Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity
LI Wei-hua, LIU Qi-zhi, CHEN Peng
College of Plant Protection, China Agricultural University, Beijing 100193, P.R.China
Abstract Long-term monoculture leads to continuous cropping (CC) problems, which complicate agricultural production, both locally and abroad. This study contrasted the different bacterial community compositions, physicochemical properties and enzyme activities of strawberry soil subjected to CC, CC rhizosphere (CCR), non-CC (NCC) and non-CC rhizosphere(NCCR) treatments. The soil physicochemical properties and enzyme activities were significantly reduced after long-term CC. In addition, five variation trends were observed for the 11 major bacterial genera in the soil. Sphingomonas was the only stable group among all treatments. The proportions of Novosphingobium, Rhodoplanes, Povalibacter, Cellvibrio and Stenotrophobacter decreased after CC. The relative abundances of Pelagibius, Thioprofundum and Allokutzneria increased only in the CC treatment. Nitrospira were more abundant in rhizosphere soil than in non-rhizosphere soil. The relative abundance of Bacillus increased after CC. Redundancy analysis revealed that Bacillus, Pelagibius and Allokutzneria had significant negative correlations with the soil physicochemical properties and enzyme activities. Therefore, these genera may be the key bacteria influenced by the physicochemical properties and enzyme activities altered by replanting. These results indicate that long-term CC of strawberry leads to less favourable rhizosphere soil conditions, which can be understood as a stress-induced response of the bacterial community diversity. Further research is needed to determine how the quality of soil is reduced by the shift in the diversity of the soil bacterial community.
Keywords: rhizosphere soil, high-throughput sequencing, biogeochemical cycle, bacterial diversity
1. Introduction
Long-term monocultures often lead to a change in the structure of soil microorganisms, such as the accumulation of soil-borne pathogens and the growth inhibition of beneficial bacteria (Wang et al. 2017). Changes in the soil microorganism structure lead to a decline in plant health and yield (She et al. 2017). Many studies have demonstrated that the yields of soybean, cotton, eggplant and garlic decline after long-term continuous cropping (CC)of the same land (Wang et al. 2014; Bai et al. 2015; Guo et al. 2015), and this problem also exists for strawberry.CC is a historical problem, particularly for horticultural crops, and there has long been a need to overcome this challenge in agricultural development, both locally and abroad (Suranyi 1998; Yin et al. 2009; Mondal et al. 2013).CC is also known as replanting and refers to a system in which certain crops are “replanted” in soils that have previously supported the same or similar plant species(Rietz and Haynes 2003). This practice has been shown to influence biotic and abiotic factors such as changes in the soil microbial community structure, the deterioration of soil physicochemical properties, decreases in soil enzyme activities and increases in soil-borne pathogens (Fuentes et al. 2009; Huang et al. 2013). Recent studies have shown that CC can decrease soil pH, shifting the soil environment from neutral to acidic. This shift promotes the growth of fungi and inhibits the reproduction of bacteria and actinomycetes in the plant growth process (Vargas Gil et al. 2011).
Soil microorganisms play vital roles in many soil processes, particularly in agricultural production (Kennedy 1999; Garbeva et al. 2004). Microbes are critical for the production of pristine soil and the decomposition of animal and plant residues into soil organic matter (OM) (Yin et al.2010). Microbes regulate nutrient cycling and provide nutrients to crops (Mondal et al. 2013; Ling et al. 2014). In addition to soil-borne plant pathogens, microbial communities also make important contributions to plant growth and health(Baek and Kim 2009), such as the carbon, nitrogen and sulfur cycles. Microbes play key roles in element transport and transformation, during which soil microorganisms are essential for nutrient cycling (Sun et al. 2004). In addition,pollutants, which are degraded by bacteria, affect microbial structure and stimulate functional microbial groups (Fetzner 1998). Many studies have shown that the ecological and environmental factors regulate microbial community and diversity, such as bacterial responses to carbon, nitrogen and phosphorus availability (Kragelund et al. 1997; Ramos et al. 2000; Koch et al. 2001; Axtell and Beattie 2002; Singh et al. 2004; DeAngelis et al. 2005). Furthermore, many of the specific antagonistic microbial communities play an important role in soil in the suppression of soil-borne plant diseases via antibiosis, competition or stimulation of plant host defences (Conway 1996).
Bacteria are the largest component of the microbial groups present in soil. They influence nutrient cycling,soil structure and biological interactions (Kennedy 1999).Bacterial community structure and diversity can be used as indicators of soil quality for agricultural land (Sharma et al. 2010). Bacteria can be classfied as autotrophic,heterotrophic, chemotrophic and phototrophic organisms according to the way they utilizing the diverse energy and carbon sources. Bacteria are more sexually compatible when living in aerobic or anaerobic environments (Mccarthy and Williams 1992). Bacteria produce extracellular polysaccharides that assist in the stabilization of the soil structure (Zelles 1999). In addition, the bacterial community structure and diversity in an ecosystem are influenced by the rhizosphere system and the soil environment (Kennedy 1999). The rhizosphere is the volume of soil under the direct influence of the roots (Singh et al. 2004). Previous studies have suggested that the rhizosphere soil contains abundant microbes that are beneficial to plant growth and plant pathogenic microorganisms, and rhizosphere organisms also influence plant growth and health, especially bacteria(Mendes et al. 2013).
A thorough analysis of the microbial community and diversity requires methodologies for analysing large amounts of information, which is expensive and timeconsuming. Soil microbial diversity analysis involves various methods. Traditional technologies have demonstrated that only a limited minority (ca. up to 5%) of microbes can be cultured, and an enormous quantity of microbial groups cannot be studied using microbial cultivation techniques(Mendes et al. 2013). New methods can be divided into biochemical, molecular and analytical methods, including phospholipid fatty acid (PLFA) and fatty acid methyl ester(FAME) profile analyses. These methods have been used to describe the communities and diversities of soil microorganisms and to distinguish broad groups of fungi and bacteria (Zelles et al. 1992, 1995). Denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (T-RFLP) target a particular locus of 16S rRNA and produce electrophoretic bands that are used to describe the diversity of microorganisms (de Oliveira et al. 2006). Although these methods have overcome the challenge of identifying unculturable microorganisms, they cannot yield accurate classification information. In addition,both techniques require a considerable amount of cloning and sequencing, which are expensive and time-consuming(Yin et al. 2010). At present, the rapid development of high-throughput sequencing (HTS) technology provides a thorough and accurate evaluation of soil microorganism groups (Zhang et al. 2014), as it enables more accurate identification of the base sequences of microbial groups than PLFA, FAME, DGGE and T-RFLP techniques in a complex environment (Sogin et al. 2006; Roesch et al. 2007). As a consequence, this approach offers a more detailed picture of the structure and dynamics of the microbial population in soil ecosystems without the limitation of needing a microbial culture.
