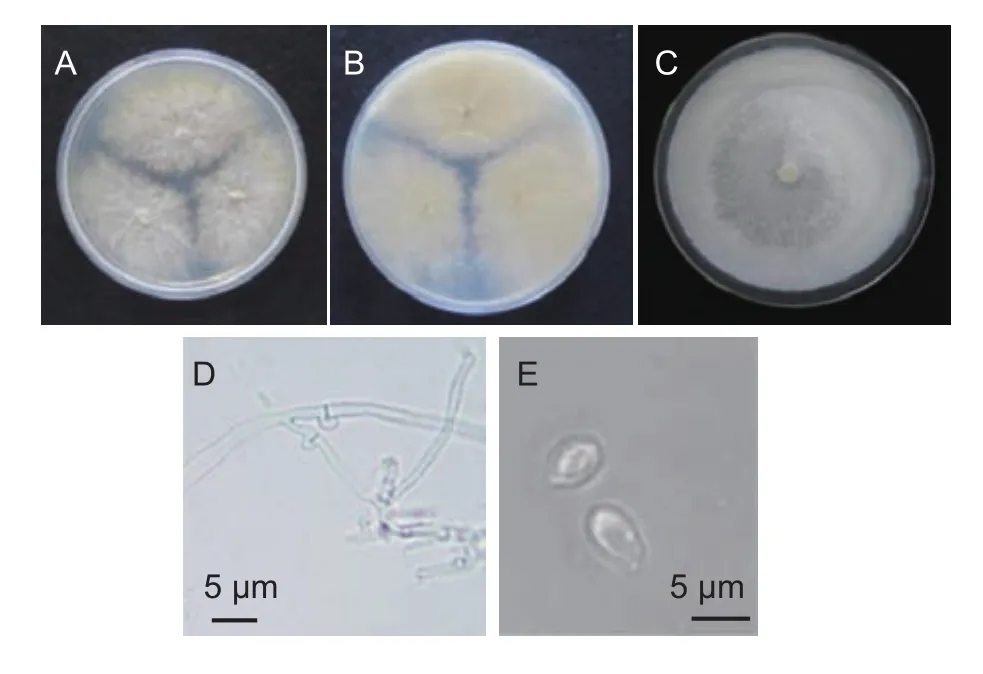
Fig. 2 Athelia bombacina. A, colony on potato dextrose agar medium (PDA) for 5 days. B, reverse colony on PDA for 5 days. C, hymenial. D, clamp connection of aerial mycelium.E, conidia.
Athelia bombacina is rarely reported as a pathogen except of stored sugar beet roots (Toda et al. 2012). However,A. bombacina was first described as an antagonistic strain of the apple scab pathogen, Venturia inaequalis, in 1983 (Heye and Andrews 1983). Athelia bombacina has been used, in a lab and field setting, as a biocontrol agent of the apple scab pathogen and spotted tentiform leafminer (Miedtke and Kennel 1990; Young and Andrews 1990; Carisse et al.2000; Vincent et al. 2004).
4. Conclusion
Based on its morphology, molecular characteristics,pathogenicity and ITS sequence, the fungus was identified as Athelia bombacina. To our knowledge, this is the first report of A. bombacina causing postharvest fruit rot on pear.The disease has caused severe economic losses to storage enterprises in China. In view of the lack of previous studies on the fungus, it is urgent to carry out research on biological characteristics of the fungus, pathogenesis, prevention and control technology of the disease.
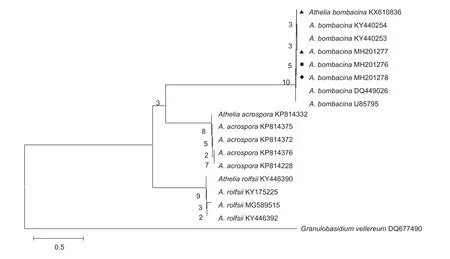
Fig. 3 Phylogenetic tree of HG-AB20170418 (MH201276) constructed based on rDNA-ITS (internal transcribed spacer) sequences.stands for the accession number of pathogen from the stored Korla Xiang pear;stands for the accession number of pathogen from the stored Huangguan pear;stands for the accession number of pathogen from the stored Hongxiangsu pear.
Acknowledgements
This study was supported by a grant from the National Key R&D Program of China (2016YFD0400903-06), the earmarked fund for China Agriculture Research System(CARS-29-19), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP).
 Journal of Integrative Agriculture2018年11期
Journal of Integrative Agriculture2018年11期
- Journal of Integrative Agriculture的其它文章
- Sustainability assessment of potato fields using the DEXi decision support system in Hamadan Province, lran
- Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity
- Alternate row mulching optimizes soil temperature and water conditions and improves wheat yield in dryland farming
- lnter-annual changes in the aggregate-size distribution and associated carbon of soil and their effects on the straw-derived carbon incorporation under long-term no-tillage
- Mycoplasma leachii causes polyarthritis in calves via the blood route but is not associated with pneumonia
- Genome-wide detection of selective signatures in a Duroc pig population
