Effect of alternate-day-fasting combined with Lingguizhugan Decoction on blood lipid profiles of hyperlipidem ic rats
Jun-Jie Zhang,Xian-Zhi He,Guo-Shun Peng,Zhen-Kun Wang,Bin Ke,Jian Qin
1Department of InternalMedicine of Cardiology,ZhuhaiHospital of Traditional Chinese andWestern Medicine,Zhuhai,China.2Department of Traditional Chinese Medicine,First Affiliated Hospital of Sun Yat-Sen University,Guangzhou,China.
Background
As a pattern of dietary restriction,alternate-day-fasting(ADF)regimen generally involves a “fast day”on which food is withheld alternating with a “feast day”on which food is consumed ad libitum.The feast and fast periods are typically 24 h each.It has been demonstrated that ADFwas able to promote lipolysis,reduce body weight,and improve lipid profiles,of which the possible mechanisms may be that ADF could to some extent improve adipose tissue metabolism,remain elusive though[1].
Lingguizhugan Decoction (LD) comes fromShanghanlunpublished in Eastern Han Dynasty of China(the third century A.D.),which played a role of warming Yang,strengthening spleen and dissipating excessive fluid,indication of phlegm stagnation and it is a major recipe for warming phlegm.Our previous clinical studies have found that LD contributed to reducing cardiovascular risk factors(including blood pressure,lipid profiles,blood glucose,and insulin sensitivity)and relieving phlegm syndrome under dietary restriction on overweight/obesity patients with phlegm dampness syndrome[3-5].Furthermore,LD combined with ADF therapy has been proved to be able to improve hyperlipidemia and enhance resistance to oxidative stress in the experimental research[6].In additional to this,LD combined calorie restriction could reduce the body weight, and alleviate high-fat induced obesity,hyperglycemia,hyperlipidemia,hypertension,hepatic injury and insulin resistance[7-8].From above all,we hypothesize that LD combined with ADF therapy maybe more effective than ADF therapy alone in lipid metabolism improvement.
MicroRNAs(miRNAs)have been recognized as a class of epigenetic regulators of metabolism and energy homeostasis in recent years.Accumulative evidence support miRNAs play a vital role in the pathological development of obesity by affecting adipocyte differentiation [9-10].In addition,miRNA-143 is up-regulated after the induction of differentiation in human preadipocytes,3T3-L1 cells and in the mesenteric fatof high-fat diet-induced obese mice[11-12].Evidence suggested that functional analysis of specific miRNAs as a target for therapeutic intervention was available[14].However,whether ADF affects miR-143 expression in adipocytes isstillunknown.
Peroxisome proliferator-activated receptor(PPAR-γ)is expressed in adipocytes, known as adipocyte differentiation marker.PPAR-γ is a ligand-activated transcription factor that belongs to the nuclear receptor super-family.In the last decade,PPAR-γwas defined as a regulator of metabolic pathways for whole body energy homeostasis and adipose tissue differentiation.Recently,it has been showed that expression of miR-143 was associated with expression of PPAR-γ in visceral adipose in high-fat-diet (HF) induced obese mice [14].Furthermore,low-calorie dietwas found to down-regulate the PPAR-γ expression of adipose tissue in obese humans[15].
Thus,we proposed that ADF therapy accompanied with LD was able to improve lipid metabolism through suppression of higher expression of miR-143 and PPAR-γ in visceral adipose tissue under HF.To test hypothesis,we aimed to determine the effect of ADF combined with LD on lipid profiles and expression of miR-143 and adipogenic gene PPAR-γprotein in adipose tissue in HF-induced hyperlipidemic rat.
Materials and Methods
Ingredients of LD
LD was formulated with medicine particle prescription provided by Huarunsanjiu Pharmaceutical Limited Company.Ingredients of LD include Fuling(Poria)12 g,Guizhi(Ramulus Cinnamomi)9 g,Baizhu(Atractylodes Macrocephaia)6 g and Gancao(Radix Glycyrrhizae)6 g.The Chinese herbs granules were dissolved in saline in accordance with the proportion above.The concentration of the decoction was 0.3625 g/m L.According to the unit conversion of rats and human body surface area,dosage of intragastric administration was 8 m L/kg[16].
Animalsand diet
52 male wistar rats weighing 250 g istarra obtained from Animal Center of Sun Yat-Sen University(SCXK Guangdong 12009-001)were fed in specific pathogen free cage in the Experimental Animal Center of Medical School of Sun Yat-Sen University.They were used for experiment after 7-day adaptive feeding.Subsequently,the experiment was divided into two main phases and that analyses were carried out accordingly.In the phaseⅠ,the rats were randomized into HF group(n=32)and normal-diet(ND)group(n=20)and fed continually for 5 wk.The ND group was fed mouse chow containing 51%carbohydrate,16%protein,4%vitamins and minerals,3%fat,while the HF group received high-fat diet including 78.3% basal diet, 10%lard,10%yolk powder,1%cholesterol,0.5%bile salts and 0.2%propylthiouracil.Total energy of the high-fat diet was 17.4 kJ/g,relative to the normal chow was 15 kJ/g.All fodders were provided by the Experimental Animal Center of Guangdong Province.
