The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1
RuiTang,Qia-Qia Li,Di Wang,Jing Chen,Jin-Hua Huang*,Qing-HaiZeng*
1Xiangya School of Medicine,Central South University,Changsha,Hunan,China.2Dermatological Department,The Third Xiangya Hospital of Central South University,Changsha,Hunan,China.
Background
Skin photoaging refers to the damage caused by long-term repeated exposure of the skin to ultraviolet(UV)rays.Long-term exposure of the skin to UV causes dermal collagen degeneration and progressive degradation and fragmentation of elastic fibers until they disappear;the skin becomes loose,dry,w rinkly,pigmented,and sensitive [1,2].The mechanism underlying skin photoaging is complicated.It has been found that various photosensitive substances or chromophores acting on the skin through UV exposure can induce the generation of reactive oxygen species(ROS),such as OH·and lipid peroxides,destroying the skin’s own antioxidant system and leading to an imbalance of oxidative stress[3,4].Oxidative stress caused by UV irradiation can promote the expression of matrix metalloproteinases(MMPs),reduce collagen synthesis and secretion,and cause skin damage[5-9].MMP-1 mainly degrades type I collagen-based skin extracellular matrix.During the process of photoaging,the expression of MMP-1 is increased,and this can be used as an indicator of skin photoaging[10].Among the many cytokines that induce collagen secretion,transforming grow th factor β (TGF-β)is currently the most recognized cytokine that induces the synthesis and secretion of extracellular matrix[11].In the TGF-bfamily,TGF-b1 has the highest expression,the strongest activity,and the most extensive range in somatic cell lines.Previous studies found that TGF-b1 could significantly increase the expression of type I procollagen mRNA in artificial skin and TGF-β1 can also reduce the expression of MMP-1 mRNA and inhibit collagen degradation[12-13].In the photoaging process,decrease in TGF-b1 can be used as an indicator of skin photo-aging.
In order to reduce UV damage to the skin,people should not only cover the skin to prevent UV radiation exposure,but also use antioxidants,anti-inflammatory agents,and other protective measures to prevent UV damage to the skin,and even repair damaged tissue.In recent years,studies found that many traditional Chinese medicines had a positive effect on the prevention and treatment of skin photoaging,with high efficiency and no side effects[14-16].Tiepishihu(Dendrobium officinaleKimura et Migo,TPSH)is an orchid and herbaceous plant that has a unique medicinal value.TPSH was first recorded inShennongbencaojingin the Qin and Han Dynasty of China(221 B.C.-25 A.D.)as being sweet,with the property of eliminating wind,cold,and dampness.Modern pharmacology studies have shown that,in addition to TPSH acting to regulate endocrine function, enhance resistance, and promote tissue regeneration,it also can scavenge oxygen free radicals and thus has a very prominent role in anti-aging therapy[17].ROS play a very important role in the photoaging of the skin;thus,we investigated whether TPSH could play a role in antagonizing photoaging by scavenging ROS.There have been no previous studies evaluating this question.This paper explored the protective effect ofDendrobiumofficinalepolysaccharides (DOP) on photoaging human skin fibroblasts and the specific underlyingmechanism.
Methods
Materials and reagents
DOP of 90%purity were purchased from Xi’an Wei Te Biotechnology Co.,Ltd.DMEM medium was purchased from Gibco.Fetal bovine serum was purchased from the United States Hyclone company.The β-galactosidase(SA-β-Gal)kit and reactive oxygen detection kit were purchased from Biyun Tian Biotechnology Research Institute.The MMP-1 detection kit and pre-human type I collagen C-terminal peptide (CICP)test kit were purchased from the Wuhan Bode Science and Technology Development Co.,Ltd.Biotech.The TGF-β1 test kitwas purchased from Wuhan Elaite Biotechnology Co.,Ltd.The UVB irradiation instrument(SS-07)was purchased from ShanghaiSigma Technology Co.,Ltd.