This research described a comparative analysis of the physiochemical properties, enzyme activities, bacterial community structures and bacterial abundances in strawberry soils subjected to CC, CC rhizosphere (CCR),non-CC (NCC) and non-CC rhizosphere (NCCR) treatments.This study was designed to: 1) explore the potential correlations among physiochemical properties, enzyme activities and soil bacteria; 2) provide a better understanding of the soil bacterial communities present during strawberry growth in a CC system and a rhizosphere soil system; and 3)determine the key bacteria present during CC of strawberry crops. A future study will investigate changes in the fungal community diversity of strawberry rhizosphere soil. This research will contribute to agricultural production and soil improvements.
2. Materials and methods
2.1. Site description and soil sampling
The study site is located at the Beijing Academy of Forestry and Pomology Sciences (40°1´21´´N, 116°16´32´´E), China.Beijing is one of the primary strawberry-producing areas in China. The agrotype in this area is fluvo-aquic soil. The average annual rainfall and temperature are 628.9 mm and 12.5°C, respectively (Wang et al. 2014). In this study, soil samples were collected from six greenhouses, including three continuously cropped greenhouses and three noncontinuously cropped greenhouses. Continuously cropped soils were those that had been cultivated with strawberries for 12 years. Non-continuously cropped soils were collected from a greenhouse that was planted with strawberries for the first time. The samples were collected on 14 November 2015 (squaring stage). The six greenhouses were at the same location and were maintained under the same climate conditions and tillage management patterns. Basic fertilizer,i.e., farm manure (29 985 kg ha-1organic matter>25%)and NPK fertilizer (300 kg ha-1, N+P2O4+K2O≥45%), was applied on July 10; the fertilizer was repeatedly mixed prior to application, and sulfur fumigation was used once per 20 days for pest control during growing season. The water supply method was drip irrigation. Each greenhouse contained 80 strawberry beds, each of which was, 800 cm length×40 cm width×40 cm height. Walnuts were planted in the continuous cropping greenhouse before the strawberries were continuously cropped for 12 years. Similarly, walnuts were planted in the non-continuous cropping greenhouse prior to the planting of strawberry.
The strawberries were carefully pulled from the ground,and the conglutinating soil from the strawberry roots was mildly crushed and shaken to collect the soil located within 2 mm of the plant root surfaces; this was defined as rhizosphere soil (continuous cropping rhizosphere soil(CCR) or non-continuous cropping rhizosphere soil (NCCR)of strawberries). Bulk soil (continuous cropping (CC) soil or non-continuous cropping (NCC) soil of strawberries)located 10-20 cm away from the strawberries and without significant root influence was collected as “non-rhizosphere”soil (Wang et al. 2014). Each sample was composed of three replicates. Each replicate, consisting of 30 samples,was collected from a different greenhouse. The subsamples of the same replicates of soil samples were taken from different sites based on a Z-pattern and were mixed in an aseptic bag. These bags were placed in ice boxes and were subsequently transported to the laboratory. A portion of the samples was stored at -80°C for microbial analysis by extracting a DNA composite sample from each replicate.The remaining samples were sieved through a 2-mm mesh sieve and were dried at 60°C to determine physicochemical properties and enzymatic activities.
2.2. Soil physicochemical properties and enzyme activities
All of the physicochemical analyses were based on published physicochemical analysis methods for soils(Sarkar 2010). OM was measured using the vitriol acidpotassium dichromate oxidation method. Soil total nitrogen(TN) was determined using an automatic Kjeldahl distillationtitration unit (Foss, Sweden). Soil available phosphorus (AP)was determined using the molybdenum blue colourimetric method. Flame photometry was used to analyse soil available potassium (AK). Soil pH and electrical conductivity(EC) were determined in a 1:2.5 (w/v) soil-water suspension(Eivazi et al. 2003). Urease and alkaline phosphatase activities were determined using methodology described by Black (1965) and Smith (2010). Invertase activities were determined on the basis of the method described by Prathasarathi and Ranganathan (2000).
2.3. Soil DNA extraction and PCR conditions
Total soil DNA was extracted from each repeat using a Powersoil®DNA Isolation Kit (Mobile Biometry,USA). The V3-V4 region of the bacteria 16S ribosomal RNA gene was amplified using universal forward(5´-GTACTCCTACGGGAGGCAGCA-3´) and reverse 806R(5´-GTGGACTACHVGGGTWTCTAAT-3´) primers. The 16S rRNA in the DNA samples was amplified by polymerase chain reaction (PCR) before Illumina MiSeq sequencing.The PCR system contained 2 μL of template DNA, 2 μL of forward primer (10 μmol L-1), 2 μL of reverse primer(10 μmol L-1), 4 μL of dNTPs (2.5 μmol L-1), 5 μL of 10×Pyrobest buffer, 0.3 μL of Pyrobest DNA polymerase (2.5 U μL-1), and 34.7 μL of ddH2O. The reaction programme was 5 min at 95°C, followed by 30 cycles of 30 s at 95°C, 30 s at 56°C, and 40 s at 72°C, with a final elongation step of 10 min at 72°C, and a hold at 4°C (Zhang et al. 2015). After PCR amplification, the products were examined using 2% agarose gel electrophoresis and spectrophotometry (determining the optical densities for the 260 nm/280 nm ratio).
2.4. High-throughput sequencing
The PCR products of the V3-V4 region in the bacterial 16S rRNA gene were sequenced using QuantiFluor™-ST (Promega, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced (Paired-end 300) on an Illumina MiSeq platform according to standard protocols.
Noisy amplicon sequences (low-quality sequences)were filtered out from the high-quality sequences using the Precluster tool in the QIIME package (Quantitative Insights into Microbial Ecology) (ver. 1.2.1). This quality filtration was performed to remove the low-quality sequences.Reads shorter than 110 bp were removed, sequences with homopolymers longer than 10 bp were filtered using a sliding window filter score of <20 over 50 bp and truncated reads shorter than 50 bp were discarded. Finally, only sequences that overlapped by more than 10 bp were assembled on the basis of their overlap sequence; reads that could not be assembled were discarded.
