Study of dual-directional regulatory effect of Banxia(Pinellia ternata)and Huanglian(Coptis chinensis)drug pair on gastrointestinal movement of m ice
Yue Ji,Jun-Chen Li,Jing-Yan Meng,Xue-Rou Yan,Jian-Liang Li,Qing-Yun Zhao,Kang Yang,Chun-Liu Liu
1Tianjin University of Traditional ChineseMedicine,Tianjin,China.
Background
Functional dyspepsia(FD),with primary symptoms of epigastric pain and postprandial abdominal distension,is a common gastrointestinal dysfunction that has serious effects on the life of the patient[1].Epidemiological data revealed that 8%-20%of people have FD in Asia[2],with a particularly high prevalence in China[3].The high susceptibility of the gastrointestinal tract to hyperchlorhydria andHelicobacter pyloriinfection is a critical risk factor for the occurrence of FD [4].Traditional therapies for FD include antacids [5],prokinetic agents,anxiolytic drugs,digestive aids,and anti-Helicobacterpyloriinfective agents that exert specific effects[6].However,most of these treatments have problems including drug dependence,side-effects,and tolerance[7].
Traditional Chinese medicine formulations that offer advantages of low side effects and high efficacy have emerged as new treatment modalities for FD.Previous studies have demonstrated that the aqueous extract of Banxia(Pinellia ternata,P)inhibited gastric secretion,decreased the acidity of the gastric juice,and increased the activity of pepsin to protect the gastric mucosa[8].Dafupi(Areca peel),Sharen(Fructusamomi),Cangzhu(Rhizomaatractylodis),and Binlang(Macrocephalae)improved gastric emptying and intestinal transmission[9].Dahuang(Rhubarb)enhanced electrical activity in the gastrointestinal tract [10]. Guizhi soup exerted bidirectional regulatory effects on atropine(ATR)-and neostigmine(NEO)-induced digestive tract inhibition and in an asthenia model[11].In addition,Guizhi soup regulated the levels of gastrin(GAS),somatostatin(SS),and vasoactive intestinal polypeptide(VIP)in the small intestine.
Banxiaxiexin soup is an ancient recipe,which was first recorded in theShanghanluncomposed by Zhang Zhongjing in the third century(Eastern Han Dynasty of China).It comprises P 9 g,Huanglian(Coptis chinensis,C)3 g,Huangqin(Scutellaria baicalensis)6 g,Ganjiang(Rhizoma zingiberis)6 g,Renshen(ginseng)6 g,Gancao(Glycyrrhiza)6 g,and Dazao(jujube)4 g.Its functions include Qi-reduction and stomach function regulation,and it has become a representative formula for the treatment of the cold-heat complex of various digestive tract diseases[12].In addition to Banxiaxiexin soup,there are two other ancient recipes,Gancaoxiexin soup,and Shengjiangxiexin soup.These formulations have the same principal drug pair of P and C but in different proportions(1:1,3:1,and 4:1).
In this study,mice were injected with ATR and NEO after they had been treated with differentproportions(1:1,3:1,and 4:1)of the P and C drug pair for 10 days.The effects of the different proportions of the drug pair were evaluated based on the alvine advance rate.In addition,we used the same modeling method used in the first experiment,treated themicewith P:C at ratio of 3:1 and at different doses(4.68 g/L,2.34 g/L,and 1.17 g/L)respectively,and then measured the levels of the gastrointestinal hormones,GAS,VIP,and SS to study the possiblemechanisms responsible for this effect.
Materials andmethods
Animalsand reagents
Male Kunming mice were purchased from Beijing HuaFuKang Bioscience Company(Beijing,China),P and C were obtained from the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine(Tianjin,China),ATR was purchased from Hunan Dongtinghu Pharm.Co.Ltd.,(Hunan,China,batch number:040418)and,NEO was purchased from Shanghai Xinyi Pharm.Co.Ltd.,(Shanghai,China,batch number:040321).
Decoction of P and C
The herbal constituents of P and C derived from Banxiaxiexin soup and its categorized formulas were weighed and mixed at different proportions(1:1,3:1,and 4:1).The herbs were immersed in eight volumes of water for 1 h,boiled for 30 min,and then filtered three times.The filtrate was collected and concentrated to a final drug concentration of 2.34 g/L,according to the body surface area conversion method for experimental zoology potions[mouse dose=adult dose(mg/kg)×70 kg×0.00262/g].All the herbal extracts were administered at a dose of 25 m L/kg.
