Screening for cyclooxygenase 2 inhibitors from natural com pounds of Radix Glycyrrhizae using com puter simulation
Ming Yang,YiJin,Li-Ping Yang
1Department of Pharmacy of Beijing Hospital,National Center of Gerontology,Assessment of Clinical Drugs Risk and Individual Application Key Laboratory;National Clinical Research Center of Respiratory Diseases,Beijing,China.
2Schoolof Life Sciencesand Biopharmaceuticals,Shenyang PharmaceuticalUniversity,Shenyang,China.
Background
Chinese herb Gancao(Radix Glycyrrhizae,GC)is the dry root and the rhizome ofGlycyrrhizaspecies.It is used by Chinesemedicine doctors to invigorate and regenerate the spleen,clear heat and relieve toxicity,relieve cough and sputum production,and to control pain[1].The history of the application of GC in traditionalChinese medicine can be traced back to the ancient book namedShennongbencaojingof the Han Dynasty of China(the third century A.D.).Besides,among the 113 prescriptions ofShanghanlun,which werew ritten by Zhang Zhongjing,more than half of the prescriptionscontained GC.
Current researches have reported that GC has several pharmacological effects,including anti-inflammatory effects, antiviral effects, liver protective effects,antitussive effects,and detoxification effects[2-7].GC protects againstboth acute and chronic inflammations[8].Themost widely used GC preparations in clinical practice include compound licorice tablets,compound licorice mixtures,glycyrrhizin tablets,glycyrrhizin injections,and monoammonium glycyrrhizinate[9].They are mainly used as antitussives and hepatoprotective drugs.The mechanism of action of these licorice preparations is not yet clear,but their actions are attributed to their anti-inflammatory effects.
Inflammation is involved in the mass production of leukotriene and prostaglandins,related to the metabolism of arachidonic acid(AA).A variety of inflammatory factors induced lipoxidaseand cyclooxygenase 2(COX-2)to converts AA into leukotriene and prostaglandins,resulting in a series of clinical symptoms of inflammation[10].The commonly used anti-inflammatory drugs in clinicmain contain steroidal anti-inflammatory drugs and non-steroidal anti-inflammatory drugs (NSAIDs).Previous studies on the anti-inflammatory effects of GC components focused on the terpenes and saponins of GC,including glycyrrhizic acid(also named glycyrrhizin),glycyrrhetinic acid and oleanolic acid,etc.Their chemical structures were similar to that of steroid and had glucocorticoids-like anti-inflammatory effect by inhibiting lipoxidase and COX-2 products[11-14].Except for terpenes and saponins,GC also contains many other components such as flavonols.Though their chemical structures differed totally from that of steroid,they also possessed the powerful anti-inflammatory effects[15,16].To explore whether the other components of GC possess the NSAIDs-like anti-inflammatory effect and their underling mechanism is related to the inhibition on COX-2,the present study designed a screening route andmethod to select the bioactive components of GC and further explore their anti-inflammatory mechanism using the three-step program, pharmacophore screening,molecular docking,and physicochemical properties analysis.
Methods
All the researches were carried out using the Discovery Studio 4.5(DS 4.5,San Diego,CA,USA)System,and AutoDock Vina software(Scripps Research Institute).Unless specified,the calculation process was carried out with default values.
The natural ingredients of GC and NSAIDs
Six hundred and fifty-three natural GC componentswere obtained in our previous studies;however,only 513 of those components,including flavonoids(166),terpenoids and saponins(114),aliphatic compounds(172),and aromatic compounds(61),were included in this study.
The inflammatory pathways of the 40 NSAIDs were obtained from the KEGG website(http://www.kegg.jp/).The SDF three-dimensional formats of the 513 GC components and those of the 40 NSAIDs were either downloaded from the PubMed database or generated with the DS 4.5 software.Finally,the Minimize Ligands module of the DS 4.5 softwarewas used to optimize the energy of the small molecular ligands,which were used for the screening and docking of the pharmacophores.
Pharmacophore screening
Using the Ligand Profiler module,under the Prepare Ligands program in DS 4.5,the smallmolecular ligands were docked with the pharmacophores corresponding to the COX-2 protein in the PharmaDB database,and the BEST parameter was chosen to generate the lowest energy conformations with an energy threshold of 20 kcal∙mol-1.When the Fit Value was high,the compounds combined better with the pharmacophore,so we chose the smallmoleculeswith Fit Values of more than 0.5 to carry onwith themolecular docking.
Preparation of target protein(COX-2)receptor
We selected the 1CVU,which is the crystal structure of the AA substrate bound to the COX-2 protein,as the docking receptor for the AutoDock Vina. The three-dimensional crystal structure(PDB ID:1CVU)of COX-2 was downloaded from the Protein Database(PDB)and processed with the AutoDock Vina software.The structure of COX-2 was optim ized by removing excess protein conformations,deleting ligands,removing water molecules,adding charges,and hydrogenating atoms.It was then stored in a PDBQT format.