The unique sequence set was classified into different operation taxonomic units (OTUs) by UCLUST Software(Edgar 2010), and the OTUs were defined based on 97%similarity. Chimeric sequences were identified and removed using USEARCH. The taxonomy of each 16S rRNA gene sequence was analysed by UCLUST against the Silva 119 16S rRNA database at a confidence threshold of 70%.
2.5. Statistical analysis
The physicochemical data and enzyme activities were analysed by one-way ANOVA using SPSS 20.0 (SPSS Inc., USA). Drawings were created using PRISM (ver.7.0). The relationship between the species diversity and environmental factors was analysed using redundancy analysis (RDA) by Canoco for Windows (ver. 4.5). The significance level for differences in this research was set at P<0.05.
3. Results
3.1. Soil physicochemical properties
The results of the soil physicochemical (Table 1) analyses showed that the concentrations of OM, ammonia nitrogen (NH4+-N), TN and AP decreased in the order of NCC>NCCR>CC>CCR. However, the difference in AK between CC and CCR was not significant, and the levels in these treatments were significantly lower than that in the control. Soil pH was not significantly different among samples with the same original soil but did differ significantly among samples with different original soils.
3.2. Soil enzyme activities
This study tested the activities of three enzymes (invertase,urease, and alkaline phosphatase) in all of the soil samples at the harvest stage (Fig. 1). The three enzyme activities were ranked in the order of CC<CCR<NCC<NCCR. In a comparison of four soil samples, the enzyme activity values showed significant differences (P<0.05) between each of the enzymes. On the basis of different soil sample tests, the order of the average value variation among the three enzyme activities was urease>invertase>alkaline phosphatase.
3.3. Bacterial community phylotypes and diversity
The results were used to compare the microbial diversity levels of four samples with corresponding data from the literature.
In total, 5 755 450 clean tag sequences were generated from the 12 sediment samples, i.e., 41 055 for the CC sample, 47 684 for the CCR sample, 35 212 for the NCC sample and 36 349 for the NCCR sample. The average high-quality sequence length was 430.3 bp. The numbers of OTUs were 1 087, 1 219, 1 415 and 1 241 for CC, CCR,NCC and NCCR, respectively.
3.4. Bacterial community structures of different soil samples
The result showed that the four treatments contained 33 types of bacteria at the phylum level (Fig. 2). The relative abundances were greater than 1% for 16 phyla (Fig. 3-A):Proteobacteria, Acidobacteria, Chloroflexi, Actinobacteria,Gemmatimonadetes, Bacteroidetes, TM7, Nitrospirae,Deinococcus-thermus, Planctomycetes, Firmicutes,Verrucomicrobia, JL-ETNP-Z39, Candidate division WS3,Cyanobacteria and Thermotogae. The average abundance of Proteobacteria, Acidobacteria and Chloroflexi was greater than 10% (Fig. 3-B-E). Proteobacteria presented the highest abundance in all samples, accounting for 24.9-38.95%(the average was 34.04%) of the total effective bacterial sequences. A comparison of all bacteria at the genus level(relative abundance>0.0001%) yielded 223 genera. Eleven genera had relative abundances greater than 1% in a single sample (Table 2): Sphingomonas, Novosphingobium,Bacillus, Rhodoplanes, Stenotrophobacter, Povalibacter,Nitrospira, Pelagibius, Thioprofundum, Cellvibrio and Allokutzneria, with average values of 3.93, 1.54, 0.98, 0.85,0.85, 0.79, 0.70, 0.58, 0.45, 0.42, and 0.35%, respectively.Novosphingobium, Rhodoplanes and Nitrospira might participate in the phosphorus cycle, and Novosphingobium,Povalibacter and Nitrospira might participate in the sulfur cycle (Liu et al. 2005; Blackburne et al. 2007; Lakshmi et al. 2009; Nogi et al. 2014). Analysis of the different relative abundances of these 11 genera, based on a heat map (Fig. 4), revealed five types of variation trends. First,in terms of relative abundance, Sphingomonas was the primary genus in all of the samples, and its proportion changed little across samples (basic group). With respect to the second type of variation trend (common group, Fig. 5),the relative abundance of Novosphingobium, Rhodoplanes,Povalibacter, Cellvibrio and Stenotrophomona was significantly higher in the control groups (NCC and NCCR)than that in the experimental groups (CC and CCR)(Table 2; Fig. 4). The third variation trend (amended group, Fig. 5) showed that the abundance of Bacillus in continuously cropped soils (CC and CCR) was higher than that in non-continuously cropped soils (NCC and NCCR)(Table 2; Fig. 4). With respect to the fourth variation trend(rhizosphere group), the abundance of Nitrospira was higher than 1% only in the rhizosphere soil. The last variation trend(exclusive groups, Fig. 5) included Pelagibius, Thioprofundum and Allokutzneria, which were all concentrated in CC (Fig. 4).