Nutritionalsem isolid carbon m ixture
Carboxymethylcellulose(5 g),milk powder(8 g),sugar(4 g),and carbon powder(4 g)were mixed in 150 m L water to obtain the nutritional semisolid carbon mixture.
Animal experimental methods
Experimental conditions.Two-hundred male Kunming mice,aged 6 weeks,with an average weight of 20±2 g were used.They were housed at room temperature(23±1)°C on a 12 h light/dark cycle(lights on from 6:00 am to 6:00 pm).Food and water were provided ad libitum.The experiments were conducted in accordance with the appropriate institutional regulations and national criteria for animal experimentation.The Institution Animal Ethics Comm ittee reviewed the entire animal protocol prior to the experimental process.
Establishmentofintestinalmovement disordermodel.One-hundred mice were random ly divided into 10 groups of 10 mice each:blank control A;NEO model;NEO 1:1;NEO 3:1;NEO 4:1;blank control B;ATR model;ATR 1:1;ATR 3:1;and ATR 4:1 groups.All mice in the PC treatment groups(NEO 1:1,NEO 3:1,NEO 4:1,ATR 1:1,ATR 3:1,and ATR 4:1 groups)were orally administered different proportions of the PC drug pair extract(1:1,3:1,4:1)at 25 m L/kg per day for 10 days,as described previously.The blank control group-A,blank control group-B,NEO model group,and ATR model group were administered normal saline at 25 m L/kg per day for 10 days by gavage.
After the above steps,all the mice were fasted for 24 h.All groups,except the blank control groups,received anintraperitoneal injection of NEO or ATR(0.02 mg/kg each)as required to establish a model of intestinal peristaltic propulsion or inhibition.After 30 min,to investigate the alvine advance rate,the mice were orally administered the nutritional semisolid carbon fuzzy at 20 m L/kg and allowed to rest for 20 min.Then,the mice were decapitated,their entire small intestines were removed,and the alvine advance rate wasmeasured.The length of the carbon powder-stained small intestine was measured aswell as the distance from the pylorus to the ileocecal segment.The alvine advance rate(%)was calculated as follows:length of carbon powder-stained small intestine/length from the pylorus to the ileocecal segment×100.
Hormone level measurementafter treatedwith PCat 3:1and different doses.The present study measured the hormone levels after administering different doses of P and C in mouse models of NEO-induced intestinal peristaltic propulsion and ATR-induced intestinal peristaltic inhibition.One-hundred mice were random ly divided into 10 groups:control(n=20);NEO model,low-,medium-,and high-dose;and ATR model,ATR low-,medium-,and high-dose groups(all n=10).Mice in the NEO low-,medium-,and high-dose,aswell as in the ATR low-dose,medium-,and high-dose groups were orally administered the PC drug pair extract in a 3:1 proportion at 1.17 g/L,2.34 g/L,and 4.68 g/L(low,medium,and high doses,respectively)for 10 days;and the control andmodel groups received normal saline(0.4 m L per day for 10 days)by gavage.The methods of establishing the ATR-and NEO-induced intestinal movement disorder models were the same as those explained in the previous section.After 30 min,the animals were decapitated,their small intestines were removed,the tissues were collected,homogenized,and then were stored at-80°C forbiochemicalanalysis.
Enzyme-linked immunosorbent assay
The levels of GAS,VIP,and SS in the small intestine homogenates were analyzed using an experimental reagent kit and detected with an enzyme-linked double antibody sandwich technique with the following parameters:detection limit,1.25-80 pg/m L;sensitivity,0.31 pg/m L;testing time,4 h;sample volume,100µL;detection wavelength,450 nm.The measurements were recorded using a plate reader
Statistical analysis
The data were analyzed using the statistical package for the SPSS 13.0 software.All the data are expressed as the means±standard deviation in tables and indicated by vertical bars in the figures.The differences between groupswere determined using the Student’st-test,and aP<0.05was considered significant.