Molecular docking by AutoDock Vina software
The GC small molecules, obtained from the pharmacophore screening,were combined with the COX-2 protein(1CVU),together with the NSAIDs,using the AutoDock Vina version 1.1.2 software(http://vina.scripps.edu/).According to the literature,the center of the COX-2 active site wasset to center_x=27.7,center_y=24.6,and center_z=46.9,and the size of the active site was set to size_x=24,size_y=24,and size_z=24.The parameters were set to num_modes=10 and exhaustiveness=50.The other parameters were set to default values.The virtual binding energy was calculated with the Lamarckian genetic algorithm and Autodock Vina software.When the docking binding energy was low,the affinity of the compound for the target was high,indicating that the inhibitory effectof small molecules on large molecules is very strong.
Comparison of physicaland chem ical properties using DS 4.5 software
Calculation of the physicochemical properties of the 513 GC components and the 40 NSAIDs waswith the DS 4.5 software.The operation steps are as follows:selected Molecular Properties→Molecular-Solubility(MS)→Molecular-Weight(MW)→Molecular-Surface-Area(MSA).Parameters were set to defaultvalues.
Note:MW is the sum of the atomic weights of all atoms in amolecule.MSA refers to the total surface area of each molecule.MS refers to the solubility of a component.The solubility was greater when the absolute MS value wassmaller.
Results
Prediction of results of physicochem ical properties for GC compounds and NSAIDs
The MW,MSA,and MS of the GC components(n=513,including aliphatics, flavonoids, aromatics, and terpenoids/saponins)and the marketed NSAIDs(n=40)were predicted by the molecular properties modules of the DS 4.5 software(Table 1).
The values of the aliphatics(MW=184.4,MSA=239.5)and those of the terpenoids/saponins(MW=468.7,MSA=532.8)differed from those of the NSAIDs(MW=293.3,MSA=277)in terms of MW and MSA.The median value(MV)of the aliphatics(MV=184.4)was less than that of the NSAIDs(MV=293.3).The MV of the terpenoids/saponins(MV=468.7)was far greater than that of the NSAIDs(MV=293.3).Otherwise,the values of the aromatics(MW=278.3,MSA=281.9)were similar to those of the NSAIDs,and the values of the flavonoids(MW=354.4,MSA=360.2)were a little higher than those of the NSAIDs.The overall value of the aromatics wassmaller than thatof the flavonoids.