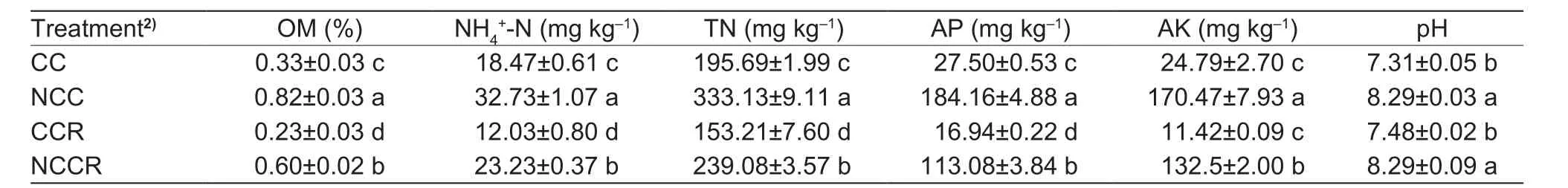
Table 1 The basic physiochemical properties of the soil1)
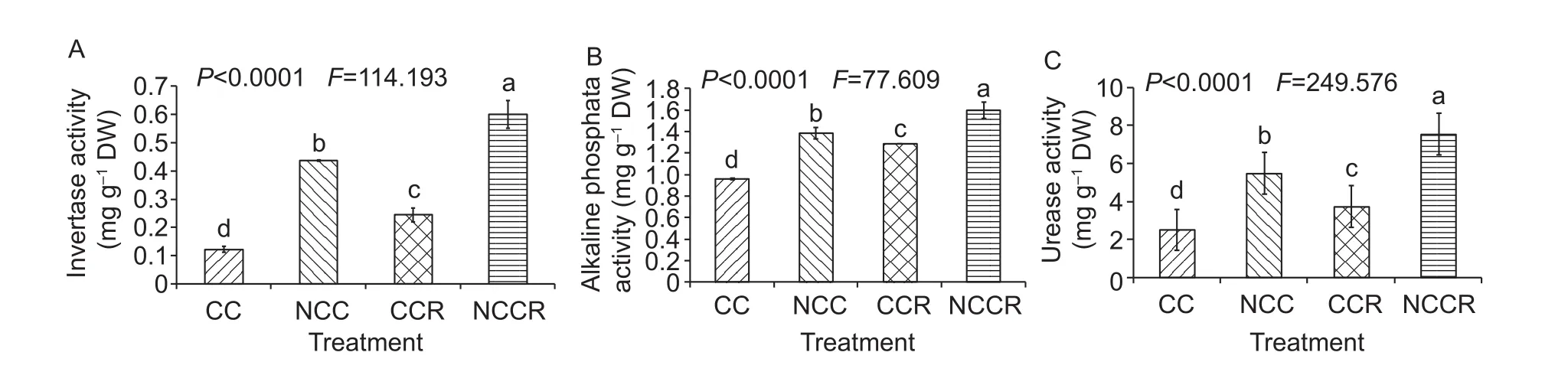
Fig. 1 Invertase, alkaline phosphate and urease activity levels in different soil samples (mg g-1 DW). CC, continuously cropped soil; NCC, non-continuously cropped soil; CCR, continuously cropped strawberry rhizosphere soil; NCCR, non-continuously cropped strawberry rhizosphere soil. Different letters indicate significant differences among the different durations of continuous strawberry strawberry (Duncan’s multiple range test, P<0.05, mean±SE).
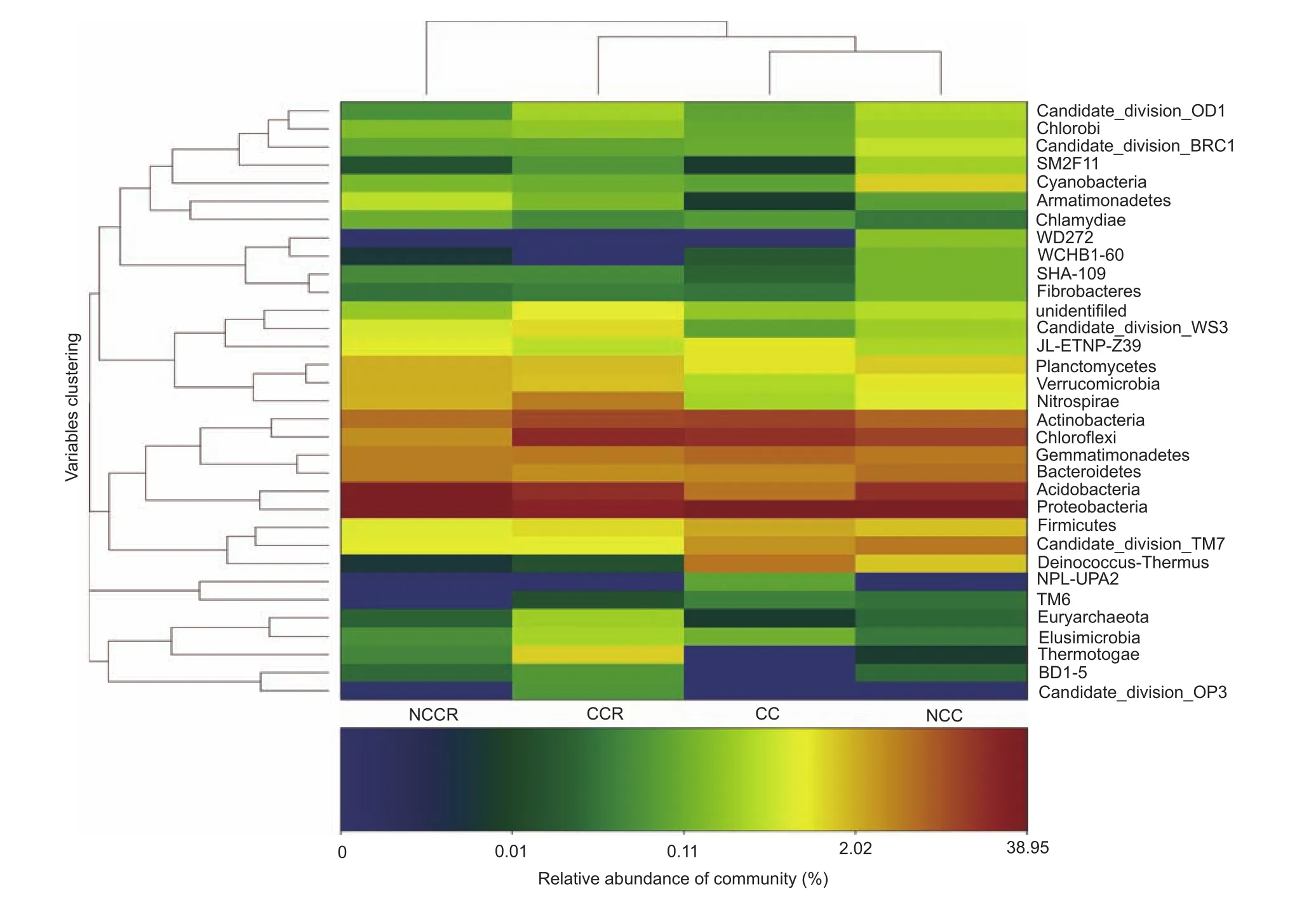
Fig. 2 Heat map at the phylum level. NCCR, non-continuously cropped strawberry rhizosphere soil; CCR, continuously cropped strawberry rhizosphere soil; CC, continuously cropped soil; NCC, non-continuously cropped soil.
