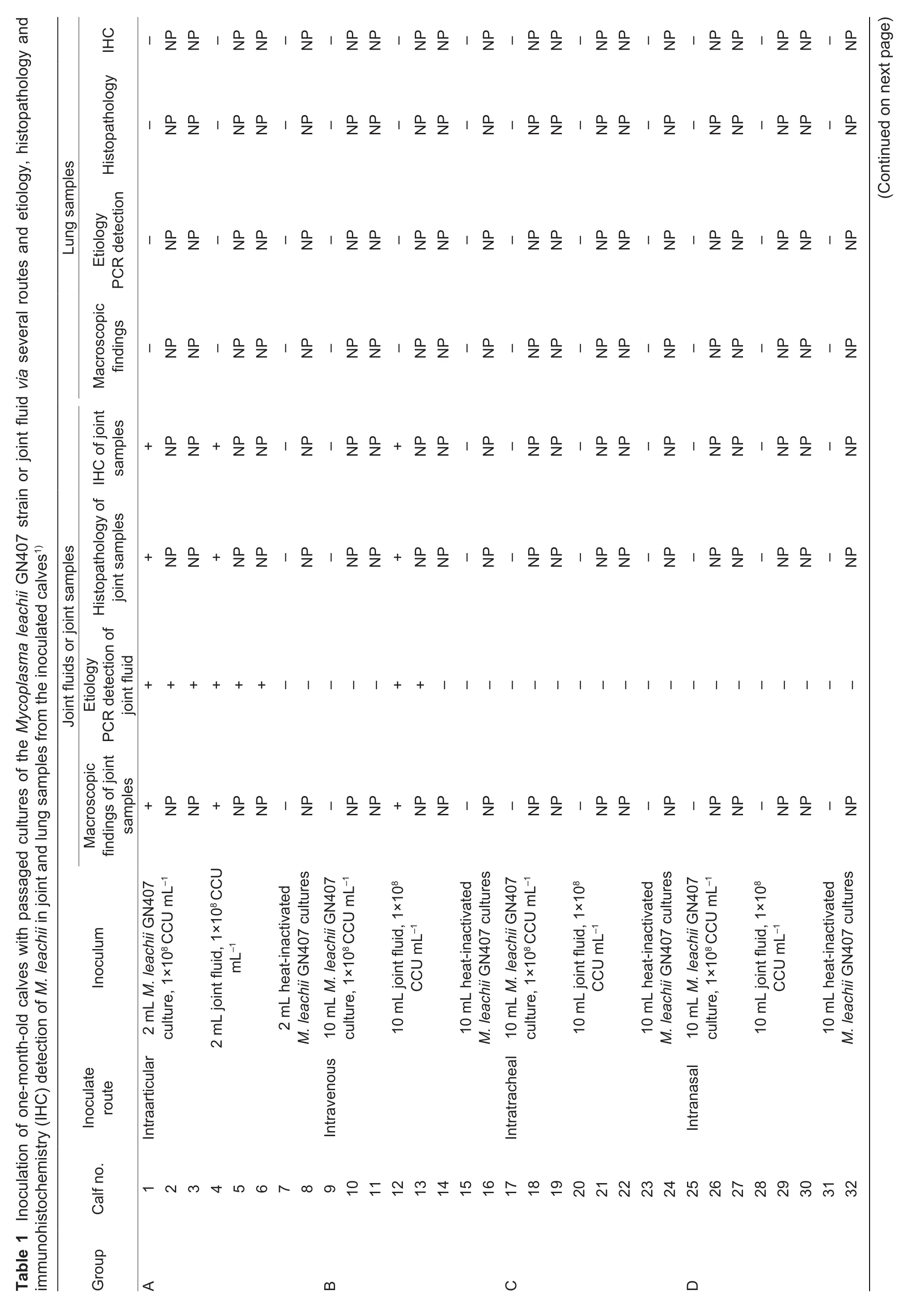
Mycoplasma leachii causes polyarthritis in calves via the blood route but is not associated with pneumonia
CHANG Ji-tao, WANG Guan-bo, ZHANG Yue, WANG Fang, JIANG Zhi-gang, YU Li
Division of Livestock Infectious Diseases/State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute,Chinese Academy of Agricultural Sciences, Harbin 150001, P.R.China
Abstract Mycoplasma leachii was initially isolated from arthritic calves in South Queensland, Australia, and its ability to cause clinical polyarthritis in calves was demonstrated by experimental infection. However, the source of M. leachii infection in calves and its means of spreading are not well known. In this study, one-month-old calves were inoculated with cultures of M. leachii or joint fluid (collected from M. leachii-infected calves) through the intraarticular, intravenous, intratracheal, intranasal or oral routes.Multidisciplinary procedures, including clinical assessment, etiology assessment, pathology and immunohistochemistry(IHC), were used to evaluate the pathogenicity of M. leachii in calves and to elucidate the transmission route of M. leachii infection in calves. The results showed that all calves inoculated intraarticularly with cultured GN407 or joint fluid and twothirds of the calves inoculated intravenously with joint fluid developed severe polyarthritis, and the M. leachii antigen was detected in the joints of all infected calves by IHC and PCR. In contrast, calves inoculated with cultured M. leachii or joint fluid through the intratracheal, intranasal or oral routes did not show any M. leachii infection in the clinical assessment,etiology assessment, or pathology and IHC results. These results indicated that polyarthritis caused by M. leachii in calves is transmitted via the blood route; however, this disease is not transmitted through the respiratory or digestive routes. In addition, the M. leachii antigen was not detected in the lungs of all the inoculated calves using IHC and PCR, indicating that M. leachii is not associated with pneumonia, even in the calves inoculated through the respiratory duct. These findings are important information for the prevention and control of calf polyarthritis caused by M. leachii.
