Polysaccharide extracts of Cirsium japonicum protect rat H 9c2 m yocardial cells from oxidative stress induced by hydrogen peroxide
Zheng-Bo Tao,Li-Yan Xiong,Li-Hui Wang,Chuan Zhang,*
1 Administrative Office for Undergraduates,Second Military Medical University,Shanghai,China.2 Department of Identification of Traditional Chinese Medicine,School of Pharmacy,Second Military Medical University,Shanghai,China.3 Business Unit of Traditional Chinese Medicine Resources,Shanghai Traditional Chinese Medicine Co.,Ltd,ShanghaiPharma,Shanghai,China.
Background
Ischemic heart disease is one of the leading causes of mortality globally[1].It is vital to preventmyocardial ischemia and reperfusion injury and to preserve myocardial cell function in coronary heartdisease surgery[2,3].A large amount of reactive oxygen species(ROS)is produced by myocardial ischemia and reperfusion injury in cardiomyocytes.ROS formation might be triggered by ATP depletion and calcium cation overload[4,5].During reperfusion,ROS formation can occur when oxygen interrupts the mitochondrial respiratory chain[6].It has been proven that ROS affect intercellular communications and cell skeletons,as well as modify the active sites of intracellular targets involved in processes such as cell detachment,morphology alteration,or even cell death[7].The blockage of excessive ROS was supposed to create a promising therapeutic approach against cardiovascular diseases[8].Mitogen-activated protein kinase(MAPK)is one of the important cell signaling pathways that is involved in the development of heart disease[9].
Daji(Cirsium japonicum),a spiny deciduous shrub of the family asteraceae,has recently gained worldwide attention due to its nutritional and medicinal potential[10].This plant is naturally distributed widely acrossAsia and Europe and is currently domesticated in various parts of the world[11].Various parts of Daji(Cirsium.japonicum),especially berries,have been traditionally utilized by the Tibetan and Mongolian people since the Tang Dynasty of China(618 A.D.-907A.D.).The earliest medical use of the plant was found in the Tibetan medicinal system [12]for the treatment of gastric disorders,cardiovascular problems,liver injury,and skin diseases[13,14].Current research has reported a wide spectrum of pharmacological properties of Daji(Cirsium japonicum)including anti-oxidative,immunomodulatory,hepatoprotective,and anti-inflammatory effects[11].Daji(Cirsium.japonicum)berries are rich in nutrients and compounds such as vitamins,carotene,flavonoids,essential oils,carbohydrates,organic acids,amino acids,and minerals.However,the specific components in Daji(Cirsium japonicum)that are responsible for these effects have not yetbeen elucidated.Hence,in the present study,we aimed to investigate the effect of Daji(Cirsium japonicum) polysaccharide extracts (CJP) against oxidative stress in the rat H9c2 myocardial cell line.Results showed that CJP had a protective effect against hydrogen peroxide(H2O2)injury in rat H9c2 myocardial cells.These results laid the preliminary foundation for the therapeutic application of Daji(Cirsium japonicum).
Methods
Materials and reagents
Daji(Cirsium japonicum)was collected from Matoushan in Liangcheng,Inner Mongolia,China,and authenticated by Professor Xuejun Jiang.A voucher specimen of Daji(Cirsiumjaponicum) (herbarium voucher number Shaji-2012-1215)was deposited at the Department of Pharmaceutical Engineering.Sephadex G-150 was purchased from Pharmacia Chemicals (Uppsala,Sweden).
Preparation of polysaccharide extracts
Polysaccharide extracts from the berries of Daji(Cirsium japonicum)were prepared as previously described with small modifications[15].Briefly,the powdered Daji(Cirsium japonicum)dry berries were defatted with light petroleum(boiling point 30-60°C)for 16 h.Then the residue was sonicated for extraction four times with distilled water(solid/liquid ratio 1:25 g/m L)at 80°C for 40 min.The extracts were combined,concentrated,and incubated with papain(0.4 g/100 m L)at 65°C for 6 h.The Sevage method [16]was further adopted to deproteinate concentrated solution, which was precipitated with four volumes of 95%ethanol at 4°C for 48 h and was followed by centrifugation at 1500×g for 10 min at room temperature(25-30°C).And the pellet was washed by ethanol followed by acetone,and then dried.The polysaccharide solution(50 g/L,pH=8.0)was dialyzed extensively against doubly distilled water with an 8 kDa cut-off dialysis bag for 48 h.And to yield the CJP fraction,the dialyzed solution was fractionally precipitated with 80%concentration of ethanol,followed by chromatograph analysis on a Sephadex G-150 column(type:1.5×50 cm),and elution with distilled water(0.5 m L/min).