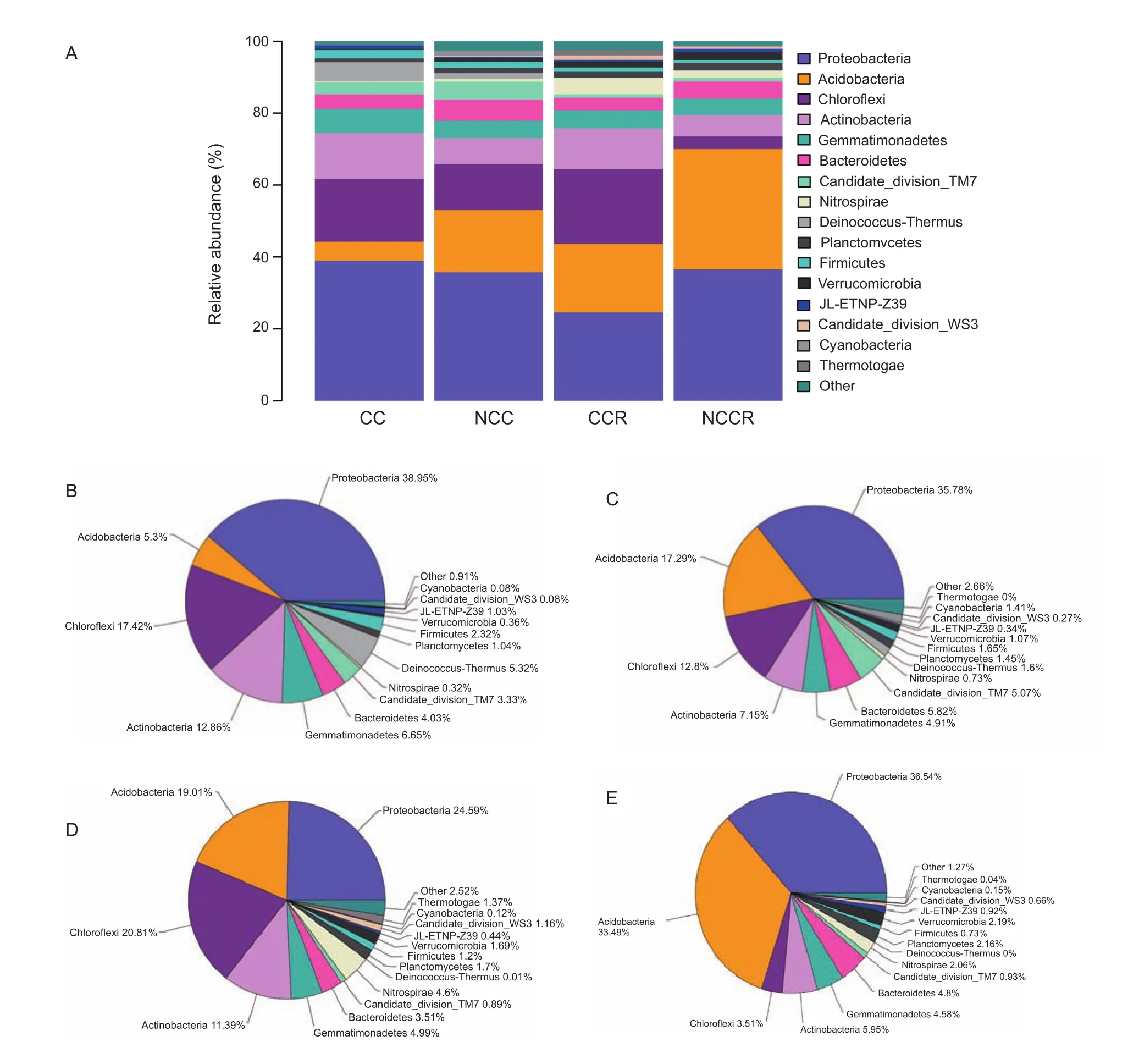
Fig. 3 Phylum-level compositions. A, the average bacterial phyla distributed across different soil samples. CC, continuously cropped soil; NCC, non-continuously cropped soil; CCR, continuously cropped strawberry rhizosphere soil; NCCR, non-continuously cropped strawberry rhizosphere soil. B, C, D and E, the relative abundance of the bacterial phyla found in CC, NCC, CCR and NCCR, respectively.
3.5. Redundancy analysis (RDA) results of microbial communities and environmental factors
RDA is based on the population abundance and environmental variables and was performed using Canoco 4.5 Software (Ithaca,NY, USA). Bacterial species are indicated by different generic letters and are shown as solid blue lines. Environmental variables are indicated by different generic letters and are shown as solid red lines with filled arrows. Samples are represented by green triangles. The axis 1 explained 75.3% and axis 2 explained 20.2% of the variance (Fig. 6). The RDA results showed that the environmental variables were positively associated with NCC and NCCR and negatively associated with CC and CCR. The environmental variables were negatively associated with Bacillus,Allokutzneria, Pelagibius and Thioprofundum and were positively related to the Novosphingobium, Povalibacter, Rhodoplanes and Stenotrophobacter genera. Sphingomonas and Nitrospira were positively correlated with pH, OM, urease, invertase and alkaline phosphatase and were negatively correlated with NH4+-N, AK,AP and TN. In addition, the bacterial groups were negatively correlated with the environmental variables, and the abundances of the bacterial groups in CC and CCR were higher than those in NCC and NCCR. Therefore, Bacillus, Pelagibius, Thioprofundum and Allokutzneria may be key bacteria involved in the replant problem.
4. Discussion
4.1. Physicochemical properties and enzyme activities
Physicochemical property analysis (Table 1) revealed that the nutrient contents of the soil decreased after strawberries were planted in the same soil. Moreover, the nutrient content of the non-continuously cropped soil was significantly higher than that of the continuously cropped soil. Agegnehu et al. (2016) found that acidic soil might limit root growth as well as the uptake of N, P and K during plant growth. These consequences originate from changes in soil pH. This result is in general agreement with previous research (Li et al. 2016). Long-term application of chemical fertilizers might be the key factor leading to soil acidification(Schroder et al. 2011). Other studies have suggested that soil enzyme activities correlate positively with SOC, soil temperature,soil moisture content, total N, P, and K contents, and microbial populations (Qi et al. 2016), which is similar to this study. Alkaline phosphate (ALP) is correlated with OM and available phosphorus(Agegnehu et al. 2016). In particular, invertase, phosphatase and urease belong to hydrolases, which can indirectly affect the degradation of organic pollutants by altering the metabolic activities of degraders through nutrient supply or limitation (Wei et al. 2017).In addition, soil properties such as the organic carbon content,pH, contamination, and land use patterns can affect the activity of soil hydrolases (Stursova and Baldrian 2011).Invertase, phosphatase and urease are closely related to the physicochemical properties of the soil, as well as the microbial biomass, and can indirectly describe the effects of physicochemical properties on microbial activity (Wei et al.2015; Agegnehu et al. 2016; Qi et al. 2016). Moreover,urease plays an important role in increasing soil nutrient solubility. Some studies have indicated that invertase can reflect the contents of stable water aggregates, OM, and humus, as well as the cation exchange capacity of arable soil (Li et al. 2012).