Keywords: Mycoplasma leachii, calf polyarthritis, transmission via blood
1. Introduction
Mycoplasma leachii sp. nov., a new species designation for Mycoplasma sp. bovine group 7 (Manso-Silvan et al. 2009),was initially isolated from the joint fluid of arthritic calves in southern Queensland, Australia, in 1963 (Simmons and Johnston 1963). Subsequently, M. leachii was also isolated from calves with polyarthritis and pneumonia, cows with signs of mastitis, aborted fetuses and small ruminant hosts(Hughes et al. 1966; Connole et al. 1967; Cottew 1970;Shiel et al. 1982; Alexander et al. 1985; Atalaia et al. 1987;Hum et al. 2000). In China, polyarthritis in calves caused by M. leachii emerged in 2009, and M. leachii was first isolated from the joint fluid of arthritic calves by our laboratory (Liu et al. 2010; Chang et al. 2011). Outbreaks of polyarthritis with concomitant isolation of M. leachii from affected joints in calves have been documented in records compiled by veterinary laboratories in New South Wales, Victoria, and Queensland in Australia (Simmons and Johnston 1963;Shiel et al. 1982; Alexander et al. 1985; Hum et al. 2000)and Heilongjiang Province in China (Liu et al. 2010; Chang et al. 2011). The ability of M. leachii to cause clinical arthritis in calves has been demonstrated by artificial experimental infection (Simmons and Johnston 1963), but direct histological and immunohistochemical evidence was not presented in previous pathogenicity study (Simmons and Johnston 1963). In addition, although M. leachii has been isolated from pneumonic bovine lungs (Alexander et al.1985), whether M. leachii is associated with calf pneumonia has not been determined in an artificial infection experiment.Thus, the detailed pathogenicity of M. leachii infection in calves and its routes of transmission are not well known. Our previous epidemiologic and clinical investigations provided clear evidence of contaminated semen as a source of infection (Chang et al. 2011), but other routes of M. leachii transmission have not been elucidated. Hence, the aim of this study was to further evaluate the pathogenicity of M. leachii in calves and to elucidate the transmission route of M. leachii infection in calves.
2. Materials and methods
2.1. Ethics statement
All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Harbin Veterinary Research Institute (HVRI) of the Chinese Academy of Agricultural Sciences (CAAS) on 15 June 2014 under approval number SQ2014072. All animals were housed and cared for in an accredited facility at the HVRI in accordance with local and institutional policies.
2.2. Mycoplasma strain and culture production
The M. leachii GN407 strain, which was originally isolated from the joint fluid of arthritic calves in the Heilongjiang Province of China in 2010, was passaged four times before triple clone purification, and the final titer was 1×108color change units per milliliter (CCU mL-1). For lppA gene sequence analyses, the GN407 strain shared 99.6 and 95.1% nt identities with the M. leachii representative strain PG50 and the M. mycoides subsp. mycoides, respectively(Liu et al. 2010). Joint fluids, which were used to inoculate the calves, were collected from calves experimentally infected with GN407 and these joint fluids were filtrated by 0.45-μm filter membrane, and were then diluted to the same titer as the cultured GN407 for inoculation.