Analysis of polysaccharide extracts
The total carbohydrate contents in the CJP fraction were determined by the phenol-sulfuric acid method as previously described[17],with d-glucose as a standard.The absorbance was measured at 490 nm.The content of uronic acid in the CJP was determined using the m-hydroxydiphenyl colorimetric method with glucuronic acid as the standard[18].The protein content was determined by the bicinchoninic acid assay using bovine serum albumin(BSA)as the standard.High-performance gel permeation chromatography was adopted to primarily determine the molecules of the polysaccharides and the monosaccharide composition of CJPwasanalyzed by gas chromatography.Briefly,2mg CJP was hydrolyzed with 2 mol/L CF3COOH(1.0 m L)at 120°C for 3 h.The monosaccharide solution of CJPwas derivatized with 0.5 mol/L 1-phenyl-3-methyl-5-pyrazolone and 0.3 mol/L NaOH.After neutralization with 0.3 mol/L HCl and extraction with chloroform,the supernatant was filtered(0.22 µm),analyzed by high performance liquid chromatography equipped with a ZORBAX Eclipse C18 column(250 × 4.6 mm,5 μm),and monitored with UV absorbance at 245 nm.For infrared spectral analysis,10 mg CJPwasmixed with 100mg potassium bromide and compressed to a tablet,followed by conduction on a fourier transform infrared spectrophotometer(Thermo Election Corporation,USA)in the range of 4000-400 cm-1.
Cell cultureand cellviability assay
H9c2 ratcardiomyocyte cells obtained from the American Type Culture Collection(Cedarlane Laboratories,Ontario,Canada)were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10%heat-inactivated fetal bovine serum,100 U/m L penicillin,and 100 g/m L streptomycin at 37°C with 5% CO2.When cell confluency reached 70-80%,H9c2 cells were used for experiments.
Cell viability was measured quantitatively using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT)assay.Briefly,the ratH9c2 cells(5×104cells/m L)were seeded in a 96-well plate followed by 24 h H2O2incubation of 25,50,100mol/L or CJP incubation of 50,100,200,400,and 600µg/m L.Then,10µLMTT solution(5 mg/m L)was added to each well,and incubated at 37°C for 4 h.After removal of the supernatant,200µL dimethyl sulfoxide was added to each well to dissolve the formazan.The absorbance was measured at 570 nm by an ELISA reader(Tecan,Männedorf,Switzerland).After subtraction of the blank,the cell viability was calculated and presented as percentagesof the control.
Effect of CJPon hydrogen peroxide injury model
The model of oxidative stress injury was established by adding a free radical donor,H2O2,to the cultured myocardial cells.To determine the protective effect of CJP against H2O2-induced cell loss in H9c2 cells,cells were incubated with increasing concentrationsof CJP(50,100,200,400,and 600µg/m L)for 24 h and then treated with 100mol/L H2O2for another 24 h.Cell viability was determined colorimetrically using MTT assay.
Fluorescence-activated cell sorting(FACS)analysis of cell apoptosis
Cell apoptosis was detected using fluorescein isothiocyanate-conjugated Annexin-V and propidium iodide double staining as described previously[19].Briefly,the rat H9c2 cells(1×106cell/m L),subjected to be shock with H2O2and treat with or without CJP of 100µg/m L,were seeded into 6-well plates.Staining was performed using an Annexin-V and propidium iodide double staining kit(Biosea Biotechnology Co.,Beijing,China), in accordance with the manufacturer’s instructions.Ten thousand cells were counted,and the data acquisition and analysis were performed using a flow cytometer FACSCaliburTM(BD Biosciences,San Diego,CA,USA)and CellQuest software(BD Biosciences,San Diego,CA,USA).The alteration of nuclearmorphology was observed under a fluorescence microscope(Olympus,IX53,Tokyo,Japan)by Hoechst33258 staining.