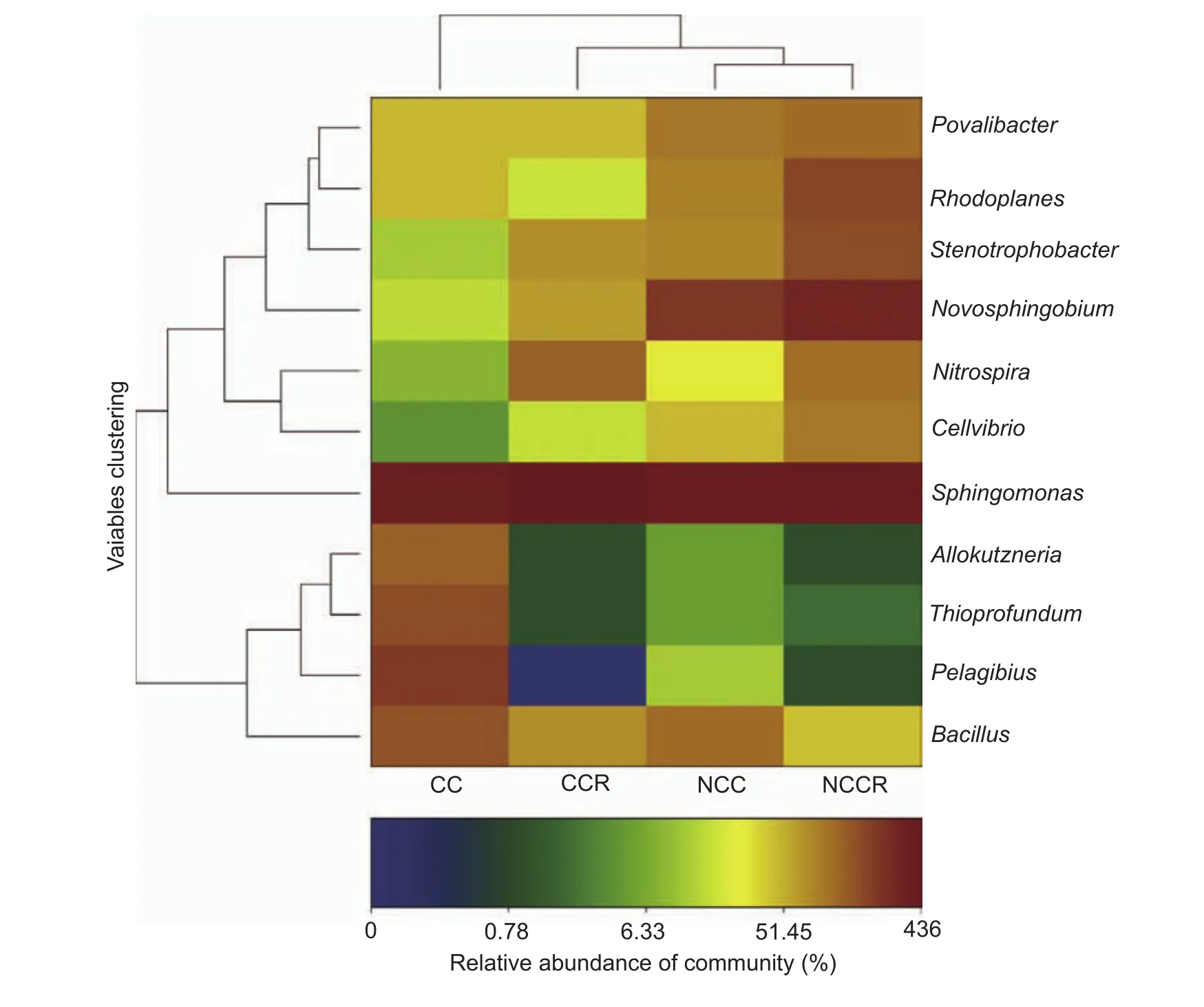
Fig. 4 Heat map at the genus level for the relative abundance of the bacterial communities among the four samples. CC,continuously cropped soil; CCR, continuously cropped strawberry rhizosphere soil; NCC, non-continuously cropped soil; NCCR,non-continuously cropped strawberry rhizosphere soil.
The decreased enzyme activities may also be attributable to reduced microbial abundance, decreased microbial activity and changes in the microbial community composition(Martens et al. 1992; Zhao et al. 2009). In addition, many studies have shown that N fertilization can accelerate the mineralization of OM by modifying soil microbial communities (Kuzyakov 2010). A lack of phosphorus in P-poor soil can inhibit microbial activity (Cleveland et al.2002), and potassium deficiencies reduce the crop quality and the capacity to resist adversity (Jenkinson et al. 1985;Kuzyakov 2010).
4.2. Changes in the bacterial community structure
In this experiment, the soil bacterial classification was obtained using HTS technology at a high certainty level.In all of the samples, Proteobacteria and Acidobacteria were the most predominant phyla (Fig. 3). In addition,the following three phyla had a single genus with an abundance greater than 1%: Firmicutes, Nitrospirae and Actinobacteria (Table 2). These five phyla contained 11 genera (Table 2). Proteobacteria constitute a major phylum of heterotrophic gram-negative bacteria in various ecosystems, including soil, plant leaf surfaces, atmosphere,seawater and freshwater (Duangma et al. 2014). This study shows that Proteobacteria was the most stable phylum that contained the most abundant genera. At the genus level, this phylum contained seven genera (Sphingomonas,Novosphingobium, Rhodoplanes, Pelagibius, Povalibacter,Cellvibrio and Thioprofundum) with abundances greater than 1% (Table 2). Proteobacteria are capable of inducing nitrogen fixation in symbiosis with plants (Emmert and Handelsman 1999). A comparison of the different treatments indicated that the abundance of Proteobacteria did not differ among the different soil environments (Fig. 3-A). In addition,Firmicutes, Nitrospirae, Acidobacteria and Actinobacteria had only one genus with an abundance greater than 1%.