Fibroblastextraction and culture
The skin tissue of prepuce was obtained from a healthy young man after circumcision.The specimen was selected in advance,and the patient provided w ritten informed consent.The study procedurewas confirmed by the hospital ethics comm ittee.The skin tissue was placed in a complex soaked in iodine for 15 min.The dirt was cleaned off with PBS solution,and the subcutaneous tissue was cut.The tissue was then cut into pieces.The tissue pieceswere allowed to attach to a 35 mm Petridish by being left undisturbed for 10 min.Two to three drops of DMEM were periodically added to prevent the tissue block from drying.The dish was placed in a carbon dioxide incubator overnight.The next day,2 m L DMEM was slow ly added to the culture dish,and the liquid medium was changed once after 2-3 days.When many cells grew around the tissue block,the fibroblasts were digested with 0.25%trypsin,collected,and washed.The isolated fibroblasts were cultured in high-glucose DMEM containing 10%fetal bovine serum and 1%double antibody.When the cultured fibroblastsgrewinto a dense monolayer,they were subcultured at a ratio of 1:3.In this experiment,3-10 generationsof fibroblasts were used.
Experimental grouping and adm inistration
The same generation of fibroblasts were random ly divided into a normal control group,photoaging model group(60 m J/cm2UVB irradiation),and UVB irradiation+ DOP groups.Fibroblasts were incubated with serum-free medium for 24 h and then irradiated with 60 m J/cm2UVB to establish the fibroblastphotoaging model[18].The control group was not irradiated,and DOP were not added to the culture solution.For the photoaging model group,cells were irradiated with UVB,and DOP were not added to the culture solution.For the UVB irradiation+DOP groups,the cells were incubated with serum-free medium containing DOP at different concentrations for 24 h before UVB irradiation.AfterUVB irradiation,the cells continued to be cultured with the culture solution containing different concentrations of DOP for 24 h.DOP powder was dissolved in PBS solution at a concentration of 1 mg/m L,stored in a stock solution at 4 °C and diluted to the appropriate concentrationwith serum-free DMEM.
Cell culture and cell viability assay
The toxic effects of different concentrationsof DOPwere studied.Fibroblasts were incubated with different concentrations of DOP in serum-free medium for 24 h.MTT(20μL,5 mg/m L)solution was added and incubated at 37°C for 4 h.The absorbance value at 490 nm was read on amicroplate reader.
SA-β-Gal assay for detection of senescent cells
The cells were stained according to the instructions of the SA-β-Gal Staining Kit.The cells were observed at 200×magnification under a lightmicroscope.Fifteen fields of view were selected for each specimen,and at least 200 cells were random ly counted.The positive cell ratios were calculated as follows:positive cell ratios=blue dyed cells/totalnumber of cells×100%).
ROS detection by flow cytometry
The cells were treated and collected according to the kit’s operating instructions.The fluorescence intensity was measured by flow cytometry.The stronger the fluorescence intensity is,the higher the ROS level is.
ELISA for expression analysis of MMP-1,CICP,and TGF-β1
The spent culturemedium was aspirated and centrifuged at 3000 rpm for 10 min.Then,according to the instructions of theMMP-1,CICP,and TGF-β ELISA Kits,the OD valuewasmeasured at450 nm with a microplate reader,and the concentrations were calculated according to the standard curve.The concentrations of MMP-1,CICP,and TGF-β1 in fluid weremeasured.CICP can be used asan indirect measure to assess the contentof type I collagen.
Statistical analysis
Statistical analyses of experimental data were performed using SPSS 19.0.The experiment was repeated three times,and data were expressed as means±standard deviations;the differences between groups were analyzed using analysis of variance or t-test.WhenP<0.05,the differencewas considered statistically significant.
Results
Drug toxicity of DOPon fibroblasts
The MTT assay was used to determine cell activity of fibroblasts after being treated with DOP.The results showed no toxicity in the groups treated with 20,40,and 80 μg/m L of DOP.The cell activity in the 160 μg/m L concentration group was significantly lower than that in the control group(P=0.003)(Figure 1).The results showed that within a certain concentration,DOP is not toxic to fibroblasts,but high concentrations have toxic effects on fibroblasts.
DOP inhibits UVB irradiation-induced fibroblast injury
As shown in figure 2,the senescent cells presented blue color after detected with SA-β-Gal assay.The percentage of senescent cells in the control group was 18.67%,while that in the photoaging model group was 66.48%,indicating a significant difference between the two groups(P<0.001).The cell senescence rates of the DOP groups(20,40,80 μg/m L)were lower than that of the photoaging model group.The senescent cell rate decreased gradually with increasing DOP concentration;the rate in the DOP 20 μg/m L group was 55.82%.There was no statistically significant difference in the senescence rate between DOP 20 μg/m L group and photoaging model group(P=0.087).The rates in the DOP 40 and 80 μg/m L groups were 50.45%and 42.63%,respectively.Compared with the photoaging model group,the difference was statistically significant(P=0.025,P=0.004 respectively).