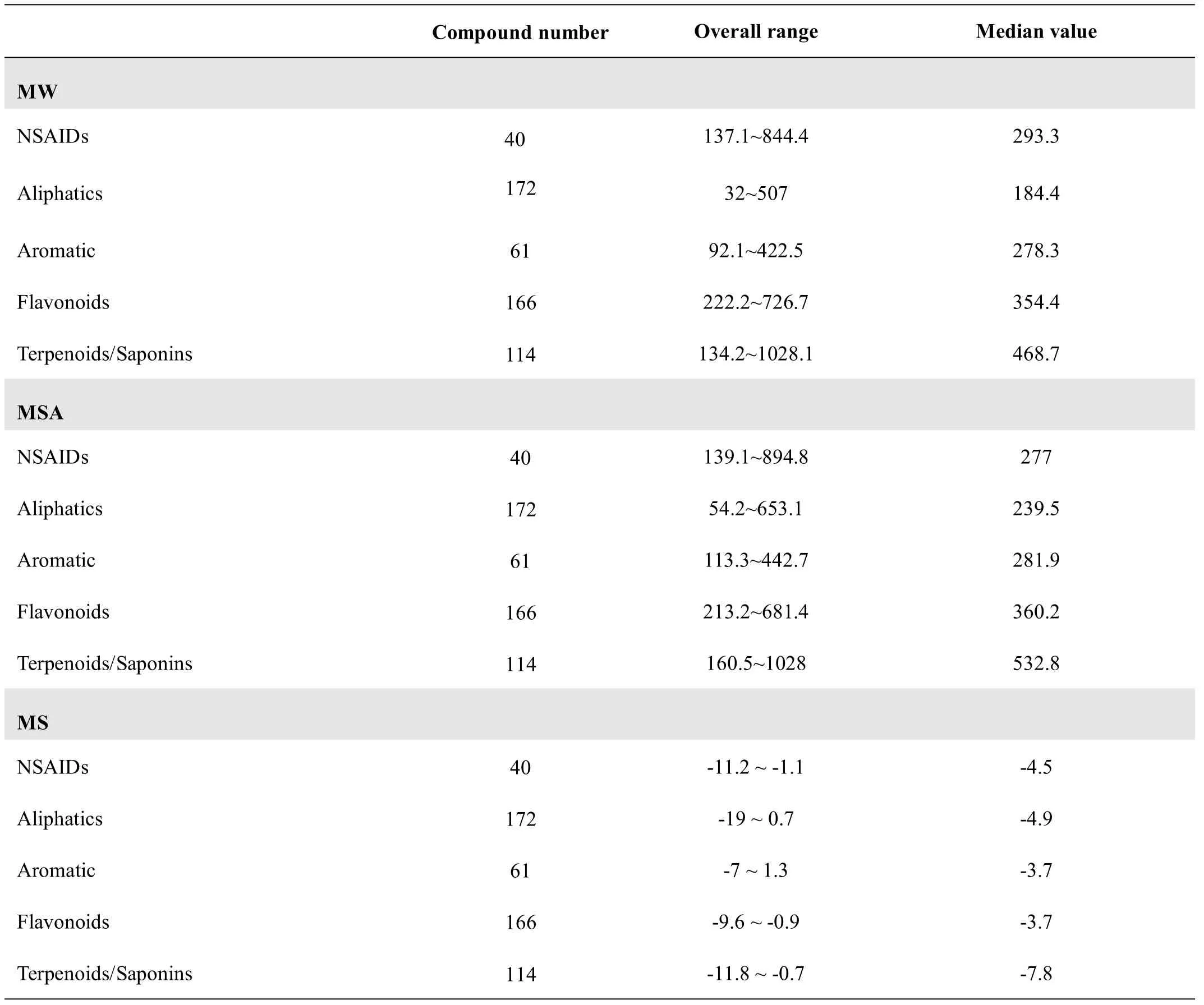
Table 1 Prediction of results of physicochem ical properties for GC compounds and NSAIDs
Themedian MS value of the terpenoids/saponins(MS=-7.8)wasmuch smaller than thatof the NSAIDs(MS=-4.5),and themedian MS value of the aliphatics(MS=-4.9)was the same as that of the NSAIDs,whereas the median MS values of the aromatics and flavonoids(MS=-3.7,MS=-3.7)were both slightly higher than that of the NSAIDs.However,the variation in the MS value of the aliphaticswas the highest,from+0.7 to-19.
These results indicated that the aromatics were the closest to the NSAIDs in terms of the three properties(MW,MSA,and MS),followed by the flavonoids.The terpenoids/saponins differed most,and there were inconsistencies in the valuesof thealiphatics.
Pharmacophorescreeningand moleculardocking Screening potential inhibitors of GCcomponents with COX-2pharmacophores.The DS 4.5 PharmaDB database contains 8 different crystals of the COX-2 protein(3mqe,3rr3,3q7d,1pxx,3ntg,3ln0,3ln1,4fm5)and a total of 59 pharmacophores.Using the Prepare Ligands program in the DS 4.5 software,the 513 GC small molecules were combined with the 59 pharmacophores and 7585 results were obtained;the corresponding figures for the various Fitvalues,FitValue=0,Fit Value>0,and Fit Value>0.5,were 6259,1326,and 680 respectively.The duplicate items of the small molecules in the 680 results were subtracted and 118 small moleculeswith a Fit Value of>0.5 were obtained.The 118 GC small molecules consisted of 43 aliphatics,44 flavonoids,24 aromatics and 7 terpenoids/saponins.The 118GC small molecules were used for the following molecular docking and physicochemical properties analyses.
Further filtration bymoleculardockingwith COX-2 crystal(1CVU).The ligand molecule,AA,was docked into the binding site of the COX-2 protein using the Autodock Vina software(Figure 1).The root mean square deviation(RMSD)values of the ligand molecules and the original ligand molecules were used to determine the rationality of the parameter settings and the suitability of the procedure for the protein receptor-ligand complex.The RMSD value for the docking of the AA into the COX-2 protein was 1.62Å(Figure 1).It is generally believed that RMSD ≤ 2 Å indicates that the docking results and the original conformation have overlapped very well,and that the design of the docking parameters was reliable and feasible.