In the phaseⅡ,rats in HF groups were random ly divided into model control(MC)group(n=8),ADF group(n=8)and alternate-day-fasting with LD(ALG)group(n=8)and fed with high-fat diet in the following 4 wk.The ND group,which served as negative control(NC)group(n=10),was provided with normal chow as before.Rats in ALG group were given LD(8 m L/kg)by intragastric administration on the fast day.Others were administrated by gastric perfusion of the same dosage of saline.Rats in ADF and ALG groups were both fed ad libitum of HF on the feast day,and then were given no access to any food on the fast day.Rats in MC groups were fed ad libitum diet.The experimental design and animal handling were approved by the Ethic Committee of the first Affiliated Hospital of Sun Yat-Sen University([2012]246).
Body weight and food intake of the ratswere recorded weekly.In the phaseІ,10 and 8 rats were chosen random ly from the ND and HF groups respectively and were sacrificed by decapitation under light intraperitoneal anesthesia with 10%chloral hydrate(3 m L/kg),followed by fasting for 14 h.Blood sample was collected from abdominal aortic blood into heparinized polyethylene tubes.
In the phaseⅡ,all of the rats were killed by decapitation under light intraperitoneal anesthetized with 10%chloral hydrate(3 m L/kg),followed by fasting for 14 h.The omental tissues were quickly removed,frozen with liquid nitrogen,and stored at-80°C until further analysis.Blood sample was collected from abdominal aortic blood into heparinized polyethylene tubes.All the experimental research procedures were shown in Figure 1.
Serum lipid profiles
Serum obtained from centrifugation of blood(3500 r/min,10min,4°C)was stored in the refrigerator at-20°C.Triglyceride(TG),total cholesterol(TC),high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol(LDL-c)were measured by automatic biochemical analyzer in the Laboratory Department of First Affiliated Hospital of Sun Yat-Sen University.
RNA extraction and quantitative real-time PCR
Total RNA from omentum tissue was extracted using RNAiso Plus according to the manufacturer’s instruction.To assess miRNA expression levels,total RNA was reverse-transcribed into cDNA using cDNA Synthesis Kit.U6 small nuclear RNA was the reference gene used to normalize cDNA.SYBR PremixEx TaqTMⅡwas used to monitor the amplification of cDNA,and ROX Reference Dye was used for normalization.miRNA PrimeScript PrimeScript○R RT Enzyme was employed to amplify cDNA.Amplification was performed using the ABI PRISM 7000 Sequence Detection System,and initiated at 50°C for 2 min and 95°C for 10 min,followed by 40 cycles consisting of denaturation at 95°C for 15 sec,and annealing and extension at 60°C for 1 min.The results were expressed relative to the ΔΔCt method.
The primersused in this study were:
miR-143:5’-GGT GAG ATG AAG CAC TGT AGC TC-3’.
U6:5’-AACGCTTCACGAATTTGCGT-3’.
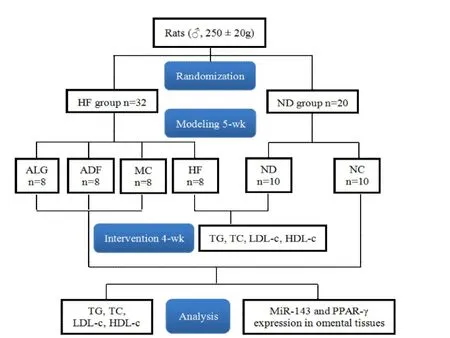
Figure 1 Flowchart
Western blot
Ratomentum tissues were homogenized and protein was extracted.The homogenates were centrifuged at 12,000 g for 15 minutes at 4°C,analyzed using 12%SDS-PAGE,and the proteins were then transferred onto PVDF membranes.The membranes were incubated with antibodies for PPAR-γ(Cell Signaling Technonlogy.,Inc,America) or GAPDH (1:5000 dilution;Abcam,Cambridge,MA)overnightat 4°C.The membraneswere incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody for 2 hours at room temperature.After the final washing,blots were probed using ECL luminescent liquid(Applygen Technologies Incorportion,China)and autoradiographed.