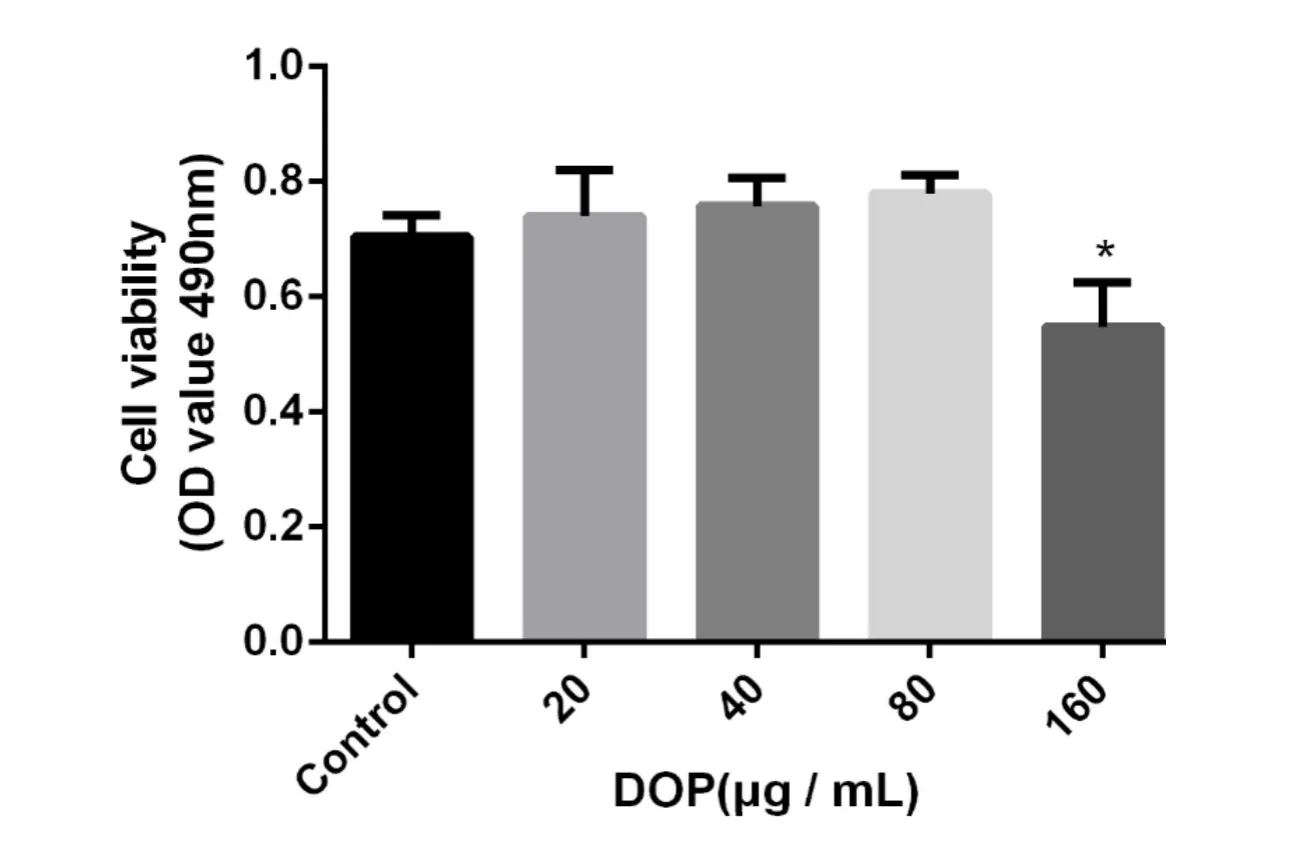
Figure1 Cytotoxicity of DOPon fibroblasts
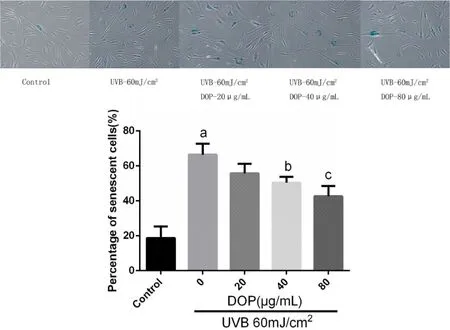
Figure 2 DOPdepressed fibroblastsaging induced by UVB
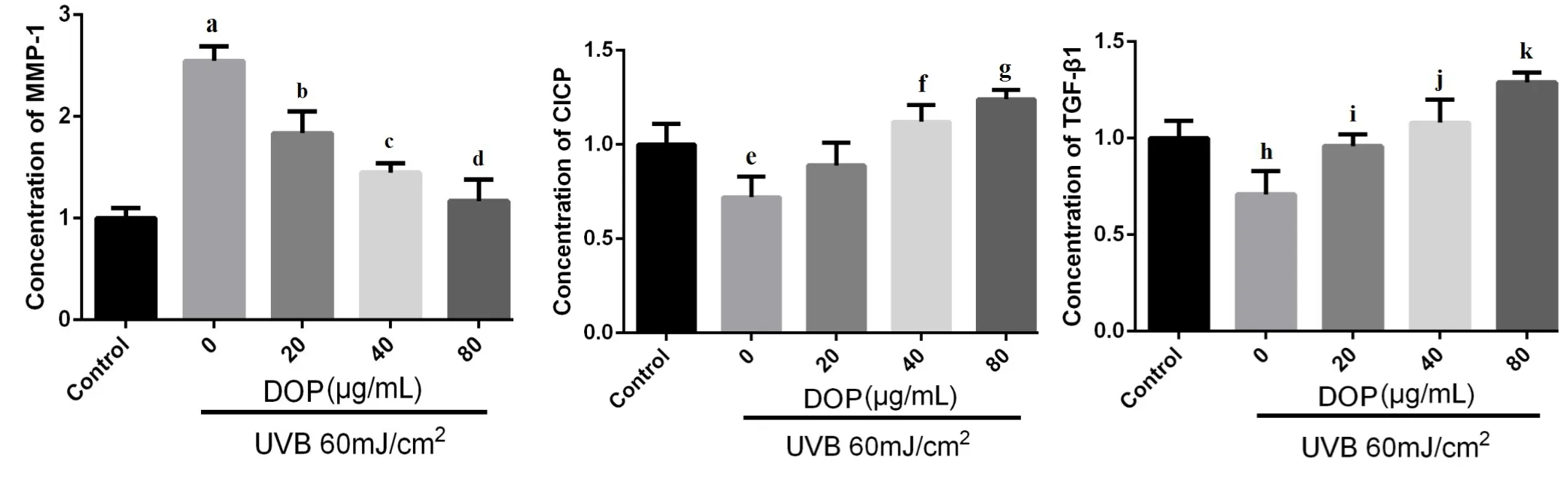
Figure 3 The effects of DOPon the expression ofMMP-1,CICPand TGF-β1.
The effects of DOP on the expression of MMP-1,TGF-β1 and CICP
The results showed thatcompared with the control group,the expression of MMP-1 in fibroblasts significantly increased,and the expression of TGF-β1 and CICP decreased significantly after UVB irradiation(P<0.001,P=0.026 andP=0.017)(Figure 3).The expression of MMP-1 in the DOP groups(20,40,80 μg/m L)was significantly lower than that in the photoaging model group(P=0.038,P=0.007 andP=0.001 respectively);the expression of CICP in the DOPgroups(40,80μg/m L)was significantly higher than that in the photoaging model group(P=0.008 andP=0.002 respectively);compared with the photoaging model group,the expression of TGF-β1 (DOP 20,40,80 μg/m L group)increased gradually,and the difference was statistically significant(P=0.015,P=0.006 andP<0.001 respectively).
DOP inhibits the production of ROS in fibroblasts after UVB irradiation
Compared with the control group,the fluorescence intensity of ROS in the photoaging model group was increased(P<0.001),indicating the higher level of ROS.While after treated with increasing concentrationsof DOP,the fluorescence intensity of ROS was decreased,indicating the lower level of ROS.Flow cytometry data also showed that the ROS content in the photoaging model group was 2.3 times higher than thatof the control group;ROS levels in the DOP 20,40,and 80μg/m L groups were lower than those in the photoaging model group(P=0.029,P=0.017 andP=0.002).This suggests that a certain concentration of DOP can inhibit UVB-induced ROS production(Figure 4).