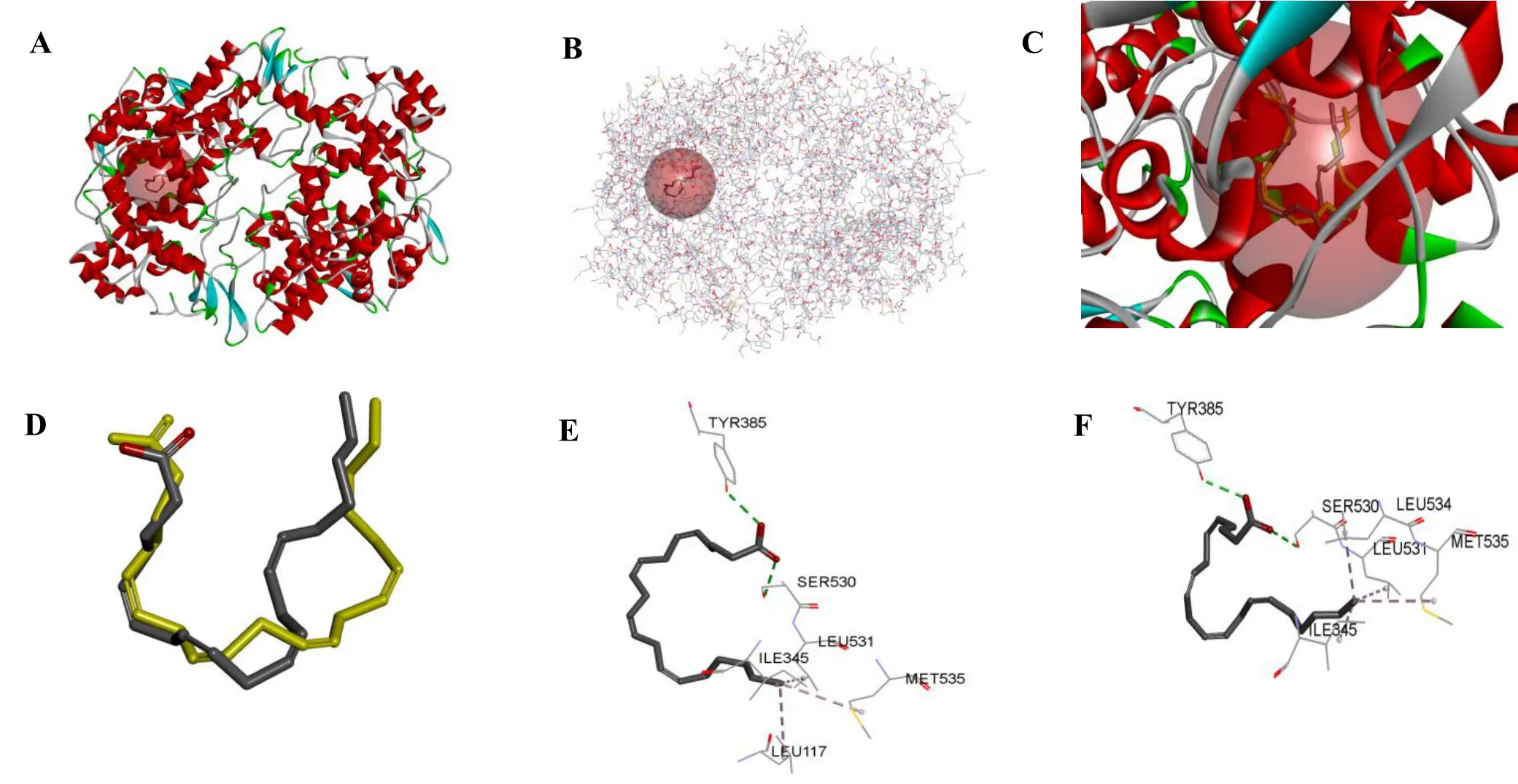
Figure 1 Active site of COX-2 crystal(1CVU)and the interaction with arachidonic acid

Table 2 Group by binding energy of GC compoundsand NSAIDs with COX-2
The 118 GC components,screened with the pharmacophores,were classified as group A small molecules and the 40 NSAIDswere classified as group B small molecules, which were docked with the macromolecules,COX-2 crystal protein(1CVU)and AA,respectively.We obtained 158 molecular binding energy(MBE)values.Among them,the MBE value of the AA and 1CVU was-8,which was used as the reference value.The two groups,A and B,were subdivided into groupsA1 and B1,with a MBE value lower than the reference value,and groups A2 and B2,with a MBE value higher than the reference value.The results showed that there were 59 GC components in the A1 group,including 43 flavonoids,14 aromatics,1 aliphatic,and 1 terpenoid;the A2 group also contained 59 GC components,including 42 aliphatics,10 aromatics,1 flavonoid,and 6 terpenoids.The NSAIDs were mainly found in group B1(34);however,six of them(ibuprofen,meloxicam,aspirin,acetaminophen,salicylamide,and sodium salicylate)were found in group B2(Table 2).
From the results of the MBE determination,the MBE value of flavonoids/aromatics,obtained from their xxxxxx binding with the COX-2 protein,was lower than that obtained from their binding with the AA,which may have a good inhibitory effect on the COX-2 protein.However,the MBE value of the aliphatics/terpenoids,obtained from their binding with the COX-2 protein,was higher than that obtained from their binding with the substrate,AA,which may have a less inhibitory effect on the COX-2 protein.The docking pattern of COX-2 and the small molecules is shown in Figure 2.