Statisticalanalysis
Data were presented as mean±standard deviation(mean±SD).The difference of measurement data between the two independent groups was compared via independent-samplettest.Themeasurement data of multiple groups were analyzed by one-way analysis of variances.The differences were considered to be significant asP< 0.05.Statistical analysis were performed with 13.0 SPSS software(SPSS,Inc.,Chicago,IL).
Results
Food and calorie intake
In phaseⅡ,calorie intake in MC group was significantly higher(MC vs.NC,P<0.001)than that in NC group.As compared with MC group,food intake in ADF and ALG groups was significantly increased in feast day(MC vs.NC,P=0.001).However,total calorie intake in ADF and ALG groups decreased significantly as compared with that in MC group.
An approximate 41.6%increase in total calorie intake during phaseⅡ was also observed in MC group.A reduction of total calorie intake in ADF and ALG groups during phase Ⅱ was also shown in Table 1(approximately 44.3%and 43% respectively),when compared with MC group.
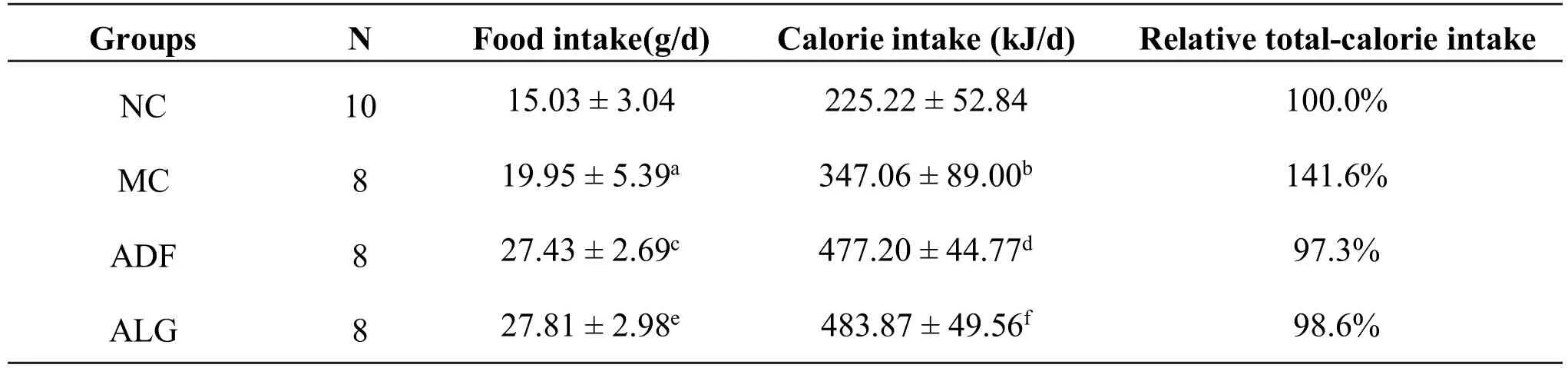
Table 1 Food intake,calorie and relative total-calorie intake
Changes in body weight
During phaseІ,as shown in Table 2,the body weight of HF group grew more rapidly than that of ND group.Additionally,italso weighedmuchmore during modeling period(HF vs.ND,P=0.032 by wk 2,P<0.001 by wk 4&6).
There had also been a noticeable weight loss in ADF and ALG groups(ADF vs.MC,P=0.028 by wk 8,P<0.001 by wk 10;ALG vs.MC,P=0.036 by wk 8,P<0.001 by wk 10)during ADF period.As to ADF and ALG groups,all of them were greatly lighter than that of NC group(ADF vs.NC,P=0.021;ALG vs.NC,P=0.036)at the end of the experimental period.However,the body weight in ALG group showed non-statistically difference in comparison with ADF group(ALG vs.ADF,P>0.05)(Table 3).
Table 2 Effect of HF on body weight changes in rats(g, ±s)
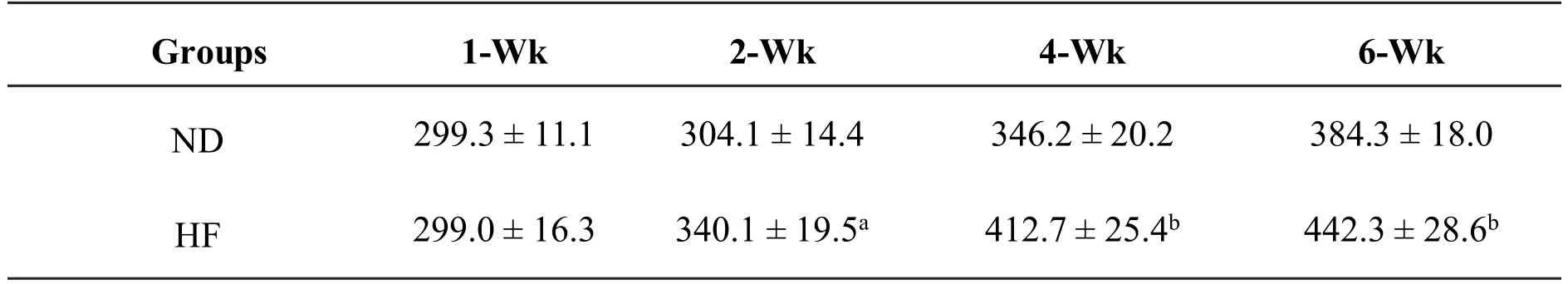
Table 2 Effect of HF on body weight changes in rats(g, ±s)
Abbreviation:ND:normal diet,HF:high-fat-diet,Wk:week of feeding.Valuesare expressed as mean±SD.HF vs.ND:a P=0.032 by wk 2,b P<0.001 by wk 4&6.