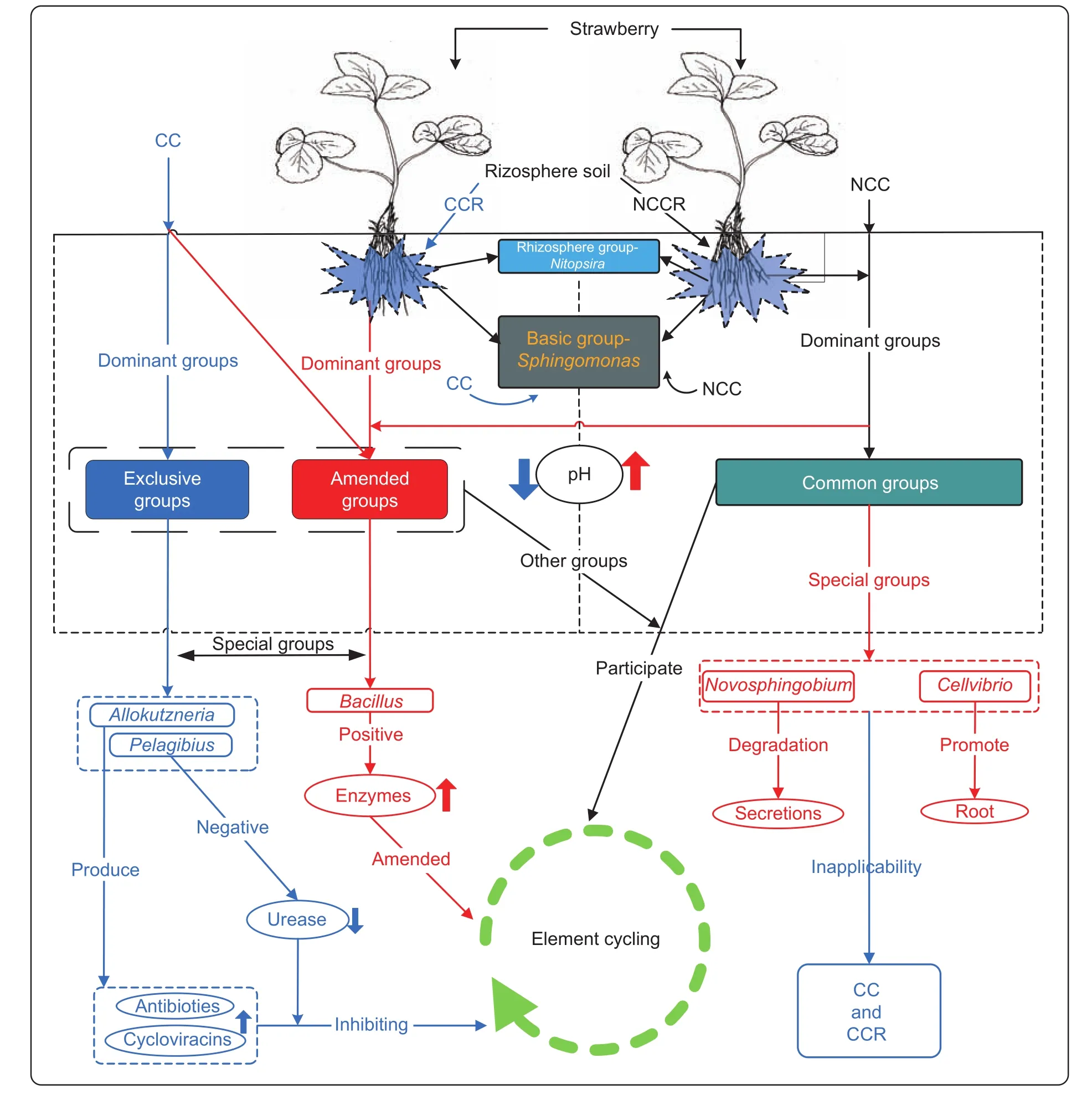
Fig. 5 Changes in the primary bacterial groups in rhizosphere soil under continuous cropping. CC, continuously cropped soil;CCR, continuously cropped strawberry rhizosphere soil; NCC, non-continuously cropped soil; NCCR, non-continuously cropped strawberry rhizosphere soil. Blue lines denote down-regulation; red lines show up-regulation, and black lines indicate normality.
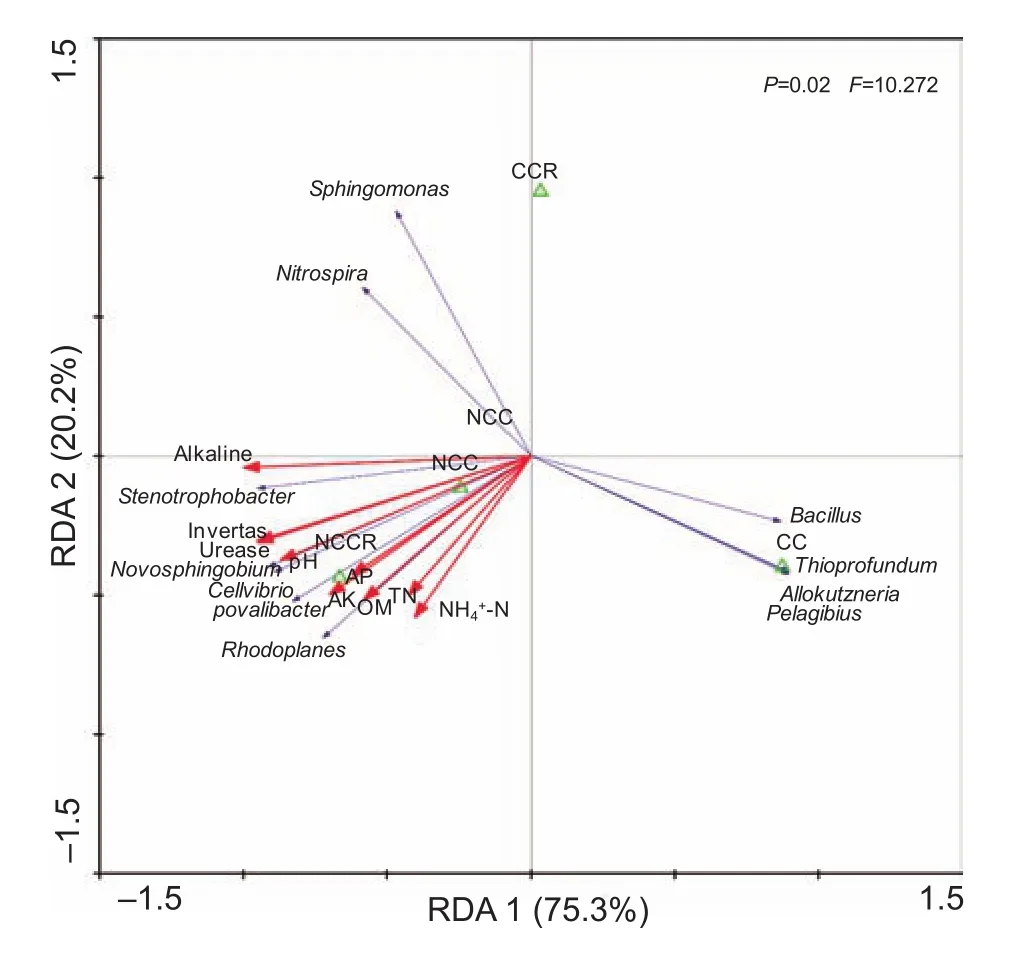
Fig. 6 Redundancy analysis (RDA) showing the relationships between environmental variables and major bacterial species.OM, organic matter; NH4+-N, ammonia nitrogen; TN, total nitrogen; AP, available phosphorus; AK, available potassium.CC, continuously cropped soil; CCR, continuously cropped strawberry rhizosphere soil; NCC, non-continuously cropped soil; NCCR, non-continuously cropped strawberry rhizosphere soil. Different dots represent different soil samples. The major bacterial species are indicated using blue lines, and the environmental variables are indicated using red lines.