Results
A lvine advance rate for different proportions of PC in mouse models of NEO-induced intestinal peristaltic propulsion and ATR-induced intestinal peristaltic inhibition
The alvine advance rate in the NEO model group was higher than that in the control group(P=0.007,Table 1),whereas P:C at ratios of 1:1,3:1,and 4:1 significantly reduced the alvine advance rate compared to that in the NEOmodel group(P=0.003,P=0.012,andP=0.021,respectively,Table 1).The alvine advance rate in the ATR model group was lower than that in the control group(P=0.001,Table 2),whereas the P:C at the ratio of 3:1 significantly increased the alvine advance rate compared with that in theATRmodelgroup(P=0.007,Table 2).

Table 1 A lvine advance rate in amousemodel of NEO-induced intestinalperistaltic propulsion treated with different proportions of PC drug pair

Table 2 A lvine advance rate in amouse model of ATR-induced intestinal peristaltic inhibition treated with different proportionsof PC drug pair
Hormone levels following PC at 3:1 and different doses in mouse models of NEO-induced intestinal peristaltic propulsion and ATR-induced intestinal peristaltic inhibition
The GAS level was lower in the controlgroup than that in the NEO model group(P=0.001,Figure 1A),but it decreased in the NEO low-,medium-,and high-dose groups compared to that in the NEO model group(P=0.001,P=0.004,andP=0.003,respectively,Figure 1A).The intestinal VIP and SS levels were higher in the control group than that in the NEO model group(P=0.004 andP=0.003,Figure 1B and C).The VIP levels were higher in the NEO medium-and high-dose groups than that in the NEO model group(P=0.004 andP=0.002,Figure 1B).Simultaneously,the SS level increased in the NEO medium-dose group compared to that in the NEOmodel group(P=0.002,Figure 1C).The GAS was negatively correlated with VIP(r=-0.356,P=0.036)and SS(r=-0.51,P=0.028),whereas SSwas positively correlatedwith VIP(r=0.779,P=0.001).
The GAS level was higher in the control group than that in the ATRmodel group(P=0.007,Figure 2A),but higher in the ATR medium-and high-dose groups than that in the ATR model group(P=0.007 andP=0.021,respectively,Figure 2A).The intestinal VIPand SS levels were lower in the control group than that in the ATR modelgroup(P=0.002 andP=0.001,Figure 2B and C).The VIP levelwas lower in the ATR low-dose,medium-,and high-dose groups than that in the ATR model group(P=0.001,P=0.001,andP=0.001,Figure 2B).Furthermore,the SS levelwas lower in the ATR mediumand high-dose groups than that in the ATR model group(P=0.001 andP=0.006,Figure 2C).The GAS was negatively correlated with VIP(r=-0.643,P=0.001)and SS(r=-0.703,P=0.001),whereas SS was positively correlated with VIP(r=0.723,P=0.001).
Discussion
The movement of the digestive tract smooth muscle is modulated by the sympathetic and parasympathetic nerves[13].ATR is a toxic,white,crystalline alkaloid(C17H23NO3)derived from Dianqie(belladonna)and other nightshade plants,which can inhibit the parasympathetic nerve by blocking the muscarinic cholinergic receptor.It ismainly used to extend the gastric emptying time and inhibit intestinal motility in clinical practice.NEO,a white crystalline alkaloid (C12H19BrN20) with an anticholinesterase effect,isoften used for the treatmentof myasthenia gravis and intestinal paralysis after abdominal surgery.Spasmolytics and anticholinesterase are essential for the modulation of gastrointestinal motility.ATR-induced gastrointestinal tract inhibition and NEO-induced gastrointestinal tract stimulation models are commonly used to study the effectof drugs on FD.
Traditional Chinese medicine(TCM)relationships demonstrate an affirmance theory foundation and compounding prescription.To investigate the possible mechanism of TCM compounds,it is necessary to begin with the drug pair.The acrid-opening and bitter down-bearing drug pair P and C for purgation is fundamental,conferring themain effects of Banxiaxiexin soup for Qi regulation, which regulates the gastrointestinal tract of patients[14].By comparatively analyzing NEO-and ATR-inducedmice,themovement of the carbon powder in the intestine was observed after treatment with different proportions of the P and C drug pair.