Groups 1-W k 2-W k 4-W k 6-W k ND 299.3±11.1 304.1±14.4 346.2±20.2 384.3±18.0 HF 299.0±16.3 340.1±19.5a 412.7±25.4b 442.3±28.6b
Table 3 Effect of ADF or combined ADF and LD therapy on body weight changes(g, ±s)
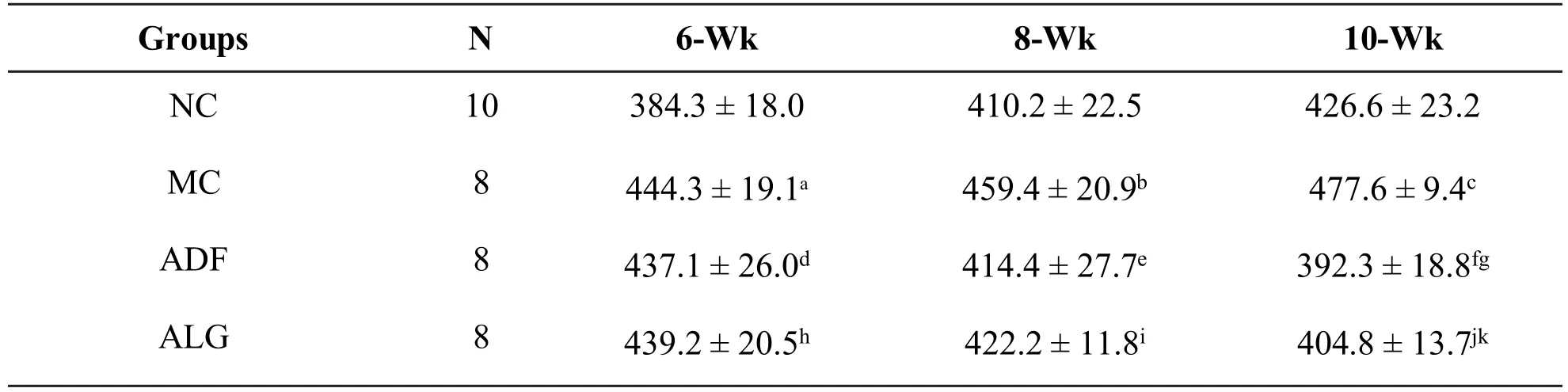
Table 3 Effect of ADF or combined ADF and LD therapy on body weight changes(g, ±s)
Abbreviation:NC:negative control,MC:model control,ADF:alternate-day-fasting,ALG:alternate-day-fasting with LD,Wk:week of feeding.Valuesareexpressed as mean±SD.MC vs.NC:a P<0.001 by wk 6,b P=0.006 by wk 8,c P=0.032 by wk 10.ADF vs.NC:d P<0.001 by wk 6,g P=0.021 by wk 10.ALG vs.NC:h P<0.001 by wk 6,k P=0.036 bywk 10.ADF vs.MC:e P=0.028 by wk 8,f P<0.001 by wk 10.ALG vs.MC:i P=0.036 by wk 8,j P<0.001 by wk 10.
Groups N 6-W k 8-W k 10-W k NC 10 384.3±18.0 410.2±22.5 426.6±23.2 MC 8 444.3±19.1a 459.4±20.9b 477.6±9.4c ADF 8 437.1±26.0d 414.4±27.7e 392.3±18.8fg ALG 8 439.2±20.5h 422.2±11.8i 404.8±13.7jk
Changes in blood lipid profiles
After the rats in HF group were fed HF for 5 wk,TC,TG,HDL-c and LDL-c concentrations were all evidently increased in comparison with ND group. TC concentration increased 68.2%(ND vs.HF,P<0.001).TG concentration increased by 84.3%(ND vs.HF,P<0.001).HDL-c concentration increased 86.9%(ND vs.HF,P<0.001).LDL-c concentration increased 2.9-fold(ND vs.HF,P<0.001)as present in Table 4.