Western blot analysis
H9c2 cells growing on 6-well plateswere pretreated with or withoutCJP(100µg/m L)for 24 h prior to exposing to 400 μM H2O2,as indicated.Then cells were harvested and lysed using Radio-Immunoprecipitation Assay lysis buffer containing 50 mM Tris-HCl(pH 7.4),150 mM NaCl,1%sodium deoxycholate,1%Nonidet P-40,1mM phenylmethylsulfonyl fluoride,1 mMethylene diamine tetra acetic acid,and protease inhibitors.The protein concentration of each sample was determined using a bicinchoninic acid protein assay kit(Pierce Chemical,Rockford,IL).A sample of 40 μg of the total protein was electrophoresed on sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene difluoride membranes.After being washed three times with phosphate-buffered saline solution, the polyvinylidene difluoridemembranes were blocked in 5%BSA for 2 h and incubated overnight at 4°C with the primary antibodies against caspase-3, caspase-8,caspase-9,p38,c-Jun N-terminal kinase(JNK)or β-actin.Subsequently, the membranes were washed in Tris-buffered saline-Tween 20 containing 1%BSA,and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature,and developed using the ECL chemiluminescence detection system(Amersham).
Statistical analysis
The data are expressed as the mean±SEM.Statistical analysis was performed using one-way analysis of variance.P<0.05 was considered to be statistically significant.
Results
Phytochem icalanalysisof CJP
CJP,a dark-brown powder,is soluble in water.CJP is comprised of carbohydrate(77.21%)and uronic acid(36.69%).Furthermore,Coomassie brilliant blue staining showing that the protein content in CJP was 6.17%.High-performance gel permeation chromatography analysis showed that CJP contained one major polysaccharide with severalminor ones(Figure 1A).The monosaccharide composition of CJP by gas chromatographic analysis found that CJP is mainly composed of glucose,galactose,arabinose,and rhamnose in the ratio of 3.40:2.14:1.17:1.00,together with trace amountsofmannose and xylose.
The fourier transform infrared spectrophotometer spectrum analysis of CJP is shown in Figure 1B.The strong stretching peak at 3398.1 cm-1represents a hydroxyl group(-OH).Peaks at 1740.7 cm-1and 1617.3 cm-1could be attributed to the aldehyde group(-CHO)stretching vibration and double bond(C=C)stretching vibration,respectively.The peak at 1383.9 cm-1might represent themethyl(-CH3)saturated C-H plane bending vibration.The peak at 1101.1 cm-1represents C-O-C glycosidic rings asymmetric vibration,indicating the existence of pyranose in CJP.The peak at 1020.6 cm-1might representC-O stretching vibration.
CJPattenuated H9c2 cell injury induced by H 2O2
H9c2 cell viability(a cell line of rat cardiomyocytes),assessed by the MTT assay,decreased dramatically after H2O2treatment for 24 h in a dose-dependent manner(Figure 2A).CJPpretreatmentalone did not significantlyalter the cell viability level of H9c2 cells.Notably,CJP(100, 200, 400, and 600 µg/m L) pretreatment significantly protected the H9c2 rat cardiomyocytes from H2O2injury dose-dependently(Figure 2B).Therefore,CJP pretreatment exhibited protective effects in free radical-induced myocardial cell injury.
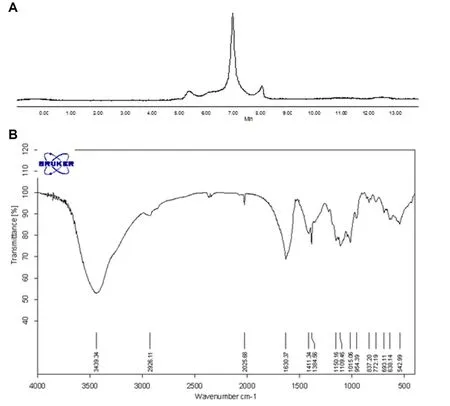
Figure1 Phytochem ical analysisof CJP
CJPattenuated H2O2-induced H 9c2 cell apoptosis
The nucleus in the normal group was showed as a typical round or oval shape;those in the H2O2-injured group were typical of karyopyknotic stain,with chromatin concentrated fragmentation(Figure 2C).Quantitative analysis using FACS analysis confirmed that the number of cells in the CJP treated group was significantly less than in the H2O2-injured group(Figure 2D).Furthermore,as shown in Figure 2E,H2O2induced caspase-3,-8,and-9 cleavages in H9c2 cells;however,the effects were inhibited by CJP,further demonstrating that CJP suppressed the induced apoptosis.