The results reveal that all three proportions(1:1,3:1,and 4:1)of the PC decoctions exerted therapeutic efficacy in the NEO-induced intestinal peristaltic propulsive model,whereas the P:C at 3:1 significantly induced the alvine advance rate compared to thatobserved in the ATR model group.A comparison of the effective treatment index among the six treatment groups(NEO 1:1,NEO 3:1,NEO 4:1,ATR 1:1,ATR 3:1,and ATR 4:1 groups),demonstrated that the P:C at3:1 significantly affected the alvine advance rate in the mouse models of ATR-and NEO-induced intestinal movement disorder.Moreover,their experimental resultswere superior to those obtained after treatmentwith otherproportions.In this experiment,the comparison of the effects of the treatment in the NEO-and ATR-induced models demonstrated that the PC drug pair improved FD by modulating the cholinergic system,but may be more effective in the NEO-induced intestinal peristaltic propulsivemodel.
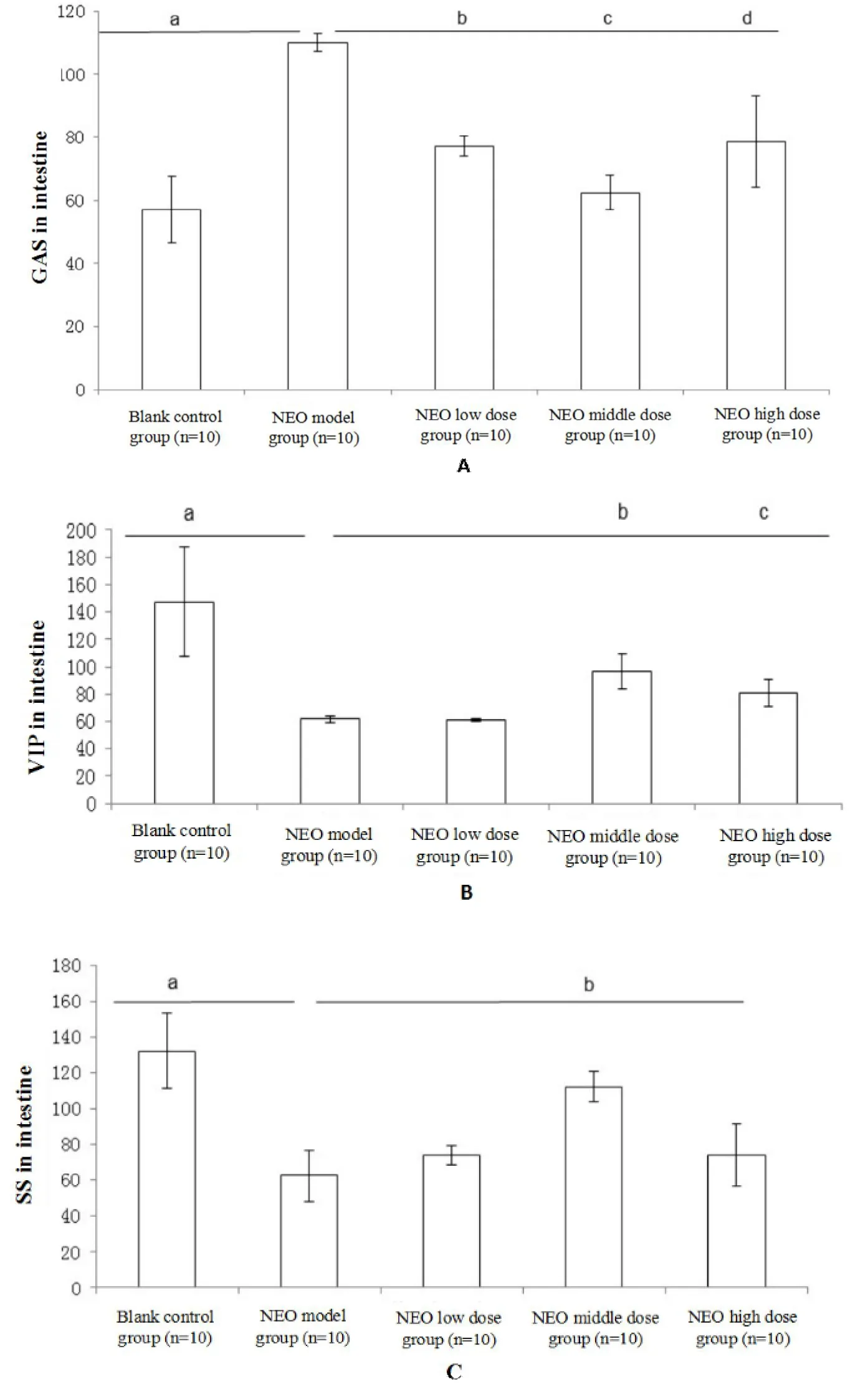
Figure 1 The hormone levelsof PC 3:1 on NEO induced intestinal peristaltic propulsive model
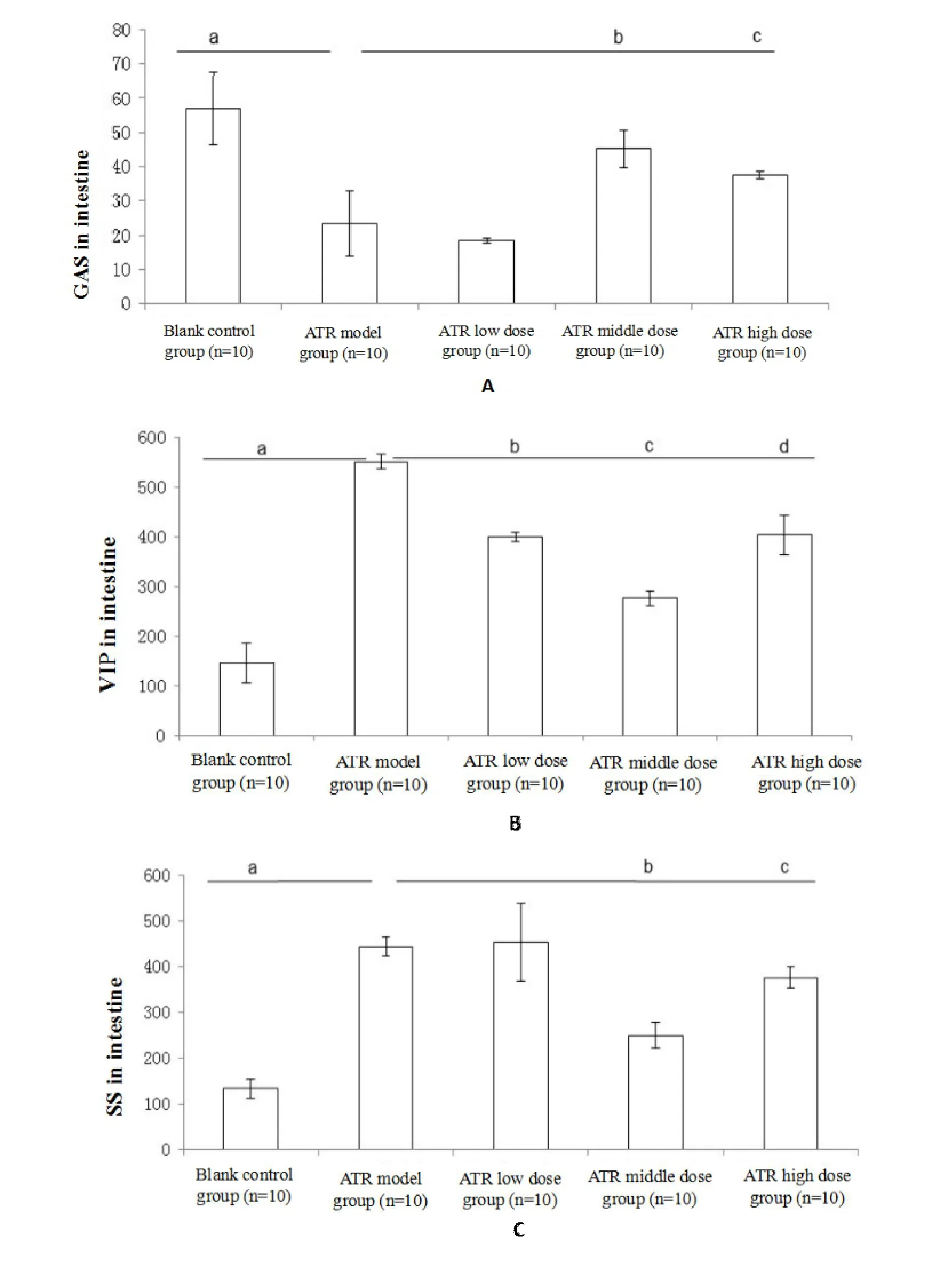
Figure 2 The hormone levelsof PC 3:1 on ATR induced intestinalperistaltic inhibitionmodel
The gut hormones can modulate gastrointestinal motility by stimulating the gastrointestinal smooth muscles [15], which regulate the gastrointestinal neurotransmitters[16]and central nervous system[17].GAS[18]is an excitatorymotor neuron neurotransmitter involved in the promotion of gastrointestinalmovement.It causes acid secretion by parietal cells and promotes not only pancreatic secretion but also the secretion of gastrointestinal mucus.It also promotes