As shown in Table 5,after a 4-wk ADF therapy,serum TC concentrations in ADF and ALG groups markedly dropped(ADF vs.MC,P<0.001 and ALG vs.MC,P<0.001)as compared to MC group.There were significantly higher TC concentrations in ADF group than that in NC group(NC vs.ADF,P=0.039).But it showed no obvious difference between ALG and NC groups(NC vs.ALG,P=0.439).
Also noted was that ADF and ALG therapy could both reduce TG concentrations significantly(ADF vs.MC,ALG vs.MC,P=0.045 andP=0.005 respectively).However,the concentration of plasma TC,TG,HDL-c and LDL-c in ALG group showed non-statistically difference in comparison with ADF group(ALG vs.ADF,P>0.05).Interestingly,ADF group had a remarkable increase in HDL-c concentrations(ADF vs.MC,P=0.01)compared to MC group,implicating that ADF therapy could exert beneficial effect on anti-atherosclerosis.However,ADF coupled with LD decoction had not achieved the expected effect on elevating HDL-c concentrations(ALG vs.MC,P=0.165).
In terms of LDL-c concentrations,in MC group it increased significantly(MC vs.NC,P=0.003)compared to NC group,but nevertheless no impactwas found with regard to ADF and ALG therapy(ADF vs.MC and ALG vs.MC,P>0.05).
Changes in m iR-143 and PPAR-γexpression in adipose tissue
As shown in Figure 2,HF induced miR-143 expression increased in adipose tissue as compared with ND(MC 225%±23%vs.NC 100%±40%,P<0.001).Interestingly,it was shown that ADF and ALG therapy was able to normalized miR-143 expression,which was elevated in MC group(ADF 128%±53%vs.MC 225%±23%and ALG 96%±17%vs.MC 225%±23%,P=0.038 andP=0.007,respectively).
Furthermore,the same trend was also noted that in MC group,which PPAR-γ protein expression in adipose tissue was evidently elevated compared with that of NC group(MC 1.24±0.07 vs.0.8±0.11 NC,P<0.001).As noted in Figure 3,ADF therapy could therefore reduce the PPAR-γexpression,which was highly expressed in MC group(ADF 0.89±0.19 vs.MC 1.24±0.07,P=0.015).In addition to this,ALG therapy was able to evidently decreased PPAR-γ expression as compared with MC group(ALG 0.60±0.13 vs.MC 1.24±0.07,P<0.001).,Moreover,expression of miR-143 and PPAR-gamma protein in adipose tissue was significantly decreased in ALG(ADF 128%±53%vs.ALG 96%±17%and ADF 0.89±0.19 vs.ALG 0.60±0.13,P=0.041 andP=0.046,respectively)group as compared to ADFgroup.
Table 4 Effectof HF on blood lipid profiles(mmol/L, ±s)
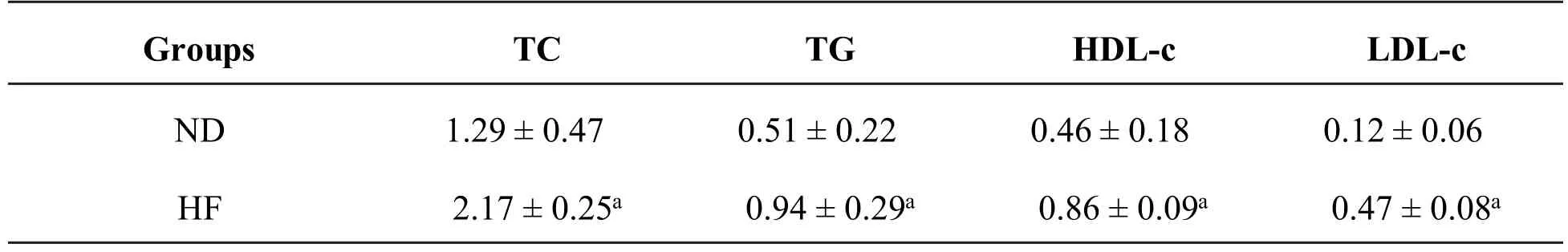
Table 4 Effectof HF on blood lipid profiles(mmol/L, ±s)
Abbreviation:ND:normal diet,HF:high-fat-diet.Values are expressed as mean±SD.a P<0.001 as compared with ND group.