2.3. Animals and experimental inoculation
For this study, a total of 40 one-month-old female Holstein calves were selected. Before inoculation, tracheobronchial lavage fluid (TBLF) was taken from all calves to exclude the presence of M. leachii by bacteriological culture and PCR(Chen et al. 2017) and antibodies to M. leachii by indirect ELISA using LPPA (lipoprotein precursor A) protein as the antigen (Wang et al. 2014). The cultures were negative. In blood samples, M. leachii-specific serum antibodies were not detected by ELISA. All the calves were randomly divided into five groups of eight calves each, infected calves and control calves were housed in separate pens, and they were fed and cared for in an accredited facility at the HVRI in accordance with uniform standard. The calves in groups A, B, C, D and E were inoculated via the intraarticular, intravenous,intratracheal, intranasal or oral routes with passaged cultures of the M. leachii GN407 strain, a Chinese isolate,as previously described by our laboratory (Liu et al. 2010)or with joint fluid collected from M. leachii-infected calves,as described in Table 1. In group A, three calves (nos. 1, 2 and 3) were inoculated with 2 mL of M. leachii GN407 liquid culture (1×108CCU mL-1in the right carpal and left toe joints;three calves (nos. 4, 5 and 6) were inoculated with 2 mL of joint fluid containing 1×108CCU mL-1of M. leachii in the right carpal and left toe joints; and two calves (nos. 7 and 8) were inoculated with 2 mL of heat-inactivated M. leachii GN407 cultures via the same route as the negative control.In group B, three calves (nos. 9, 10 and 11) were inoculated with 10 mL of fresh culture containing 1×108CCU mL-1of the M. leachii GN407 strain via the intravenous route; three calves (nos. 12, 13 and 14) were intravenously inoculated with the same volume of joint fluid containing 1×108CCU mL-1of M. leachii; and two calves (nos. 15 and 16) were intravenously inoculated with 10 mL of heat-inactivated M. leachii GN407 cultures as a negative control. In group C, three calves (nos. 17, 18 and 19) were inoculated with 10 mL of fresh culture containing 1×108CCU mL-1of the M. leachii GN407 strain via the intratracheal route; three calves (nos. 20, 21 and 22) were intratracheally inoculated with the same volume of joint fluid containing 1×108CCU mL-1of M. leachii; and two calves (nos. 23 and 24) were intratracheally inoculated with 10 mL of heat-inactivated M. leachii GN407 cultures as a negative control. In group D, three calves (nos. 25, 26 and 27) were inoculated with 10 mL of fresh culture containing 1×108CCU mL-1of the M. leachii GN407 strain via the intranasal route; three calves(nos. 28, 29 and 30) were intranasally inoculated with the same volume of joint fluid containing 1×108CCU mL-1of M. leachii; and two calves (nos. 31 and 32) were intranasally inoculated with 10 mL of heat-inactivated M. leachii GN407 cultures as a negative control.In group E, three calves (nos. 33, 34 and 35) were inoculated with 10 mL of fresh culture containing 1×108CCU mL-1of the M. leachii GN407 strain via the oral route; three calves (nos. 36, 37 and 38)were orally inoculated with the same volume of joint fluid containing 1×108CCU mL-1of M. leachii; and two calves (nos. 39 and 40) were orally inoculated with 10 mL of heat-inactivated M. leachii GN407 cultures as a negative control. For intranasal and intratracheal inoculation, calves were sedated by intramuscular injection of 0.05 mg of xylazine per kg body weight before inoculation. The inoculant was slowly instilled at 1 drop every 2 s.
?

2.4. Necropsy and sampling
The study design and the timeline are displayed in Fig. 1. On postinoculation days (PIDs) 5, 7, 14 and 21, joint fluid was collected from all inoculated calves for PCR detection and culture isolation of M. leachii, and the samples were simultaneously plated on Columbia agar (Oxoid, Basingstoke, UK) with 5% sheep blood and incubated at 37°C with 5% CO2to isolate other bacteria. Because all infected calves exhibited similar clinical features according to M. leachii PCR detection and M. leachii isolation, some calves (nos. 1, 4, 7, 9, 12,15, 17, 20, 23, 25, 28, 31, 33, 36 and 39) were randomly selected from each group and were euthanized and submitted for necropsy on PID 14. The animals were first anesthetized by intramuscular injection of 0.05 mg of xylazine per kg body weight, and then,50 mL of 40% (w/v) potassium chloride was intravenously injected into each animal. Joint and lung samples were collected and fixed in 4% neutral-buffered formalin for histology and immunohistochemistry(IHC) assays.
2.5. Histopathology
Formalin-fixed tissues were embedded in paraffin wax, sliced to 4-μm thickness and stained with hematoxylin and eosin (H&E) for histological examination. Analyses were subsequently conducted by the Pathology Laboratory of Animal Infectious Disease Diagnostic Centre, HVRI, CAAS.
2.6. PCR
The specific primer pair 17U26: ATCCTAATAACCCAGAAACTAAA CCG/556L22: GATCTTGACTAT ATAACAACAT was used for PCR amplification of the lppA gene with an annealing temperature of 55°C to detect M. leachii in samples according to our described method (Chen et al. 2017).
2.7. IHC
The monoclonal antibody (MAb) 1F2, which was prepared by Wang Guanbo and co-workers in our laboratory (Wang and Liu 2014), was used for IHC detection. The isotype of 1F2 is of the IgG1/κ-type subclass. Western blotting showed that MAb 1F2 specifically bound to M. leachii GN407 but not to other Mycoplasma (such as Mycoplasma bovis, Mycoplasma ovipneumoniae, Mycoplasma mycoides subsp. mycoides or Mycoplasma capricolum subsp. capripneumoniae) (data not shown), and the titer of this antibody was 1:105based on ELISA detection. For all immunohistochemical reactions,the avidin:biotinylated enzyme complex (ABC) method was applied. Formalin-fixed samples, MAb 1F2 and normal mouse (BALB/c) serum were provided by our laboratory,and IHC was subsequently performed by the Pathology Laboratory of Animal Infectious Disease Diagnostic Center,HVRI, CAAS.