gastrointestinal stimulation and enables principal cells to secrete pepsinogen.VIP[19]is a neurotransmitter expressed in the central and enteric nervous systems.It causes smooth muscle relaxation by promoting nitric oxide synthesis produced by the main cells[20].SS[21]is an inhibitory neurotransmitter in motor neurons and inhibits the release of a variety of gastrointestinal hormones such as GAS[22],motilin[23],VIP,and other hormones.SS not only inhibits the secretion of gastric acid,pepsin,and gastric emptying but also affects the contraction of smooth muscles and the gallbladder,simultaneously increasing the absorption of water and electrolytes in the large intestine.Therefore,VIP functions in contrast to GAS,which exerts a substantial adverse effect on the contraction of the gastrointestinal smooth muscle,but a weak effect on the secretion of gastric juices and pancreatic secretions.SS inhibits the digestive tract by inhibiting the production of GAS and VIP.Therefore,we administered GAS to examine intestinal peristaltic propulsion,and VIP and SS to exclude the intestinal peristaltic inhibition index.
ATR increased the level of VIP and SS but decreased that of GAS.NEO has opposite effects to those of ATR.The administration of NEO induced gastric emptying and intestinal motility in addition to decreasing levels of VIP and SS and increasing levels of GAS.Our study demonstrated that PC at 3:1 ameliorates the NEO-and ATR-induced FD through the modulation of GAS,VIP,and SS levels in the intestine by bidirectionaladjustment.However,dose-dependent changes were not observed after the administration of PC at 3:1.P and C have opposite properties