Protection of CJP against H2O2 injury m ight involve the MAPK pathway
Apoptosis was finally induced by H2O2,which could be partially attributed to the activated MAPK signaling pathway by H2O2.To further investigate the mechanism underlying the effect of CJP on MAPK,the intracellular signal transduction cascade wasexamined(Figure 3).The activation of p38 MAPK and JNK was detected by western blotanalysis.The phosphorylation of p38 MAPK and JNK were significantly enhanced in the H2O2group,demonstrating that pretreatment with CJP 100µg/m L could ameliorate the phosphorylation levels.
Discussion
In the present study,we demonstrated that the polysaccharide extracts of Daji(Cirsium japonicum)exerted a protective effect against oxidative stress in rat H9c2 cells treated with H2O2.It was found that CJP attenuated H2O2-induced cell apoptosis via inhibiting the MAPK pathway.These results imply that CJP could regulate the apoptosis-related pathway in response to oxidative stress.
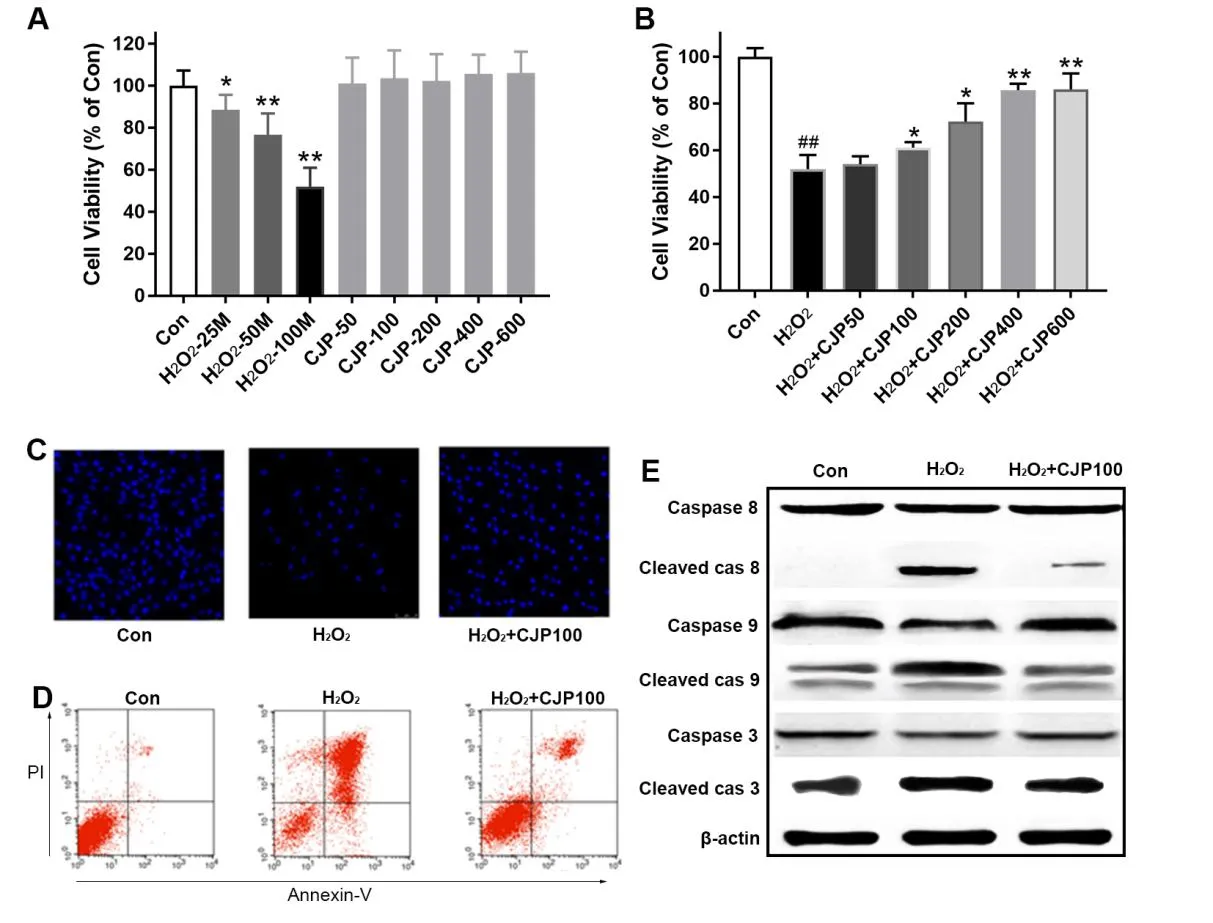
Figure 2 CJPsignificantly m itigated the H2O2-induced injury through inhibition of apoptosis
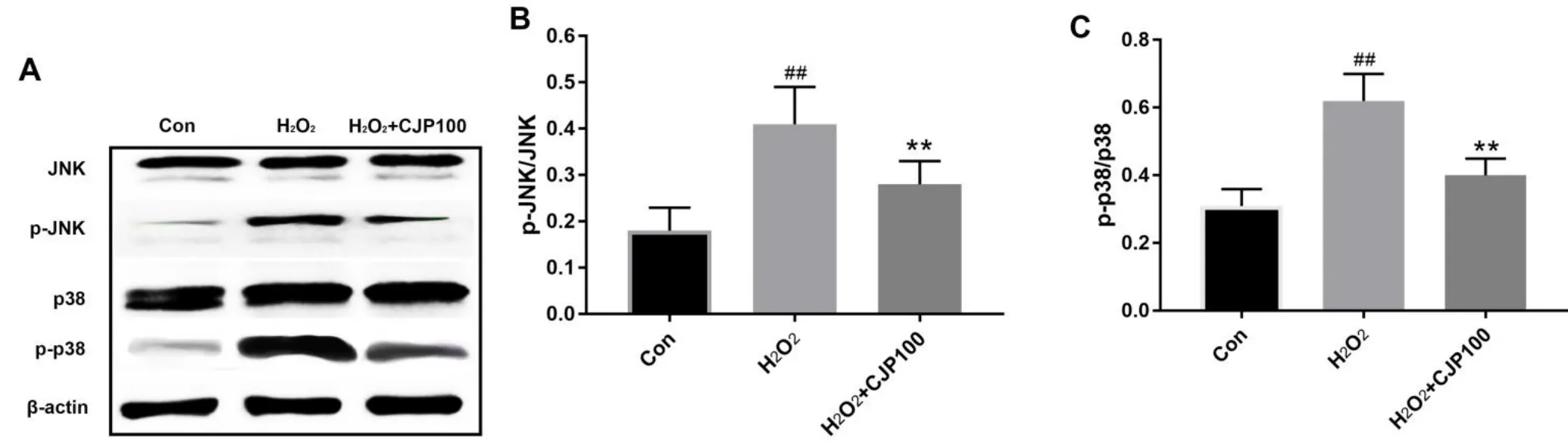
Figure 3 CJPsignificantly inhibited the phosphorylation of p38 and JNK MAPK pathway in H9c2 cardiomyocytes treated by H2O2
Polysaccharidesare reported to have pleiotropic effects,including hypolipidemic [20], anti-radiation [21],pro-immune[22],anti-oxidative[23],and other effects.As a long applied herbal medicine,Daji(Cirsium japonicum)has been extensively studied for its possible anti-inflammatory [24], anti-diabetic [25], and hepatoprotective effects[11].Additionally,the cytotoxic effect of the planthas been investigated[26].One reason that could not be neglected