3. Results
3.1. Clinical findings
Six calves inoculated via the intraarticular route with passaged cultures of the M. leachii GN407 strain (nos. 1,2 and 3) or joint fluid (nos. 4, 5 and 6) and two of the three calves inoculated via the intravenous route with joint fluid(nos. 12 and 13) showed serious polyarthritis involving all limb joints, whereas all other inoculated calves and all control calves were clinically normal (Table 1). The infected calves clinically exhibited a gradual progression to severe polyarthritis. In detail, on PID 3, the calves were lame in the right front and left hind legs (the inoculated legs). By PID 5,the inoculated joints were enlarged and hot, and on PID 7,nearly all of the diarthroidal joints (including the uninoculated joints of the affected calves) were greatly enlarged due to the accumulation of intraarticular fluid (Fig. 2-A), whereas all of the control and uninfected calves were normal(Fig. 2-B). The body temperatures of all of the calves were normal during the clinical course of the disease. Although the appetites of the affected animals remained normal, the infected calves showed significant emaciation compared with the control and uninfected calves.
3.2. Etiology
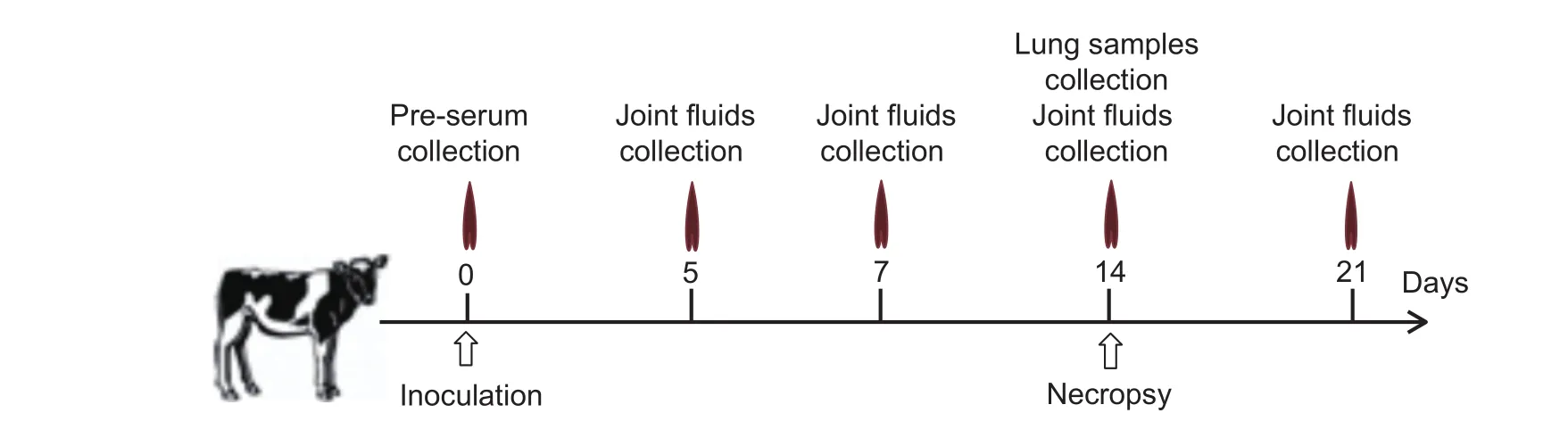
Fig. 1 Study design and the study timeline.

Fig. 2 Clinical and macroscopic findings of representative Mycoplasma leachii-inoculated calves. A, the tarsal joints of the infected calves were greatly enlarged due to the accumulation of intra-articular fluid (arrow). B, the tarsal joints of the control and uninfected calves. C, the joint cavities of the infected calves contained yellow-gray, turbid synovial fluid and large, yellow fibrin plaques (arrow)or flocculant, fibrinopurulent material. D, the joint cavities of the control and uninfected calves.
On PIDs 5, 7 and 14, the M. leachii PCR detection signals of the joint samples of all the infected calves (nos. 1, 2, 3, 4, 5,6, 12 and 13) were strong, and the isolated M. leachii titers reached 1×108CCU mL-1; however, on PID 21, the M. leachii PCR signals of the joint samples were quite weak, and the M. leachii titers decreased to 1×102CCU mL-1, although there was a large volume of synovial fluid in the joint cavity(Table 2). The inoculated and uninoculated joints of all the infected calves were similar based on M. leachii PCR detection and isolation, indicating that M. leachii could easily disseminate from the inoculated joints to the uninoculated joints in vivo. Routine bacterial cultures of the joints fromall infected calves were negative, indicating no bacterial co-infection in the joints. The PCR detection and isolation of M. leachii of the joint samples from all of the control and uninfected calves were also negative (Table 2).