and tastes.P has an acrid-opening characteristic and C has a bitter down-bearing characteristic.For this reason,we speculated that as the concentration increased,the efficacy of treatment may be shifted from a bi-directional to a unidirectional mechanism.However,further confirmation is required.Research on the effects of Tongxie Yaofang[24]and Sijunzi decoction[25]on gastrointestinal motility has demonstrated that they did not exhibit concentration-dependent effects.
In conclusion,the PC drug pair exerted bidirectional adjustments in NEO-and ATR-induced FD models through themodulation of GAS,VIP,and SS levels in the intestine,which isa characteristic of TCM.
1.Delgado-Aros S,Camilleri M,Camilleri M,et al.Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia.Gastroenterol 2004,127:1685-1694.
2.Ghoshal UC,Rajan S,Chang FY,et al.Epidemiology of uninvestigated and Functional Dyspepsia in Aisa:factsand fiction.J NeurogastroenterolMotil 2011,17:235-244.
3.Wu BY,Zhang F.Epidemiology of functional dyspepsia.Chin J Gastroenterol Hepatol 2013,22:85-90.
4.Halder SL,Rd LG,Talley NJ,et al.Impact of functional gastrointestinal disorders on health-related quality of life:a population based case-control study.Aliment Pharmacol Ther2004,19:233-242.
5.Collen MJ,Loebenberg MJ.Basal gastric acid secretion in nonulcer dyspepsia with or without duodenitis.Dig Dis Sci1989,34:246-250.
6.Pineda LF,Rosas MC,Amaya MT,et al.Clinical practice guideline for the diagnosis and treatment of dyspepsia in adults.Rev Col Gastroenterol 2015,30:9-16.
7.Miwa H,Watari J,Fukui H,etal.Current understanding of pathogenesis of functional dyspepsia.JGastroenterol Hepatol 2011,26:53-60.