Groups TC TG HDL-c LDL-c ND 1.29±0.47 0.51±0.22 0.46±0.18 0.12±0.06 HF 2.17±0.25a 0.94±0.29a 0.86±0.09a 0.47±0.08a
Table 5 Effectof ADF or combined ADF and LD therapy on blood lipid profiles(mmoL/L, ±s)
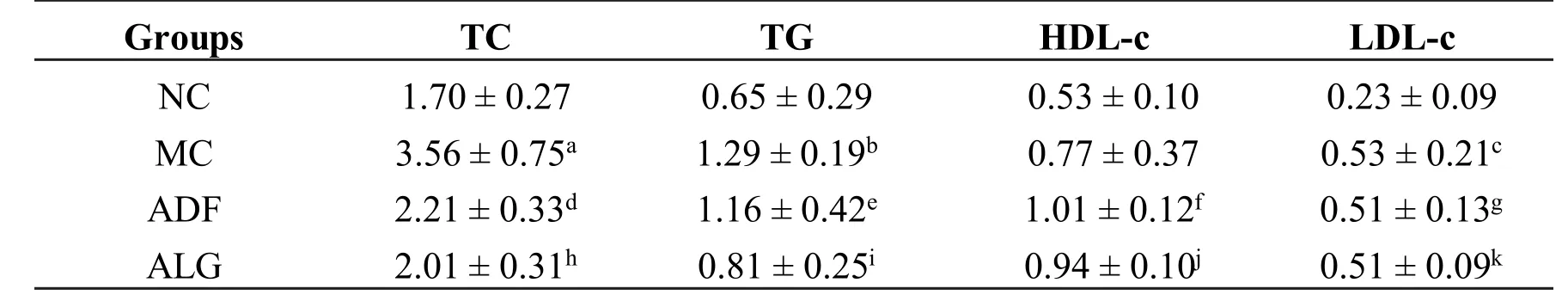
Table 5 Effectof ADF or combined ADF and LD therapy on blood lipid profiles(mmoL/L, ±s)
Abbreviation:NC:negative control group,MC:model control group,ADF:alternate-day-fasting group,ALG:alternate-day-fasting with LD group.Valuesareexpressed as mean±SD.MC vs.NC:a P<0.001,b P=0.005,c P=0.003.ADF vs.NC:d P=0.039,f P<0.001,g P=0.005.ALG vs.NC:j P=0.001,k P=0.002.ADF vs.MC:d P<0.001,e P=0.045,f P=0.01.ALG vs.MC:h P<0.001,i P=0.005.
?
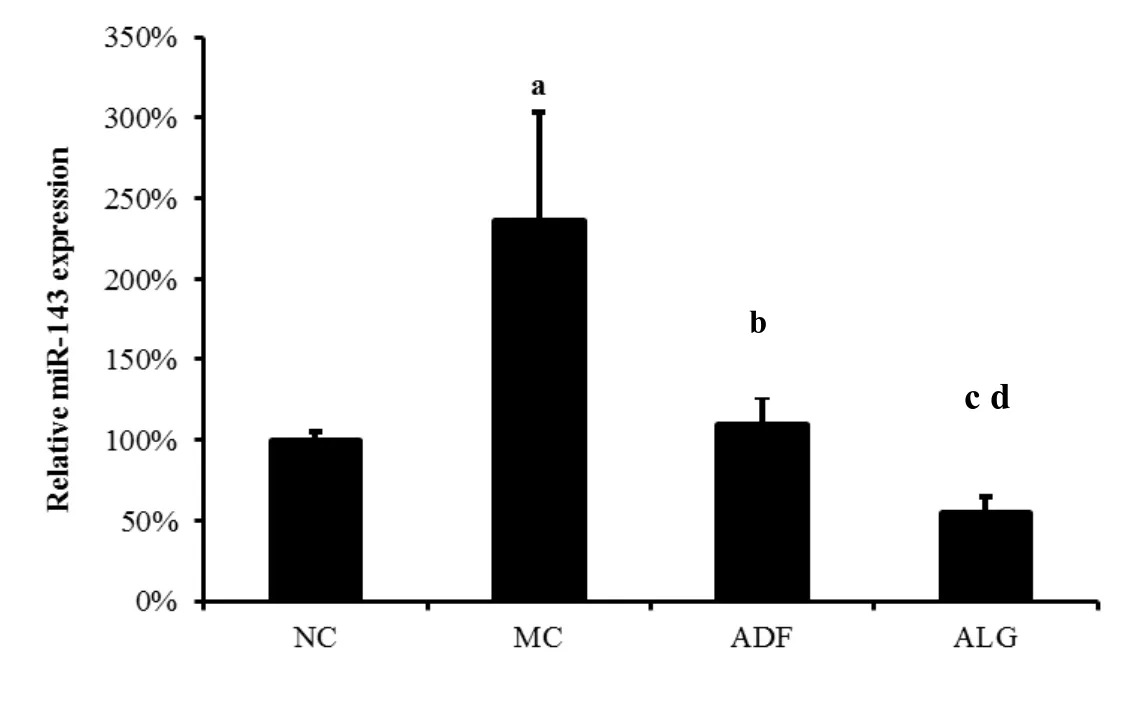
Figure 2 Relative m iR-143 expression in adiposity tissue
a,MC vs.NCP<0.001;b,ADF vs.MCP=0.038;c,ALG vs.MCP=0.007;d,ALG vs.ADFP=0.041.