Further filtration based on the physicochem ical properties and the MBE of the smallmolecules
The 118 GC components and the 40 NSAIDs were grouped into four ranges,including small range,medium range,large range,and extra-large range according to the MW,MSA,and MS(small range MW<200,MSA<200,and MS>-3;medium range MW:200 to 300,MSA:200 to 300,MS:-4 to-3;large range MW:300 to 400,MSA:300 to 400,MS:-6 to-4;and extra-large range MW>400,MSA>400,MS<-6).The analysis and statistics were carried out according to molecular docking results(Table 3).
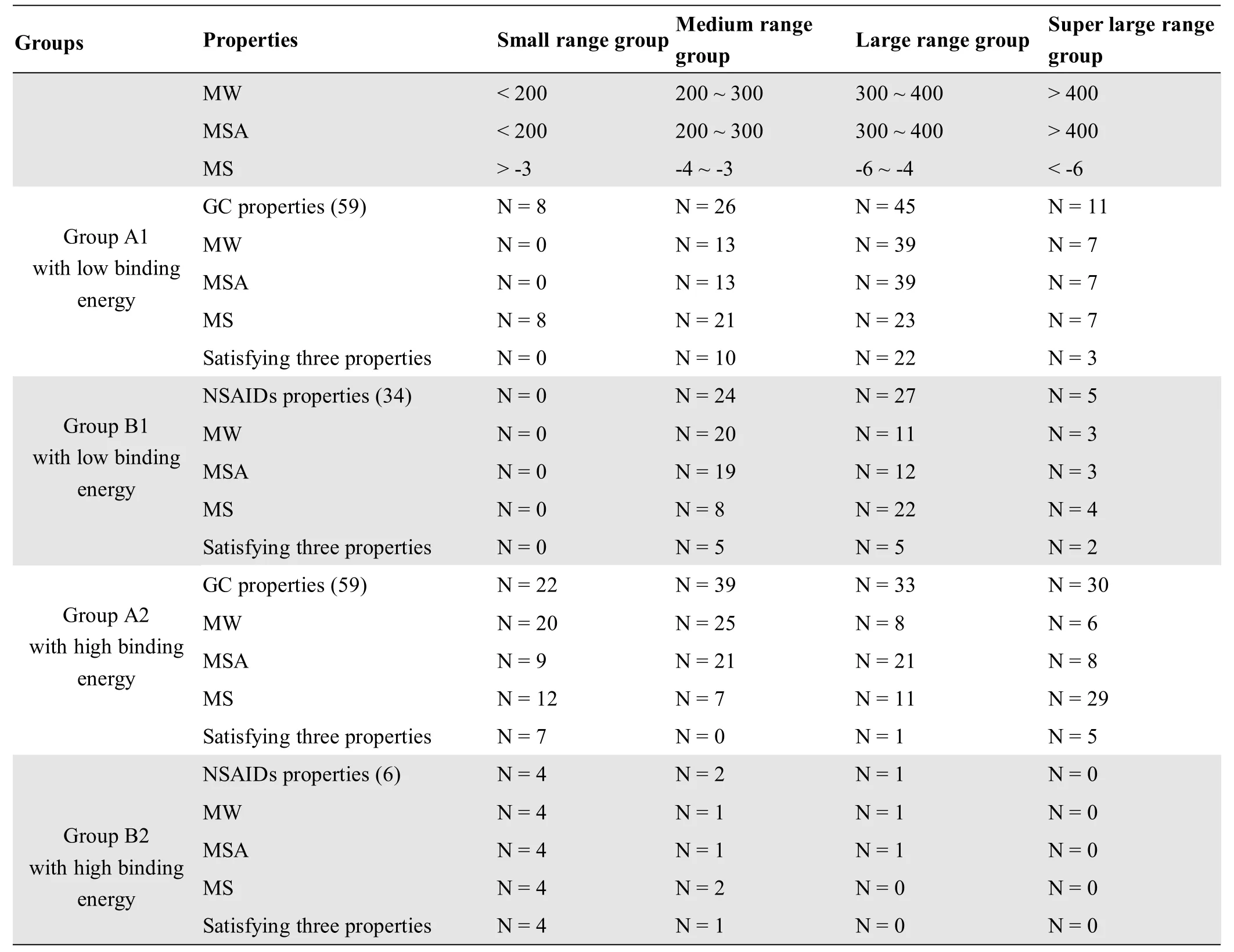
Table 3 GC components and NSAIDs were grouped according to their physicochem ical properties
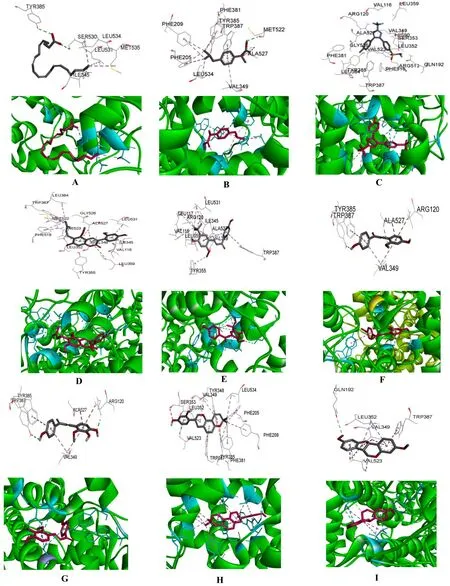
Figure 2 The am ino acid residues binding with GC compounds or NSAIDs in COX-2 active site
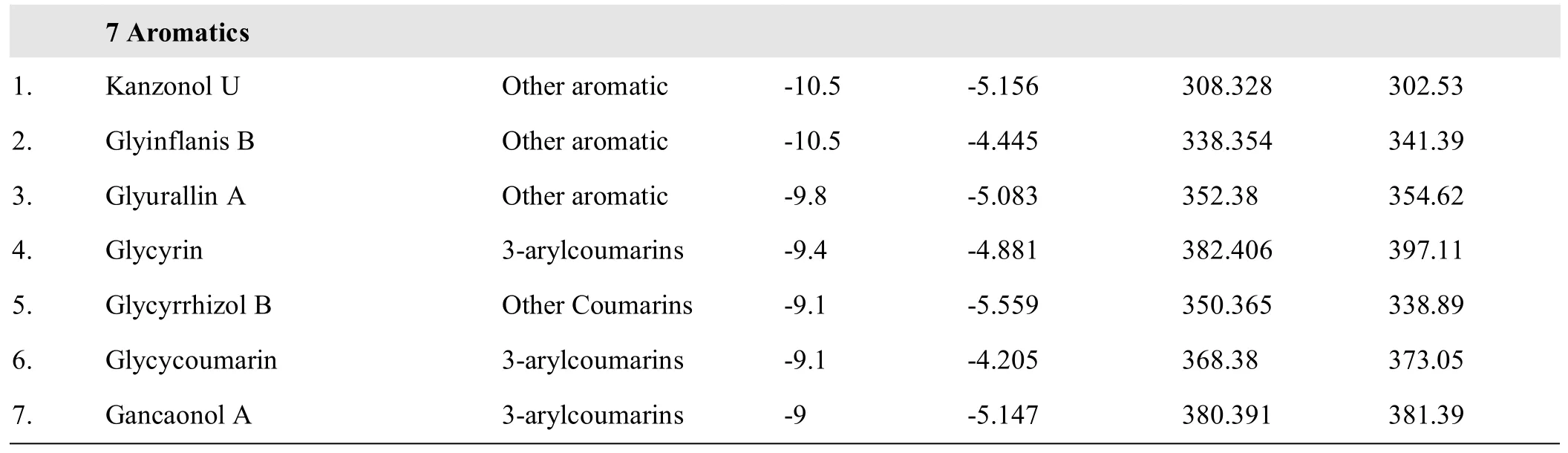
The results showed that the GC components were mainly distributed in the large-range group,followed by the medium range group,small range group,and extra-large range group.Flavonoidsmainly fell to group A1,whereas aliphatics mainly fell to group A2.The aromaticsmainly fell to the large range and extra-large group,A1,and the terpenoids were predominantly found in the extra-large range group,A2.If the binding energy values had been used to predict the inhibitory effect,the inhibitory effects of aliphatics and terpenoids on COX-2 would have been weaker,and the inhibitory effect of flavonoids and some aromatics on COX-2 would have been stronger.The MW and MSA of the flavonoids with low binding energies were between 200 and 400,and the molecular solubility was between-5 and-3.The MW and MSA of the aromatic molecules with low binding energies were between 300 and 400,and the molecular solubility was between-6 and-4.
The 34 NSAIDs in group B1were in the medium,large range,and extra-large range groups,and the 6 NSAIDs in group B2 were in the small range group(Figure 3,Table 4).Most of them were in the following ranges;MW<400,MSA<400,and MS>-6.In the 59GC components with low COX-2 binding energy,the MW and MSA of the 35 flavonoids were distributed in the range of 200~400 and the MSwas in the range of-6~-3.The MW and MSA of the 7 aromatic components were distributed in the range of 300-400 and the MS was in the range of-6~-4.Thus,we predicted that the 35 flavonoids and 7 aromaticsmay have a strong inhibitory effect on COX-2(Figure 4,Table 5).