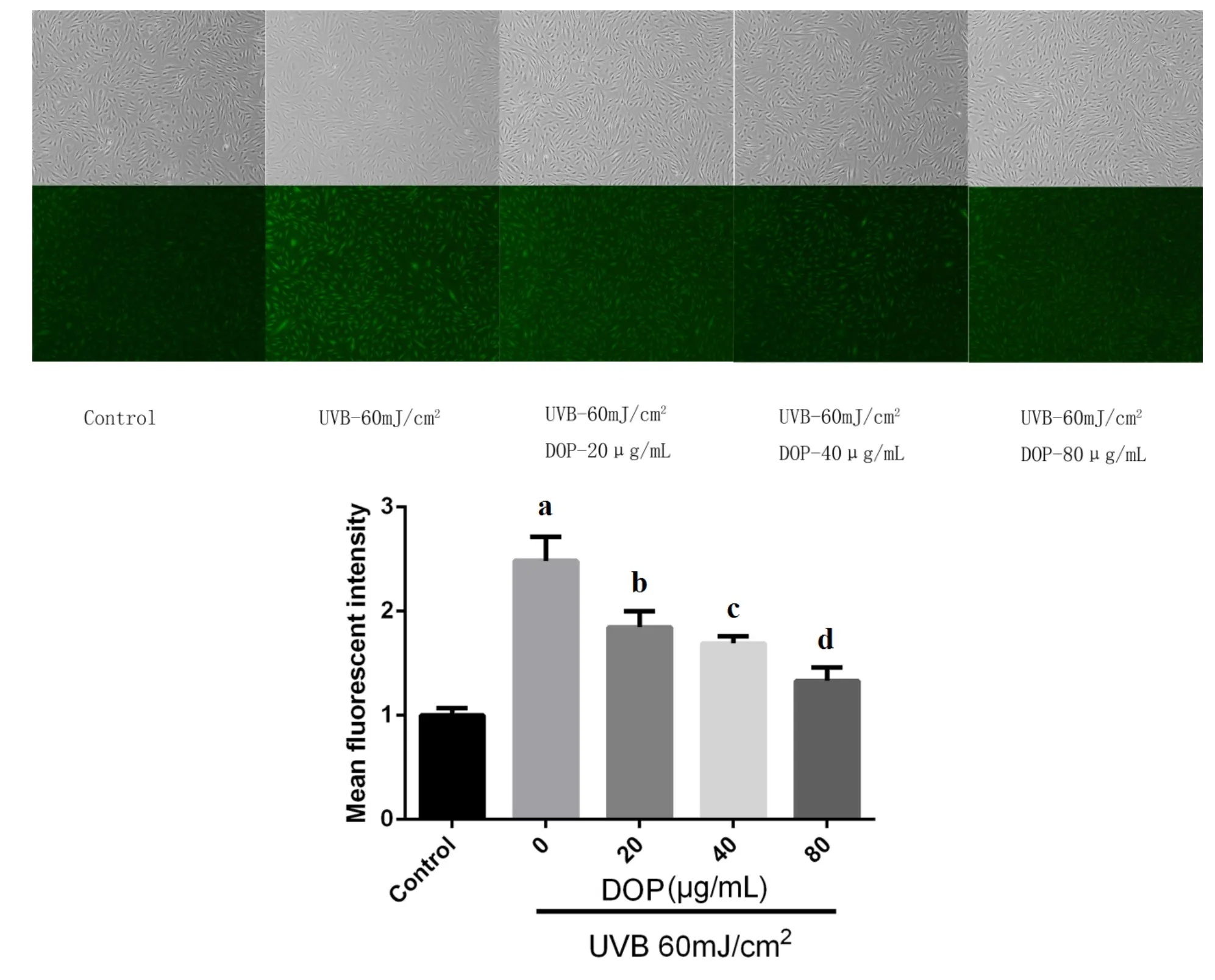
Figure4DOPdepressed the levels of UVB-induced ROS
Discussion
TPSH has a long history of medicinaluse.It is one of the most effective medicinal products among the more than 70 kinds of dendrobium in China.The polysaccharides and dendrobins are several times richer in TPSH than in other varieties of dendrobium.Dendrobium has a unique medicinal value,with hypoglycemic,anti-oxidation,anti-tumor,and anti-aging properties,while it has also been shown to improve immunity and have other beneficial effects[1,19-23].DOP is one of the main active components of TPSH,and has antioxidant and anti-aging effects,as it scavenges oxygen free radicals[24-29].ROS damage is currently recognized as one of themajor mechanisms leading to photoaging[30-36].We speculated that DOP could protect the photoaging of fibroblastsby inhibiting the production of ROS.