(NC:negative control,MC:model control,ADF:alternate-day-fasting,ALG:alternate-day-fasting group with LD)
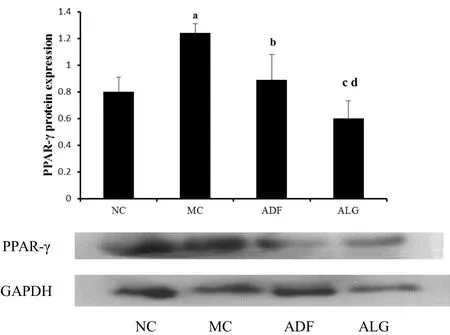
Figure 3 PPAR-γ/GADPH expression in adiposity tissue
Discussion
As far as we known,there is no related nomenclature of hyperlipidemia in TCM.Recently,it has been found that hyperlipidemia is closely associated with “phlegm syndrome”in TCMin the light of its causes,mechanisms and clinical manifestations.LD,which comes fromShanghanlun, consists of Fuling (Poria), Guizhi(RamulusCinnamomi), Baizhu (Atractylodes Macrocephaia)and Gancao(Radix Glycyrrhizae).The whole recipe has an effect of warming Yang and dissipating phlegm.
After introduced the LD combined with ADF therapy,we found that the symptom of phlegm-dampness retention like dizziness,fatigue,tick and greasy tongue coating appearing and developing in human bodies during fasting period could be obviously relieved[2].Ithas been reported that ADF could promote fat hydrolysis,decrease free fatty acids concentrations and lead to reduction of TG concentration[17].Besides,it is able to reduce TC concentration by inhibiting cholesterol synthesis.Our data demonstrates that ADF decreases TG and TC concentrations in hyperlipidemic rats evidently,but exert no obvious effect on LDL-c level,which may be relevant to high cholesterol and propylthiouracil in diet.Aswe known,serum LDL-c is a very important factor which participates in the process of atherosclerosis.Therefore it can be inferred from the study that dietary restriction without improving the components of food,such as lowering the cholesterol in diet,could not exert beneficial effect on reducing LDL-c concentration.Interestingly,ADF therapy could increase the HDL-c concentration significantly,which was involved in the process of reversing cholesterol transportation from atherosclerotic lesion.
Accordingly,we postulated that ADF therapy could help organisms resist the atherosclerosis induced by HF by the promotion of reversing cholesterol transportation.However,our present study showed that LD combined with ADF therapy couldn’t reduce the concentration of TG,TC,LDL-c and HDL-c s more effectively than using ADFalone.Interestingly,our resultsalso showed that LD combined with ADF therapy exerted no effect on weight loss and food intake,so we suggested that adipose tissue might be responsible for lipid profiles mildly improvement.However,the precise mechanism of the beneficial effect still needs to be further studied.
In our study,we revealed that ADF reduced miR-143 and PPAR-γprotein expression in mesenteric fat with significantweight loss for the first time.Recently,Esauet al.showed that antisense oligonucleotide inhibition of miR-143 prevented adipocyte-specific gene expression of PPAR-γand the accumulation of triglycerides,suggesting that miR-143 is normally involved in promoting adipocyte differentiation[9].Furthermore,Takanabeet al.demonstrated that expression of miR-143 in the mesenteric fat was up-regulated in mice fed a high-fat diet.Increased miR-143 expression was associated with an elevated body weight and mesenteric fat[12].They also revealed that miR-143 expression levels were correlated with adipose expression levels of PPAR-γ.Parra P and his colleagues demonstrated that conjugated linoleic acid could mildly influence miR-143 expression,suggesting miRNAs expression may be influenced by dietary manipulation[18].Bae IS and his colleagues identified that miR-143 was a target of PPAR-γ,and found that PPAR-γmediated G-protein coupled receptor 120 signaling pathway promoted transcriptional activation of miR-143 in adipocytes[19].And we further demonstrated that ADF could inhibit miR-143 expression accompanied with inhibition of PPAR-γprotein expression in visceral adipose and LD was able to enhanced ADF effect on miR-143 and PPAR-γexpression.