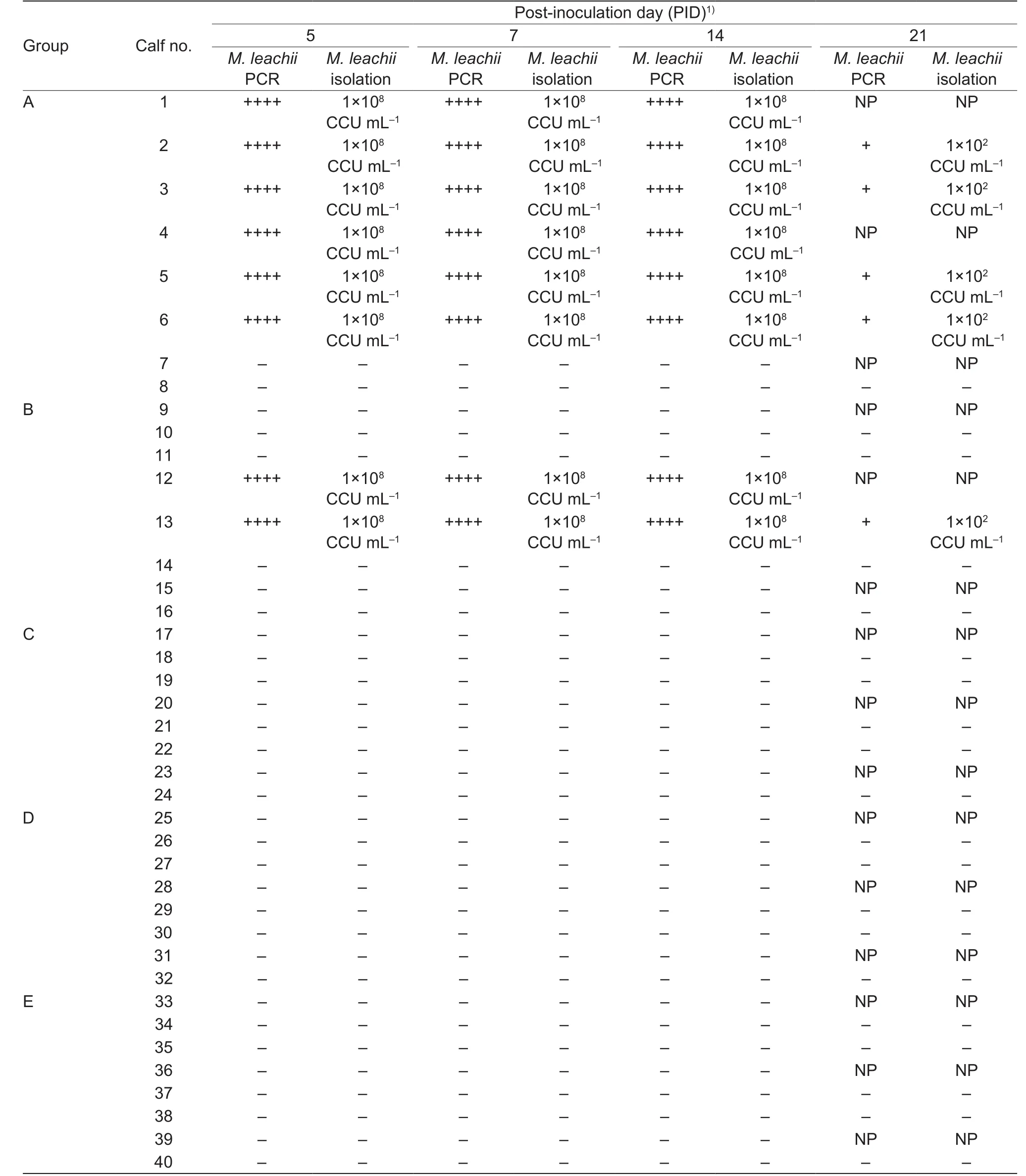
Table 2 PCR detection and isolation of Mycoplasma leachii from the joint fluids of the inoculated calves
3.3. Macroscopic pathology and histopathology
Because all infected calves exhibited similar clinical features according to M. leachii PCR detection and isolation, some calves (nos. 1, 4, 7, 9, 12, 15, 17, 20, 23, 25, 28, 31, 33,36 and 39) were randomly selected from each group,euthanized and submitted for necropsy on PID 14. The joints of randomly selected infected calves (nos. 1, 4 and 12) exhibited similar macroscopic and histopathological features; therefore, the images of calf No. 1 were selected as representative images in this article (Figs. 2-C and 3-A). The joints of the limbs were enlarged and contained yellow-gray,turbid synovial exudate and large yellow fibrin plaques or flocculant fibrinopurulent material. The synovial membranes were slightly thickened and congested (Fig. 2-C). In contrast, the joints of the control and uninfected calves appeared normal (Fig. 2-D). Histopathological examination revealed that the affected articulations displayed severe,diffuse, subacute arthrosynovitis, tenosynovitis and bursitis.Necrosis of the muscle tissue under the synovial membrane,degeneration of synovial epithelial cells and plasmocyte infiltration were detected in the joint samples of the infected calves (Fig. 3-A). The joint samples from the control and uninfected calves were histopathologically normal(Fig. 3-B).