for the pleiotropic effects is the complex content of active components present in the plant.Therefore,itwas vital to examine the effect of an individual component rather than the whole plant,or even the medical part of the plant.Our study showed that the polysaccharide extracts inhibited cell apoptosis in H2O2-treated rat H9c2 cells,which was at least partially involved in theMAPK pathway.
Oxidative stress-induced cardiomyocyte damage plays an important role in the pathophysiology of cardiovascular diseases[27].It is a state when the free radical production exceeds the defense ability of the antioxidative system.ROS is one main class of free radical,among which H2O2is a metabolite generated by various enzyme-catalyzed redox reactions.Cardiac oxidative stress is reported to be associated with increased cardiac fibrosis and hypertrophy,and reduced cardiac performance and contractility,which might lead to severe cardiac dysfunction and potentially fatal cardiac events[28].ROS could be produced in response to alcohol, nonsteroidal anti-inflammatory drugs,ischemia-reperfusion injury,chronic infections,and inflammatory disorders[29].Agents that reduce ROS production and inhibit oxidative stress that produce little adverse effects could have great therapeutic potential in the prevention or treatment of cardiovascular diseases.The present study provides CJP as a promising therapy for oxidative stress-related cardiovascular diseases.The detailed mechanism underlying the protection of CJP against oxidative stress warrant further research.It was reported by Nickeletal.[30]that rather than the cytoplasmic ROS,the mitochondrial ROS play a more important role in the pathogenesis of cardiovascular diseases.