Conclusion
ADF therapy alone could reduce blood lipids and further inhibit miR-143 and PPAR-γ protein expression in visceral adipose tissue.However,LD enhanced the effect of ADF therapy in the inhibition ofmiR-143 and PPAR-γ protein expression in visceral adiposewithout significant effect in the prevention of hyperlipidemia.Maybe the combination of ADF and LD therapy need further exploration in the prevention and treatment of hyperlipidemia.
1.Varady KA,Hellerstein MK.Alternate-day fasting and chronic disease prevention:a review of human and animal trials.Am JClin Nutr 2007,86:7-13.
2.Chen DS,Ke B,Huang YJ,et al.Effects of the modified lingguizhugan decoction (flavoread lingguizhugandecoction)combined with short-term very low calorie diets on glycemic control in new ly diagnosed type 2 diabetics.JTradit Chin Med 2011,31:185-188.
3.Chen DS,Meng J,Ke B,et al.Effect on fasting therapy for dedicine subjects with blood pressure.Gansu J TraditChin Med 2010,26:11-12.
4.Chen DS,Li CY,Qin J,etal.Modified Lingguizhugan Decoction combined with short-term fasting improves therapeutic response in type 2 diabetic patients.Eur JIntegr Med 2012,73:32-35.
5.Ke B,Shi L,Qin J,et al.Protective effects of modified Lingguizhugan Decoction combined with short-term very low calorie diets on cardiovascular risk factors in obese patients with impaired glucose tolerance.JTraditChin Med 2012,33:185-188.
6.Zhang JJ,Huang YJ,Ke B,et al.Effect of alternate-day fasting therapy combined with Linggui zhugan Decoction on hepatic oxidative stress in hyperlipidemic rat.Chin J Integr Med 2015:1-6.In press.
7.Wang YY,Jin MH,Ke B,et al.Effects of Linggui zhugan Decoction combined calorie restriction on the insulin resistance of model rats and mechanisms research.Chin JIntegr Tradit and Western Med 2013,33:356-60.
8.Yao L,Wei J,Shi S,et al.Modified Lingguizhugan Decoction incorporated with dietary restriction and exercise ameliorates hyperglycemia,hyperlipidemia and hypertension in a rat model of the metabolic syndrome.BMC Complem Altern Med 2017,17:132.
9.Esau C,Kang X,Peralta E,et al.MicroRNA-143 regulatesadipocyte differentiation.JBiolChem 2004,279:5361-5365.
10.Xie H,Lim B,Lodish HF.MicroRNAs induced during adipogenesis that accelerate fat cell development are down regulated in obesity.Diabetes 2009,58:1050-1057.
11.Kajimoto K,Naraba H,Iwai N.MicroRNA and 3T3-L1 pre-adipocyte differentiation.RNA 2006,12:1626-1632.
12.Takanabe R,Ono K,Abe Y,et al.Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet.Biochem Bioph Res Co 2008,376:728-732.
13.Vidal-Puig AJ,Considine RV,Jimenez-Linan M,et al.Peroxisome proliferator activated receptor gene expression in human tissues:effects of obesity,weight loss, and regulation by insulin and glucocorticoids.JClin Invest 1997,99:2416-2422.
14.Petri A,Lindow M,Kauppinen S.MicroRNA silencing in primates:towards the development of novel therapeutics.Cancer Res2009,69:393-395.
15.Takasawa K,Kubota N,Terauchi Y,et al.Impact of increased PPAR gamma activity in adipocytes in vivo on adiposity,insulin sensitivity and the effects of rosiglitazone treatment.Endocr J2008,55:767-776.
16.Zhao W,Sun GZ.The dosage conversion between different kinds of experimental animals.Animal Husbandry Vet2012,5:52-53.
17.Zhang JJ,Ke B,Qin J.Intermittent fasting research progress.Med Recap 2012,18:1332-1335.
18.Parra P,Serra F,Palou A.Expression of adipose microRNAs is sensitive to dietary conjugated linoleic acid treatment inmice.PLoSOne 2010,5:e13005.
19.Bae IS,Park PJ,Lee JH,et al.PPARγ-mediated G-protein coupled receptor 120 signaling pathway promotes transcriptional activation of miR-143 in adipocytes.Gene 2017,626:64-69.
 Traditional Medicine Research2018年3期
Traditional Medicine Research2018年3期
- Traditional Medicine Research的其它文章
- Screening for cyclooxygenase 2 inhibitors from natural com pounds of Radix Glycyrrhizae using com puter simulation
- The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1
- Polysaccharide extracts of Cirsium japonicum protect rat H 9c2 m yocardial cells from oxidative stress induced by hydrogen peroxide
- Study of dual-directional regulatory effect of Banxia(Pinellia ternata)and Huanglian(Coptis chinensis)drug pair on gastrointestinal movement of m ice