Among 11 genera, only five bacterial groups(Sphingomonas, Pelagibius, Thioprofundum, Bacillus,and Allokutzneria) had abundances greater than 1% in the continuously cropped soil, and two bacterial groups(Sphingomonas and Nitrospira) had abundances greater than 1% in the CCR soil (Table 2). Five variation trends(Fig. 4) were observed at the genus level for the CC treatment compared to the NCC treatment, e.g., the basic group (Sphingomonas), common group, amended group(Bacillus), rhizosphere group (Nitrospira) and exclusive group (Pelagibius and Allokutzneria). Previous research reported that Sphingomonas is widely distributed in nature,and it has been isolated from many different environments,such as land, water habitats and plant root systems.Under these conditions, Sphingomonas can survive at low nutrient concentrations and can metabolize a wide variety of carbon sources. Various strains of Sphingomonas have been isolated from environments contaminated with toxic compounds, displaying the capacity to utilize the contaminants as nutrients (Yabuuchi et al. 1990).Therefore, the relative abundance of Sphingomonas did not change significantly and was higher than that of the other genera (Table 2 and Fig. 4). Thioprofundum can utilize sulfur, but its abundance was not significant in the NCC soil environment (Mori et al. 2011). The experimental data for Pelagibius were significantly negatively correlated with urease and alkaline phosphatase activity (Fig. 5), similar to previous research (Choi et al. 2009). Duangma et al.(2014) reported that Allokutzneria from plant rhizosphere soil produces highly active antiviral antibiotics and cycloviracins.Bacillus is a common beneficial bacterium and is often considered as a primary soil conditioner. Many species of Bacillus can produce different antibiotics and different amounts of enzymes, which can act as biocontrol agents that kill soil-borne pathogens (Emmert and Handelsman 1999). Hence, Bacillus might play a key role in amending continuously cropped soil. In addition, Pelagibius and Allokutzneria might play a key role in inhibiting elements of the soil cycle (Choi et al. 2009; Duangmal et al. 2014). The abundance of saprophytic bacteria (Novosphingobium and Povalibacter) in continuously cropped soil was significantly decreased to less than 1% in the non-continuously cropped soil. The cause of the decrease in Novosphingobium and Povalibacter was potentially the lack of organic material after CC. Nitrospira uncharacteristically increased in rhizosphere soil relative to non-rhizosphere soil, which may be explained as follows: Nitrospira utilize a variety of strategies (r- and K-strategist models) under different nitrite proportions in the soil environment (Kim and Kim 2006; Blackburne et al.2007; Dytczak et al. 2008).
All tests and analyses show that bacteria can be influenced by other soil environmental factors, such as the physicochemical properties and enzyme activities (Fraser et al. 2015; Ye et al. 2015; Zhalnina et al. 2015). In addition,the physicochemical properties were positively correlated with each other and were negatively correlated with the CC soil environment based on the RDA results (Fig. 6). The soil enzyme activities were positively correlated with the soil physicochemical properties and were negatively correlated with the CC soil environment (Fig. 6).
4.3. Redundancy analysis
Bacillus, Allokutzneria and Pelagibius significantly increased after CC. RDA showed that these genera were significantly negatively correlated with environmental variables. Bacillus might play a key role in amending continuously cropped soil, and Allokutzneria and Pelagibius might play a key role in leading to more serious CC problems according to their respective characteristics (Tomita et al. 1993; Satomi et al. 2006; Choi et al. 2009). Nitrospira can achieve normal growth in rhizosphere soil, but the contribution of this genus was not evident in this environment. The other eight bacterial genera were affected by the environmental variables and participated in the biogeochemical cycles, but they were not the key bacteria involved.
5. Conclusion
These research findings provide insights into the bacterial changes at the genus level based on high-throughput analysis of the CC, CCR, NCC and NCCR treatments associated with strawberry plants. Based on the results and the data available in the literature, a hypothetical model was designed that described the transformation processes of the biological community that was affected after replanting(Fig. 6). The results showed that Bacillus might have a significant effect in amending the soil environment under continuously cropped strawberry, and Allokutzneria and Pelagibius might play a key role in leading to more serious CC. The CC phenomenon that may be caused by the change in the soil pH from environmental factors.
Dissecting the bacterial mechanisms that affect plant growth is regarded as one of the future challenges of agricultural ecological research. Exploring the complexity of the interactions between the plant roots and bacteria within the soil environment will be particularly difficult. The results from this research will provide insights into the functions and actions of fungi in continuously and non-continuously cropped soil.
Acknowledgements
The present study was supported by the National Science and Technology Support Program (2014BAD16B07). We thank the support of Beijing Academy of Forestry and Pomology Sciences.
 Journal of Integrative Agriculture2018年11期
Journal of Integrative Agriculture2018年11期
- Journal of Integrative Agriculture的其它文章
- First report of Athelia bombacina causing postharvest fruit rot on pear
- Sustainability assessment of potato fields using the DEXi decision support system in Hamadan Province, lran
- Alternate row mulching optimizes soil temperature and water conditions and improves wheat yield in dryland farming
- lnter-annual changes in the aggregate-size distribution and associated carbon of soil and their effects on the straw-derived carbon incorporation under long-term no-tillage
- Mycoplasma leachii causes polyarthritis in calves via the blood route but is not associated with pneumonia
- Genome-wide detection of selective signatures in a Duroc pig population