Continue
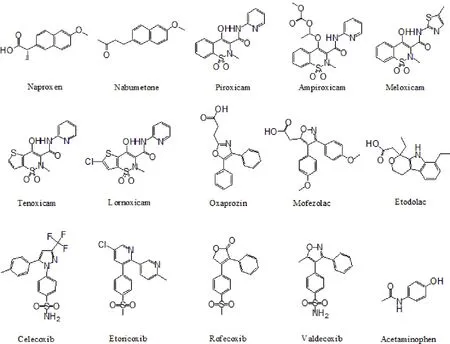
Figure 3 The structures of 40 non-steroidal anti-inflammatory drugs
Continue
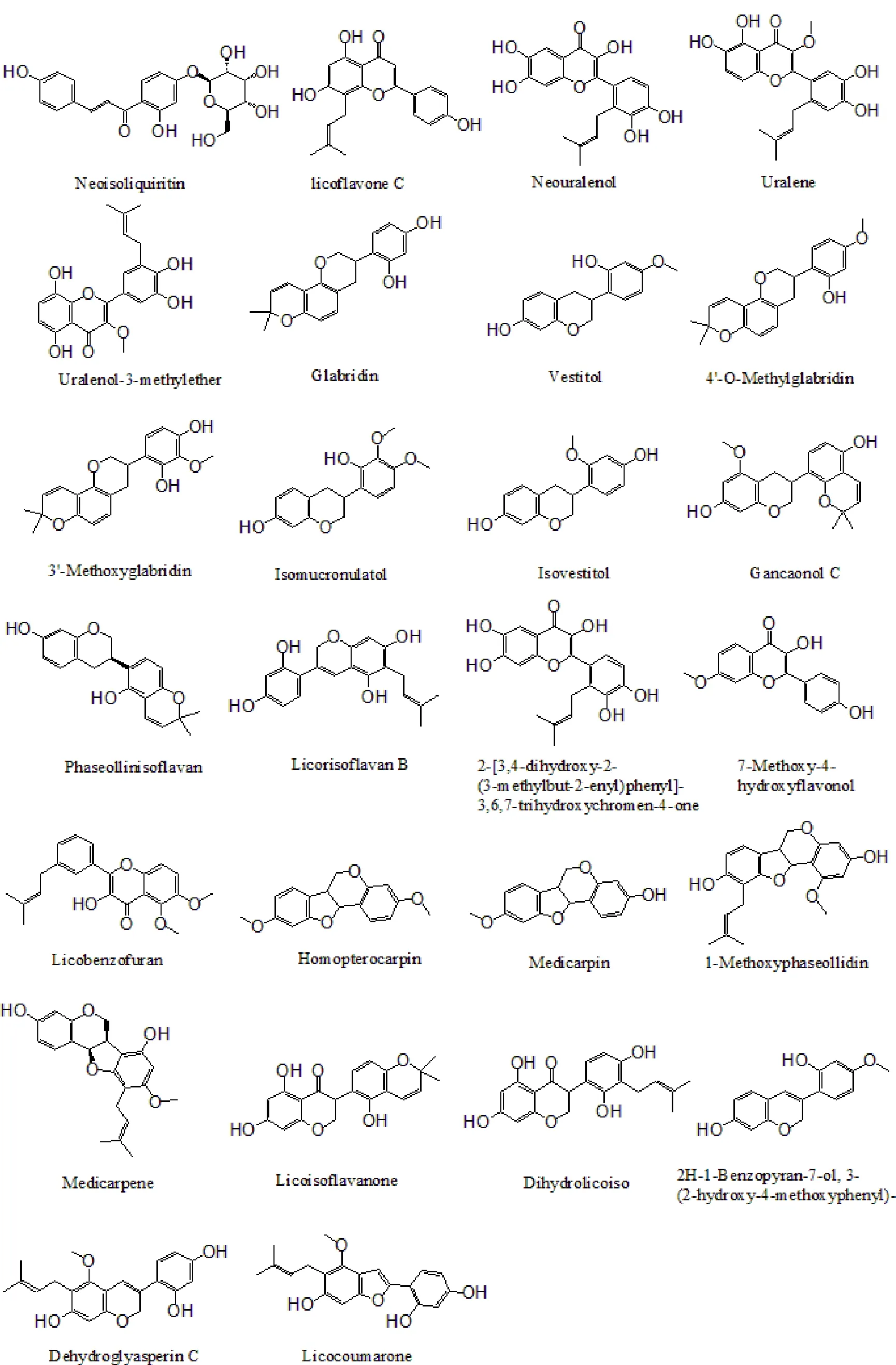
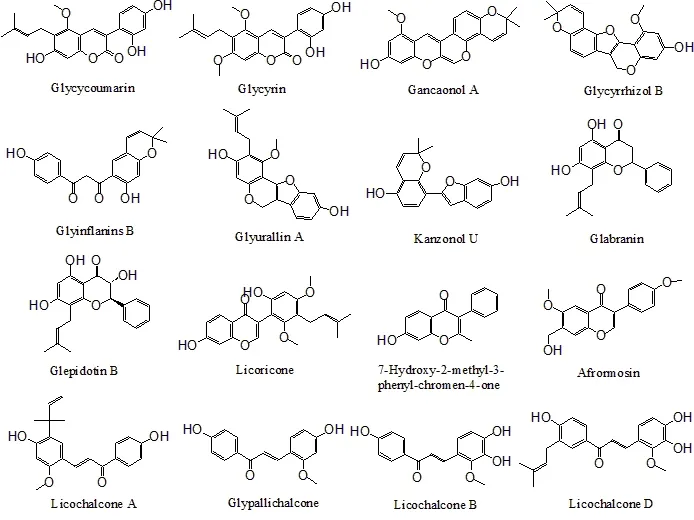