At 60 m J/cm2UVB irradiation,the cell activity was about 75%of that in the normal controlgroup.Therefore,60 m J/cm2was chosen as the irradiation dose for inducing the photoaging model.The toxicity of different concentrations of DOP(20,40,80,160 μg/m L)was explored.The results showed that 20,40,and 80μg/m L DOP had no cytotoxic effect on fibroblasts,and these concentrations were chosen for the study.The results of SA-β-Gal staining showed that 20,40,and 80 μg/m L DOP could reduce cell senescence induced by UVB irradiation,and the number of senescent cells in 40 and 80μg/m L DOP groups decreased significantly.Thus,DOPcan protect fibroblasts from photoaging.
UV can induce cells to produce ROS;ROS can directly damage cells.Increased ROS promotes the expression of MMPs and educes collagen secretion and synthesis by activating mitogen-activated protein kinase cell-mediated pathways including ERK,JNK,and p38 kinases[7,37].The four main types of MMPs include MMP-1,72,000 gelatinase(MMP-2),stromelysin-I(MMP-3),and 92,000 gelatinase (MMP-4).They cooperate to degrade extracellular matrix of dermal dermis with or without collagen components.The extracellular matrix of skin mainly contains type I collagen,which is mainly degraded by MMP-1.During the process of photoaging,the expression of MMP-1 is elevated,a large amount of collagen is degraded and disintegrated,and fibroblast proliferation and collagen synthesis are also inhibited[10,38].Studies have shown that inhibiting production of UV-induced ROS,and reducing the expression of MMP-1,MMP-2,and MMP-9,can protect the skin[14,16].The results showed that UVB irradiation reduced the expression of CICP in fibroblasts and increased the expression of MMP-1.However,the expression of MMP-1 in the DOP groups was inhibited and the expression of CICP was increased.Compared with the photoaging model group,the levels of ROS in the DOP groups were decreased,and ROS levels gradually decreased with increasing concentrations of DOP.DOP can reduce ROS levels in photoaging cells,inhibit the expression of MMP-1,and increase the expression of CICP,thereby protecting the photoaging cells.
TGF-β is a fibroblast chemokine that promotes fibroblast proliferation and produces type I and type III collagen[39-41].TGF-β1 is a major component of the TGF-β family.TGF-β combines with cell surface receptors to form heterologous trimers,which activate R-Smad protein and transmit signals to the cytoplasm.UV light can inhibit the synthesis of dermal collagen by affecting the transduction of TGF-β/Smad signaling pathway[42,43].TGF-β1 can induce the formation of extracellular matrix.Gambichleretal.found that the level of TGF-β1 protein in fibroblasts significantly decreased after exposure to UVA for 24 h[45,46];Sunet al.found that increasing the expression and secretion of type I procollagen by the TGF-β/Smad signaling pathway could prevent UV-induced acute skin damage and photoaging[47,48].In addition,Yong-Jinget al.found that TGF-β1 could reduce the expression of MMP-1 and MMP-3 mRNA and decrease type I procollagen degradation in skin fibroblasts during photoaging[13,44].We found that DOP could also promote the expression of TGF-β1.We speculate that DOP can reduce ROS and inhibit MMP-1 expression,thereby reducing type I collagen degradation.In addition,it can promote type I collagen production by up-regulating the expression of TGF-β1.In addition,the up-regulated expression of TGF-β1 by DOP may also reduce type I collagen degradation by inhibiting the expression ofMMP-1.
Conclusion
DOP may protect photoaged fibroblasts by scavenging UVB-induced ROS,inhibiting the secretion of MMP-1,increasing the expression of TGF-β1,and regulating the balance of collagen in the skin.
1.Pan LH,Li XF,Wang MN,et al.Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species.Int JBiol Macromol 2014,64:420-427.
2.Zhou BR,Zhang LC,Permatasari F,et al.ALA-PDT elicits oxidative damage and apoptosis in UVB-induced premature senescence of human skin fibroblasts.Photodiagnosis Photodyn Ther 2016,14:47-56.
3.Wenk J,Brenneisen P,Meewes C,et al.UV-induced oxidative stress and photoaging.Karger Publishers,2000:83-94.
4.Sharma D,Kober MM,Bowe WP.Anti-Aging Effects of Probiotics.J Drugs Dermatol 2016,15:9-12.
5.Kim HK. Garlic supplementation ameliorates UV-Induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression.Molecules 2016,21:70.
6.Jung SK,Su JH,Chang HJ,et al.Naringenin targets ERK2 and suppresses UVB-induced photoaging.J Cell Mol Med 2016,20:909-919.