MAPK is a family of serine/threonine kinases that mediate intracellular signals in response to various stimuli.The activation of the MAPK pathway,linked to oxidative stress,has been demonstrated to be involved in the process of cell apoptosis[31].Mammals typically express at least three distinct groups of MAPKs,including p38MAPK,ERK 1/2 and JNK.Itwas reported thatantagonism of the expression of JNK and p38 or their phosphorylation might inhibitmyocardial infarction and apoptosis,restoring cardiac function subsequent to myocardial ischemia[32,33].Similar to these previous studies,our study showed that H2O2significantly enhanced the phosphorylation levels of p38 MAPK and JNK,indicating activation of theMAPK pathway.
Conclusions
In the presentstudy,we investigated the protective effects of Daji(Cirsium japonicum)polysaccharide extracts against H2O2-induced oxidative stress.The results confirmed that,in H2O2-treated rat H9c2 cardiomyocytes,cell apoptosis was attenuated via the inhibition of the MAPK pathway.The present study provides a foundation for further exploration of agents against cardiovascular disease-related oxidative stress.
Acknow ledgement
The current study was designed by Dr Zhang Chuan and Mr Wang Li-Hui.The experiments were conducted by Mr Tao Zheng-Bo with the direction of Dr Xiong Li-Yan.The data was collected and analyzed by Mr Tao and Dr Xiong.The manuscript was drafted by Mr.Tao and Dr Xiong,and edited by MrWang and Dr Zhang.
1.Nichols M,Townsend N,Scarborough P,et al.Cardiovascular disease in Europe 2014:epidemiological update.Eur Heart J 2014,35:2950-2959.
2.Monassier JP.Reperfusion injury in acute myocardial infarction.From bench to cath lab.Part I:Basic considerations.Arch Cardiovasc Dis 2008,101:491-500.
3.Rodriguez-Porcel M,Zhu XY,Chade AR,et al.Functional and structural remodeling of the myocardial microvasculature in early experimental hypertension.Am J Physiol Heart Circ Physiol 2006,290:H978-H984.
4.Zweier JL,Duke SS,Kuppusamy P,et al.Electron paramagnetic resonance evidence that cellular oxygen toxicity is caused by the generation of superoxide and hydroxyl free radicals.FEBS Lett 1989,252:12-16.
5.Ambrosio G,Flaherty JT.Effects of the superoxide radical scavenger superoxide dismutase,and of the hydroxyl radical scavenger mannitol,on reperfusion injury in isolated rabbit hearts.Cardiovasc Drugs Ther 1992,6:623-632.
6.Ahmet I,Spangler E,Shukitt-Hale B,etal.Blueberry-enriched diet protects rat heart from ischemic damage.PLoSOne 2009,4:e5954.
7.Chen YW,Chou HC,Lin ST,et al.Cardioprotective effects of Quercetin in cardiomyocyte under ischemia/reperfusion injury.Evid Based Complement AlternatMed 2013,2013:364519.
8.Inagi R.Oxidative stress in cardiovascular disease:a new avenue toward future therapeutic approaches.Recent Pat Cardiovasc Drug Discov 2006,1:151-159.
9.Lefer DJ,Granger DN.Oxidative stress and cardiac disease.Am JMed 2000,109:315-323.
10.Wan Y,Liu LY,Hong ZF,et al.Ethanol extract of Cirsium japonicum attenuates hepatic lipid accumulation via AMPK activation in human HepG2 cells.Exp TherMed 2014,8:79-84.
11.Ma Q,Wang LH,Jiang JG.Hepatoprotective effect of flavonoids from Cirsium japonicum DC on hepatotoxicity in comparison with silymarin.Food Funct2016,7:2179-2184.
12.Zhang P,Ye Y,Yang X,et al.Systematic review on Chinese herbal medicine induced liver injury.Evid Based Complement Alternat Med 2016,2016:3560812.