3.4. Immunohistochemistry for M. leachii antigen
Positive labeling with the specific MAb 1F2 against M. leachii was observed in the joint samples from the inoculated and uninoculated joints of the infected calves. The IHC signals in the inoculated and uninoculated joints of randomly selected infected calves (nos. 1, 4 and 12) showed similar features; therefore, the images of calf no. 1 were selected as representative images in this article (Fig. 4-A and B). The M. leachii antigen was present extensively on the surface or in the cytoplasm of synovial epithelial cells (Fig. 4-A)and was also detected in the necrotic tissues under the synovial membrane (Fig. 4-B), while the joint samples from the control and uninfected calves displayed no IHC signal (Fig. 4-C). These findings, particularly detection of the M. leachii antigen in the synovial epithelial cells of the inoculated calves and no detection of the M. leachii antigen in the synovial epithelial cells of the control and uninfected calves, indicated that the joint lesions were directly caused by M. leachii.
3.5. M. leachii causes polyarthritis in calves via the blood route
All calves inoculated via the intraarticular route with passaged cultures of the M. leachii or joint fluid (containing the same titer of M. leachii) and two of the three calves inoculated via the intravenous route with joint fluid showed serious polyarthritis involving all limb joints. In addition, all uninoculated joints of the infected calves also developed serious polyarthritis, indicating that the pathogenicity of M. leachii in calves was strong. Meanwhile, none of the calves that were orally, intratracheally and intranasally inoculated with cultured M. leachii or joint fluid developed any clinical symptoms, including polyarthritis or pneumonia,and the M. leachii antigen was not detected in the joint or lung samples (Fig. 4-C and D). These results suggested that M. leachii could not be transmitted through the oral or respiratory routes. Intravenous inoculation of calves with cultured M. leachii did not cause polyarthritis. However,intravenous inoculation of calves with joint fluids that were collected from experimentally infected calves did cause polyarthritis in two-thirds of the calves, and M. leachii was also detected and recovered from the joints of these animals(Table 2). These findings demonstrated that M. leachii infection could cause polyarthritis in calves and it can be transmitted through the blood route.
3.6. M. leachii is not associated with bovine pneumonia
All the inoculated calves did not develop pneumonia, and no histopathological changes were found in the lung samples of any of the M. leachii-inoculated calves (Fig. 3-C), and the M. leachii antigen was not detected by IHC in the lungs of any of the M. leachii-inoculated calves (Fig. 4-D), indicating that M. leachii did not cause pneumonia in the calves.
4. Discussion
The findings in this study demonstrated that M. leachii infection in calves can be transmitted through the blood route;however, before infecting calves through the blood route, the pathogen must be propagated on the synovial epithelium in vivo. The mechanism underlying this phenomenon is unclear, possibly due to differences in the expression and/or structure of the related surface lipoproteins anchored in the membrane between in vitro-cultured M. leachii and in vivo-replicated M. leachii. The surface lipoproteins anchored in the membrane of Mycoplasma mediate hostpathogen interactions, and high-frequency phase and size variations in surface components frequently occur under the selection associated with different culture conditions (Citti et al. 2010; Citti and Blanchard 2013).
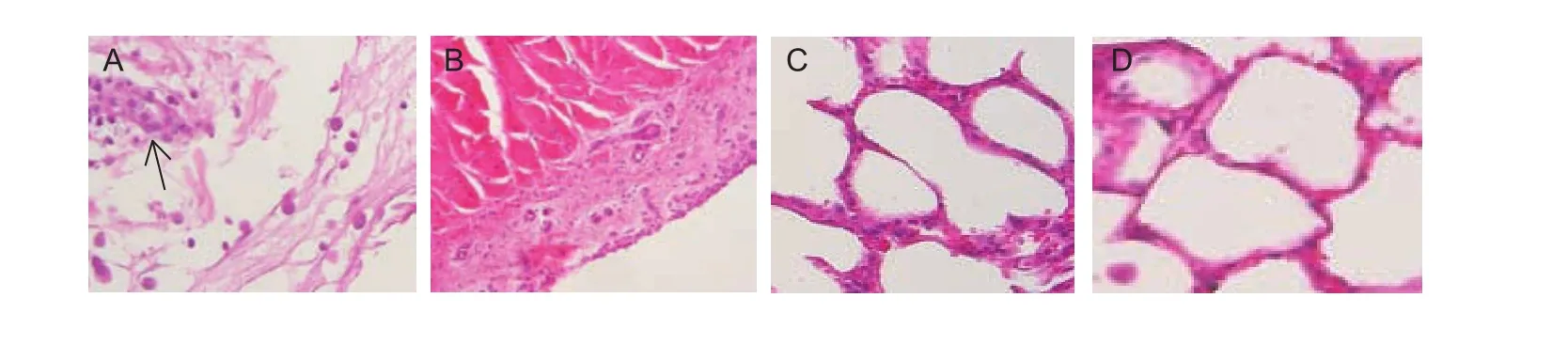
Fig. 3 Histopathology of joint and lung samples from representative Mycoplasma leachii-inoculated calf (no. 1). A, acute histopathological changes, including necrosis of the synovial membrane and muscle tissue under the synovial membrane,degeneration of synovial epithelial cells and plasmocyte infiltration (arrow), were present in the joint samples from the infected calves.B, the joints of the control calves and uninfected calves. C, the lungs of the inoculated calves. D, the lungs of the control calves.