7.Brenneisen P,Sies H,Scharffetter-Kochanek K.Ultraviolet-B irradiation and matrix metalloproteinases:from induction via signaling to initialevents.Ann N YAcad Sci2002,973:31-43.
8.Kang S,Fisher GJ,Voorhees JJ.Photoaging and Topical Tretinoin.Arch Dermatol 1997,133:1280-1284.
9.Sander CS,Chang H,Salzmann S,et al.Photoaging is associated with protein oxidation in Human skin in vivo.JInvestig Dermatol2002,118:618-625.
10.Inomata S,Matsunaga Y,Amano S,et al.Possible involvement of gelatinases in basement membrane damage and w rinkle formation in chronically ultraviolet B-exposed hairless mouse.J Investig Dermatol 2003,120:128.
11.Sorg O,Janer V,Antille C,et al.Effect of intense pulsed-light exposure on lipid peroxides and thymine dimers in human skin in vivo.Arch Dermatol 2007,143:363-366.
12.Gambichler T,Skrygan M,Tomi NS,etal.Significant downregulation of transforming grow th factor-βsignal transducers in human skin following ultraviolet-A1 irradiation.Br J Dermatol 2007,156:951-956.
13.He YJ,Liu Y,Wang JH,et al.Effects of TGF-β1 on the expression of MMP-1 and MMP-3 mRNA in skin fibroblasts induced by UVA in vitro.Chin J Aesthetic Med 2010,19:53-55.
14.Hwang IK,Yoo KY,Kim DW,et al.An extract of Polygonummultiflorum protects against free radical damage induced by ultraviolet B irradiation of the skin.Braz JMed Biol Res2006,39:1181-1188.
15.Sun S,Ping J,Su W,et al.Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging.Cytotechnology 2016,68:1-12.
16.Lee KE,Mun S,Pyun HB,et al.Effects of macelignan isolated from Myristica fragrans(Nutmeg) on expression of matrix metalloproteinase-1 and type I procollagen in UVB-irradiated human skin fibroblasts.Biol Pharm Bull 2012,35:1669-1675.
17.Luo QL,Tang ZH,Zhang XF,et al.Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale.Int J Biol Macromol 2016,89:217-227.
18.Zeng Q,Zhou F,Lei L,et al.Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging.Mol Med Rep 2017,15:111-116.
19.Xing X,Cui S W,Nie S,et al.A review of isolation process,structural characteristics,and bioactivities of water-soluble polysaccharides from Dendrobium,plants.Bioact Carbohydr Diet Fibre 2013,1:131-147.
20.Lin X,Shaw PC,Sze SC,et al.Dendrobium officinale polysaccharides ameliorate the abnormality of aquaporin 5,pro-inflammatory cytokines and inhibit apoptosis in the experimental Sjögren’s syndrome mice.Int Immunopharmacol 2011,11:2025-2032.
21.Xia L,Liu X,Guo H,et al.Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale,(Tiepishihu)in vitro.J Funct Foods 2012,4:294-301.
22.Yang LC,Lu TJ,Hsieh CC,et al.Characterization and immunomodulatory activity of polysaccharides derived from Dendrobium tosaense.Carbohydr Polym 2014,111:856-863.
23.He TB,Huang YP,Yang L,et al.Structural characterization and immunomodulating activity of polysaccharide from Dendrobium officinale.Int J Biol Macromol2015,83:2616-2642.
24.Yang LC,Lu TJ,Hsieh CC,et al.Characterization and immunomodulatory activity of polysaccharides derived from Dendrobium tosaense.Carbohydr Polym 2014,111:856.
25.Wang JH,Luo JP,Zha XQ,et al.Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl.Carbohydr Polym 2010,79:114-118.
26.Luo AX,He XJ,Zhou SD,et al.Purification,composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl.Carbohydr Polym 2010,79:1014-1019.
27.Tian CC,Zha XQ,Pan LH,et al.Structural characterization and antioxidant activity of a low-molecular polysaccharide from Dendrobium huoshanense.Fitoterapia 2013,91:247-255.
28.Pan LH,Li XF,Wang MN,et al.Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium,species.Int JBiol Macromol 2013,64:420-427.
29.Luo QL,Tang ZH,Zhang XF,et al.Chemical properties and antioxidantactivity of a water-soluble polysaccharide from Dendrobium officinale.Int J Biol Macromol2016,89:219-227.
30.Grimsrud PA,Xie H,Griffin TJ,et al.Oxidative stress and covalent modification of protein with bioactive aldehydes.J Biol Chem 2008,283:21837-21841.