13.Eccleston C,Baoru Y,Tahvonen R,et al.Effects of an antioxidant-rich juice(sea buckthorn)on risk factors for coronary heart disease in humans.J Nutr Biochem 2002,13:346-354.
14.Gupta S,Gupta BM.Metabolic syndrome:diabetes and cardiovascular disease.Indian Heart J 2006,58:149-152.
15.Liu H,Zhang W,Dong S,et al.Protective effects of sea buckthorn polysaccharide extracts against LPS/d-GalN-induced acute liver failure in mice via suppressing TLR4-NF-kappaB signaling. J Ethnopharmacol2015,176:69-78.
16.Li H,Lu X,Zhang S,et al.Anti-inflammatory activity of polysaccharide from Pholiota nameko.Biochemistry(Mosc)2008,73:669-675.
17.Saha SK and Brewer CF.Determination of the concentrations of oligosaccharides,complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method.Carbohydr Res 1994,254:157-167.
18.Blumenkrantz N,Asboe-Hansen G.New method for quantitative determination of uronic acids.Anal Biochem 1973,54:484-489.
19.Wang XD,Li CY,Jiang MM,et al.Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine,a unique alkaloid isolated from Catharanthus roseus.Phytomedicine 2016,23:641-653.
20.Nakamura M,Miura S,Takagaki A,etal.Hypolipidemic effects of crude green tea polysaccharide on rats,and structural features of tea polysaccharides isolated from the crude polysaccharide.Int J Food Sci Nutr 2017,68:321-330.
21.Shi J,Cheng C,Zhao H,et al.In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron (III) complex. Int J Biol Macromol 2013,60:341-346.
22.Sundberg-Kovamees M,Grunewald J,Wahlstrom J.Immune cell activation and cytokine release after stimulation of whole blood with pneumococcal C-polysaccharide and capsular polysaccharides.Int J InfectDis2016,52:1-8.
23.Zhang W,Huang J,Wang W,et al.Extraction,purification, characterization and antioxidant activitiesof polysaccharides from Cistanche tubulosa.Int J BiolMacromol2016,93:448-458.
24.Shin MS,Park JY,Lee J,et al.Anti-inflammatory effects and correspondingmechanisms of cirsimaritin extracted from Cirsium japonicum var.maackii Maxim.Bioorg Med Chem Lett 2017,27:3076-3080.
25.Liao Z,Chen X,Wu M.Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats.Arch Pharm Res 2010,33:353-362.
26.Shang DL, Ma QG,Wei RR. Cytotoxic phenylpropanoid glycosides from Cirsium japonicum.JAsian NatProd Res2016,18:1122-1130.
27.Valko M,Leibfritz D,Moncol J,et al.Free radicals and antioxidants in normal physiological functions and human disease.Int J Biochem Cell Biol 2007,39:44-84.
28.Faria A and Persaud SJ.Cardiac oxidative stress in diabetes:Mechanisms and therapeutic potential.Pharmacol Ther2017,172:50-62.
29.Bhattacharyya A,Chattopadhyay R,Mitra S,et al.Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases.PhysiolRev 2014,94:329-354.
30.Nickel AG,von Hardenberg A,Hohl M,et al.Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure.Cell Metab 2015,22:472-484.
31.Lan A,Liao X,Mo L,et al.Hydrogen sulfide protects against chemical hypoxia-induced injury by inhibiting ROS-activated ERK1/2 and p38MAPK signaling pathways in PC12 cells.PLoS One 2011,6:e25921.
32.Akhtar S,Yousif MH,Chandrasekhar B,et al.Activation of EGFR/ERBB2 via pathways involving ERK1/2,P38 MAPK,AKT and FOXO enhances recovery of diabetic hearts from ischemia-reperfusion injury.PLoSOne 2012,7:e39066.
33.Chambers JW,Pachori A,Howard S,et al.Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats.J Biol Chem 2013,288:4000-4011.
 Traditional Medicine Research2018年3期
Traditional Medicine Research2018年3期
- Traditional Medicine Research的其它文章
- Screening for cyclooxygenase 2 inhibitors from natural com pounds of Radix Glycyrrhizae using com puter simulation
- The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1
- Study of dual-directional regulatory effect of Banxia(Pinellia ternata)and Huanglian(Coptis chinensis)drug pair on gastrointestinal movement of m ice
- Effect of alternate-day-fasting combined with Lingguizhugan Decoction on blood lipid profiles of hyperlipidem ic rats