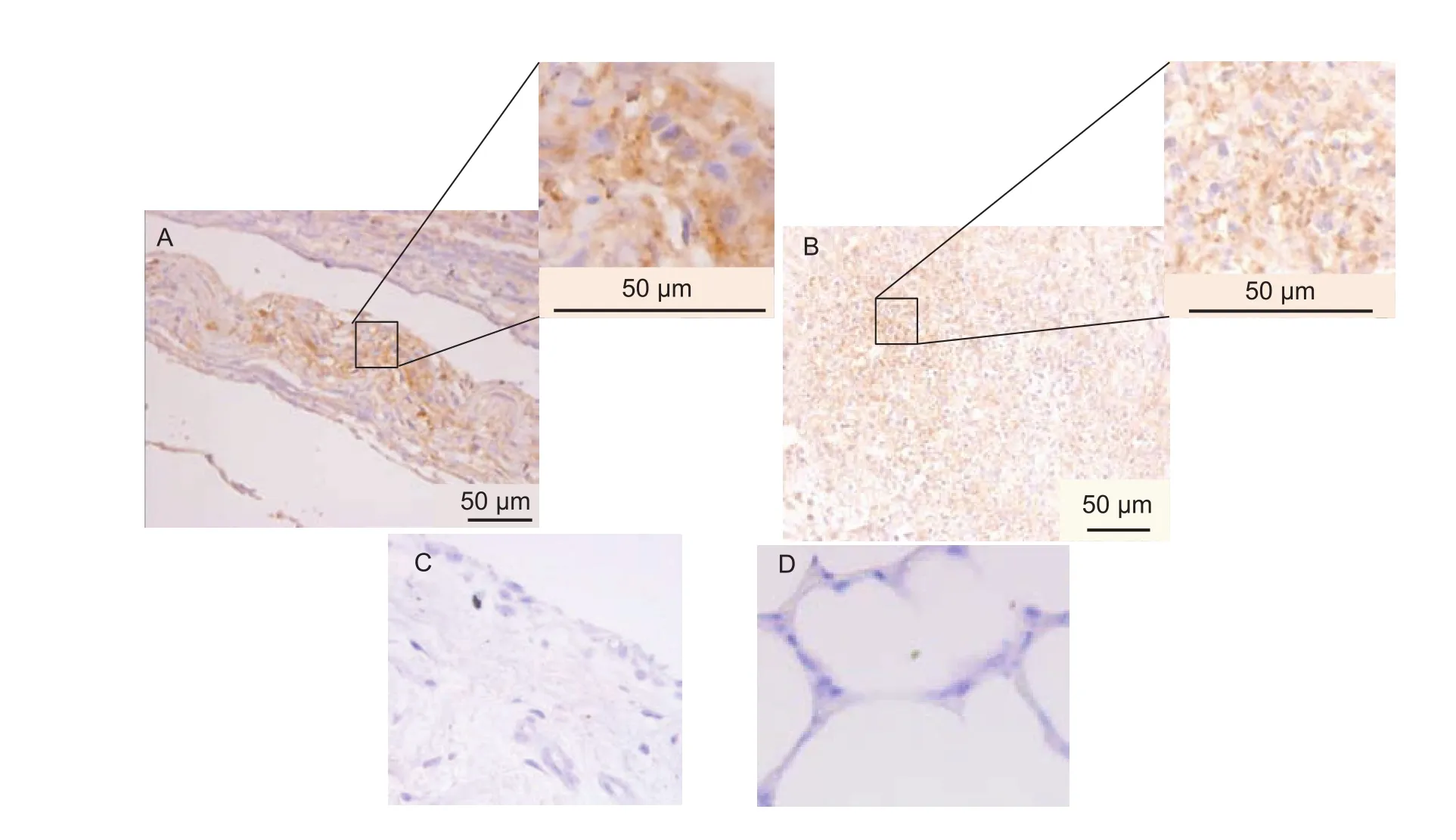
Fig. 4 Immunohistochemistry (IHC) assay of the Mycoplasma leachii antigen in joint and lung samples from representative M. leachii-inoculated calf (no. 1) and representative control calf (no. 7). A, M. leachii antigen on the surface or cytoplasm of synovial epithelial cells from the representative M. leachii-inoculated calf no. 1. B, M. leachii antigen in the necrotic tissues under the synovial membrane surface from the representative M. leachii-inoculated calf no. 1. C, no M. leachii antigen was present in the joint samples from the control calves and uninfected calves (the representative M. leachii-inoculated calf no. 7). D, no M. leachii antigen was present in the lung samples from the inoculated calves and control calves (the representative M. leachii-inoculated calf no. 7).