31.Jang J,Ye BR,Heo SJ,et al.Photo-oxidative stress by ultraviolet-B radiation and antioxidative defense of eckstolonol in human keratinocytes.Environ Toxicol Pharmacol2012,34:926-934.
32.Wölfle U,Seelinger G,Bauer G,et al.Reactive Molecule Species and Antioxidative Mechanisms in Normal Skin and Skin Aging.Skin Pharmacol Physiol2014,27:316-332.
33.Matsumura Y,Ananthaswamy HN.Toxic effects of ultraviolet radiation on the skin.Toxicol Appl Pharmacol2004,195:298-308.
34.Pillai S,Oresajo C,Hayward J.Ultraviolet radiation and skin aging:roles of reactive oxygen species,inflammation and protease activation,and strategies for prevention of inflammation-induced matrix degradation-a review.Int J Cosmet Sci 2005,27:17-34.
35.Bosch R,Philips N,Suárez-Pérez JA,etal.Mechanisms of Photoaging and Cutaneous Photocarcinogenesis,and Photoprotective Strategieswith Phytochemicals.Antioxidants2015,4:248-268.
36.Zhan JY,Wang XF,Liu YH,et al.Andrographolide Sodium Bisulfate Prevents UV-Induced Skin Photoaging through Inhibiting Oxidative Stress and Inflammation.Mediators Inflamm 2016,2016:1-12.
37.Wenk J,Btenneisen P,Meewes C,et al.UV-induced oxidative stressand photoaging.Curr Probl Dermatol 2001,29:83-94.
38.Ohnishi Y,Tajima S,Akiyama M,et al.Expression of elastin-related proteins andmatrixmetalloproteinases in actinic elastosis of sun-damaged skin.Arch Dermatol Res2000,292:27-31.
39.Quan T,He T,Voorhees J,et al.Uhraviolet irradiation blocks cellular responses to transforming grow th factor-β by down-regulating its type-II receptor and inducing Smad7.JBiol Chem 2001,276:26349-26356.
40.Qua T,He T,Voorhees J,et al.Ultraviolet irradiation induces smad7 via induction of transcription factor AP-1 in human skin fibroblasts.J Bio Chem 2005,280:8079-8085.
41.Quan T,He T,Kang S,et al.Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming grow th factor-beta typeⅡreceptor/Smad signaling.Am J Pathol 2004,165:741-751.
42.Miyazono K.Positive and negative regulation of TGF-beta signaling.J Cell Sci2000,113:1101-1109.
43.Hanyu A,Ishidou Y,Ebisawa T,et al.The N domain of Smad7 is essential for specific inhibition of transformation grow th factor-beta signaling.J Cell Biol 2001,155:1017-1027.
44.Luo W,Yao L,Hua GU,et al.The Effect of TGF-β/Smad Signal Transduction Pathway on the Expression of MMP-1 and Pro-collagenⅠmRNA in the Process of Photoaging.J Dermatol Venereol 2011,33:125-128.
45.Yin L,Morita A,Tsuji T.Tobacco smoke extract induces age-related changes due tomodulation of TGF-beta.Exp Dermatol 2003,12:51-56.
46.Gambichler T,Skrygan M,Tomi NS,etal.Significant downregulation of transforming grow th factor-βsignal transducers in human skin following ultraviolet-A1 irradiation.Br J Dermatol 2007,156:951-956.
47.Sun S,Ping J,Su W,et al.Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging.Cytotechnology 2016,68:1-12.
48.Lee KE,Mun S,Pyun HB,et al.Effects of macelignan isolated from Myristica fragrans(Nutmeg) on expression of matrix metalloproteinase-1 and type I procollagen in UVB-irradiated human skin fibroblasts.Biol Pharm Bull 2012,35:1669-1675.
 Traditional Medicine Research2018年3期
Traditional Medicine Research2018年3期
- Traditional Medicine Research的其它文章
- Screening for cyclooxygenase 2 inhibitors from natural com pounds of Radix Glycyrrhizae using com puter simulation
- Polysaccharide extracts of Cirsium japonicum protect rat H 9c2 m yocardial cells from oxidative stress induced by hydrogen peroxide
- Study of dual-directional regulatory effect of Banxia(Pinellia ternata)and Huanglian(Coptis chinensis)drug pair on gastrointestinal movement of m ice
- Effect of alternate-day-fasting combined with Lingguizhugan Decoction on blood lipid profiles of hyperlipidem ic rats