8.Liang GH,Li XJ.Effects of Banxia Xiexin Decoction on Expression of Inflammative Cell Factor in UC Rats.Lishizhen Med Mater Medica Res 2007,18:3028-3029.
9.Zhao ZJ,Ren LE,Feng CD,et al.A screening study of the kinetogenic effects of 15 Chinese herbal medicines on the gastrointestinal tract in rats.Disanjunyidaxue xuebao 2000,22:436-438.
10.Li YY,Wei Y,Li LJ,et al.The Effective Machanisms of CCB Chinese Herbs on Gastrointestinal Motility.Chin J Integr TraditWest Med 1997,3:87-90.
11.Huo HR,Tan YQ,Zhou AX,et al.Experimental Study of Active Fraction B of Guizhi Tang(Cinnamon Twig Decoction)on Dual-Direction Regulation of Gastrointestinal Motility VI:Effet on cAMP,Protein Kinase A and Protein Kinase C.Chin J Exp Tradit Med Formulae 2005,11:51-53.
12.Tong RS,Xiao KC,Li JQ,et al.Study on Material Basis of Banxia Xiexin Tang in Reguluating Gastrointestinal Motility.Chin J Exp Tradit Med Formulae 2015,21:160-163.
13.Katschinski M,Steinicke C,Reinshagen M,et al.Gastroin-testinal motor and secretory respones to cholinergic stimulation in human, Differential modulation by muscarinic and cholecystokinin receptor block.Eur JClin Investig 1995,25:112-113.14.Sanger GJ,Hellstom PM,Naslund E.The Hungry Stomatch: Physiology, Disease, and Drug DevelopmentOpportunities.Front Pharmacol2011,1:145.
15.Marinova EK,Nikolova DB,Popova DN,et al.Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine.Immunopharmacol2000,48:9-16.
16.Wildersmith CH.The balancing act:endogenous modulation of pain in functional gastrointestinal disorders.Gut 2011,60:1589-1599.
17.Van OL,Aziz Q.The role of psychosocial factors and paychiatric disorders in functional dyspepsia.Nat Rev GastroenterolHepatol2013,10:158-167.
18.Koloski NA,Jones M,Kalantar J,et al.The brain-gut pathway in functional gastrointestinal disorders is bidirectional:a 12-year prospective population-based study.Gut 2012,61:1284-1290.
19.Hurwitz A,Robinson RG,Herrin WF,et al.Oral anticholinergics and gastric emptying. Clin Pharmacol Ther 1982,31:168-174.
20.Rashid MU,Bateman DN.Effect of intravenous atropine on gastric emptying,paracetamolabsorption,salivary flow and heart rate in young and fit elderly volum teers.Br J Clin Pharmacol1990,30:25-34.
21.Pfeiffer A,Schmidt T,Holler T,et al.Effect of trospium chloride on gastrointestinal motility in human.Eur J Clin Pharmacol 1993,44:219-223.
22.Guerrerolindner E,Arruebo MP,Murillo MD,et al.Effect of motilin on Gastrointestinal myoelectric activity in conscious rabbits.Peptides 1996,17:901-907.
23.Shibata C,Sasaki I,Naito H,et al.Effect of motilin on colonic motor activity in the interdigestive state in conscious dogs.Tohoku J Exp Med 1995,176:53-60.
24.Wang JW,Zhao WJ,Li K.Experimental Study on Dual-Direction Regulation of Tongxie Yaofang on Gastrointestinal Movement of Mice. Chin J Information on TCM 2008,15:32-35.
25.Wang RJ,Hu YJ,Du Qet al.Studies of the chemical basis of bidirectional regulation of SijunziDecoction on gastrointestinal motility.Pharmacol Clin Chin Materia Medica 2001,11:3-4.
 Traditional Medicine Research2018年3期
Traditional Medicine Research2018年3期
- Traditional Medicine Research的其它文章
- Screening for cyclooxygenase 2 inhibitors from natural com pounds of Radix Glycyrrhizae using com puter simulation
- The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1
- Polysaccharide extracts of Cirsium japonicum protect rat H 9c2 m yocardial cells from oxidative stress induced by hydrogen peroxide
- Effect of alternate-day-fasting combined with Lingguizhugan Decoction on blood lipid profiles of hyperlipidem ic rats