The results of this study showed that M. leachii causes calf polyarthritis through the blood route. However, the transmission of M. leachii via the blood route was not highly effective, as indicated in the inoculation experiment(affecting only 2/3 of calves, Tables 1 and 2). Nevertheless,outbreaks of polyarthritis in calves caused by M. leachii have been reported in Australia and China (Hum et al.2000; Liu et al. 2010). Thus, it is hypothesized that there are other routes of M. leachii infection in calves. Our previous epidemiologic and clinical investigations showed that all calves (approximately 350 female calves) with arthritis came from dams that were fertilized using the same batch of semen, whereas cows in the same herd that were fertilized using a different batch of semen delivered healthy calves, indicating that contaminated semen was the most likely source of M. leachii infection in young calves (Chang et al. 2011). Although an artificial infection experiment using contaminated semen through artificial insemination was not performed due to consideration of animal welfare requirements, our epidemiologic investigation is sufficient to propose a transmission route of M. leachii infection via artificial insemination of contaminated semen. In addition,the results of clinical analyses conducted in our previous study showed that some calves suffered from polyarthritis immediately after birth, whereas cows that delivered sick calves were clinically normal. This finding further suggested that the most effective source of M. leachii infection in young calves is contaminated semen.
The results confirmed that M. leachii causes polyarthritis in calves only through the contaminated semen and blood routes, which is consistent with the epidemiological characteristics of M. leachii-induced disease (outbreaks or sporadic cases). In general, the infection of calves through contaminated semen usually causes outbreaks of polyarthritis, and the pathogen is subsequently excreted into the environment and causes sporadic cases through blood contact during injury or umbilical exposure. Thus,the establishment and application of a PCR method for the detection of M. leachii in semen is an effective strategy for the prevention and control of M. leachii infection in calves.
In a previous study, M. leachii was isolated from pneumonic bovine lungs (Alexander et al. 1985); however,whether M. leachii is associated with calf pneumonia has not been directly determined. In this study, one-month-old calves were inoculated with cultures of M. leachii or joint fluid through multiple routes (including intraarticular, intravenous,intratracheal, intranasal or oral routes). However, all the inoculated calves did not develop pneumonia, and histopathological changes and the M. leachii antigen were not found in the lung samples of the M. leachii-inoculated calves. Furthermore, our artificial intraarticular infection experiment demonstrated that M. leachii could infect calves and cause polyarthritis and that it easily disseminates from the inoculated joints to the uninoculated joints in vivo. However, M. leachii did not infect the lungs using this transmission procedure. Thus, the results in this study showed that lungs of all inoculated calves seem to be never invaded by M. leachii whatever the inoculation route, which means a poor dissemination capacity of M. leachii in calves.These results suggested that M. leachii is not associated with pneumonia in calves. In addition, M. leachii was not detected in approximately 120 nasal swab samples that were collected from cattle with pneumonia in China from 2011 to 2016 (Chen et al. 2017). This finding further suggested that M. leachii is not associated with bovine pneumonia.
Because the evolution of M. leachii is much conserved,and we compared the partial high variable lppA gene, the two Chinese isolates of M. leachii from the same epizootic shared 100% nt identity, and the Chinese isolates shared 99.6% nt identity to M. leachii representative strain PG50(Liu et al. 2010; Chang et al. 2011). Thus, we thought that the results in this study extend to any other strains of M. leachii.
5. Conclusion
In summary, the pathogenicity of M. leachii in calves and its routes of transmission were investigated in this study through artificial infection experiments. The results in this study confirmed that M. leachii causes polyarthritis in calves through blood route, and M. leachii is not associated with pneumonia in calves.
Acknowledgements
We thank Prof. He Xijun (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences) for his assistance in the histopathological and IHC examinations.This study was supported by the Natural Science Fund Project of Heilongjiang Province, China (C201348), the grants from the Central Public-interest Scientific Institution Basal Research Fund, China (1610302016001), and the National Science and Technology Program Topics of China(2012BAD12B03-3).
 Journal of Integrative Agriculture2018年11期
Journal of Integrative Agriculture2018年11期
- Journal of Integrative Agriculture的其它文章
- First report of Athelia bombacina causing postharvest fruit rot on pear
- Sustainability assessment of potato fields using the DEXi decision support system in Hamadan Province, lran
- Effect of long-term continuous cropping of strawberry on soil bacterial community structure and diversity
- Alternate row mulching optimizes soil temperature and water conditions and improves wheat yield in dryland farming
- lnter-annual changes in the aggregate-size distribution and associated carbon of soil and their effects on the straw-derived carbon incorporation under long-term no-tillage
- Genome-wide detection of selective signatures in a Duroc pig population