Figure 4 The structures of 42 Gancao(Radix Glycyrrhizae)compounds
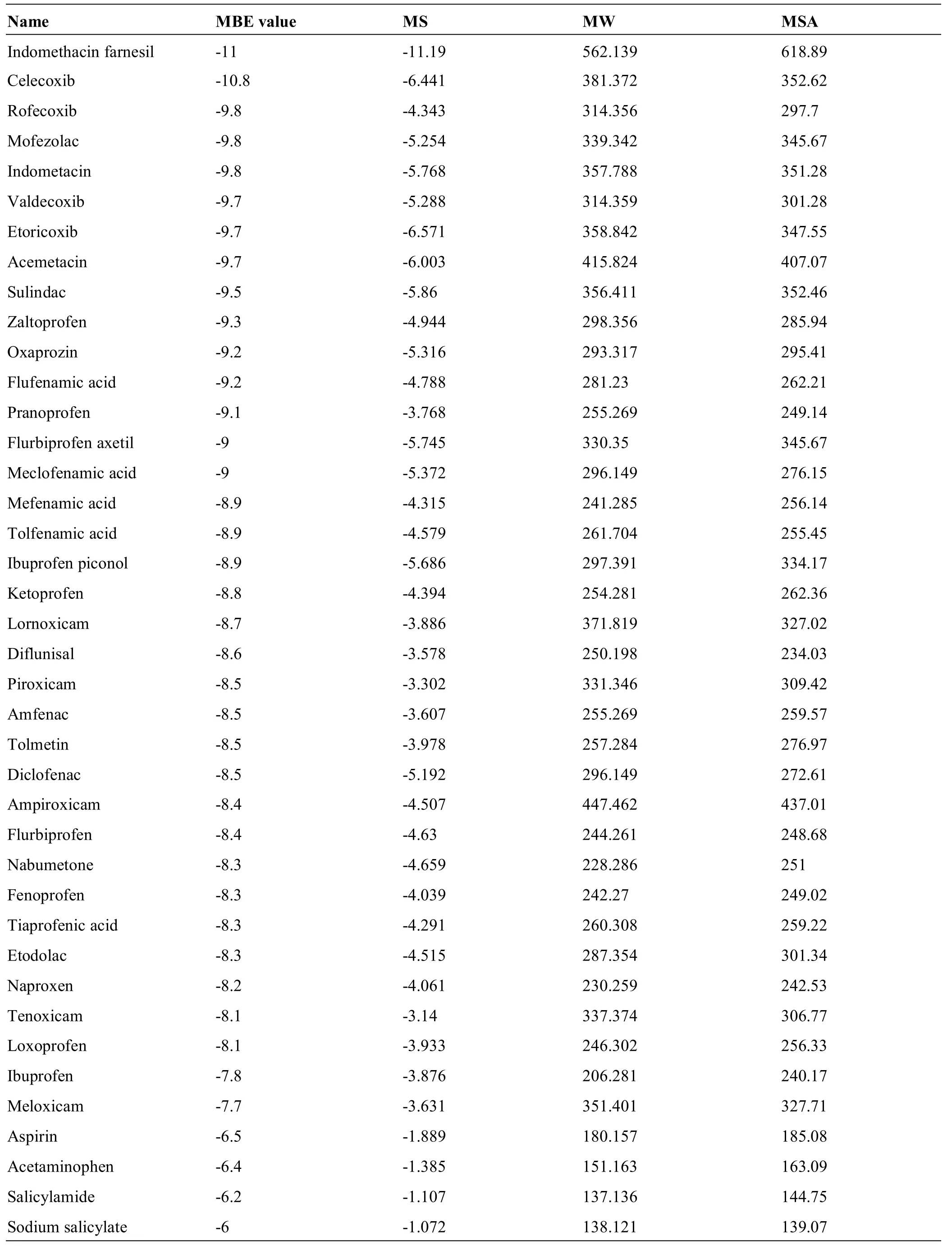
Table 4 Prediction of physicochem ical properties and binding energy with COX-2 of NSAIDs
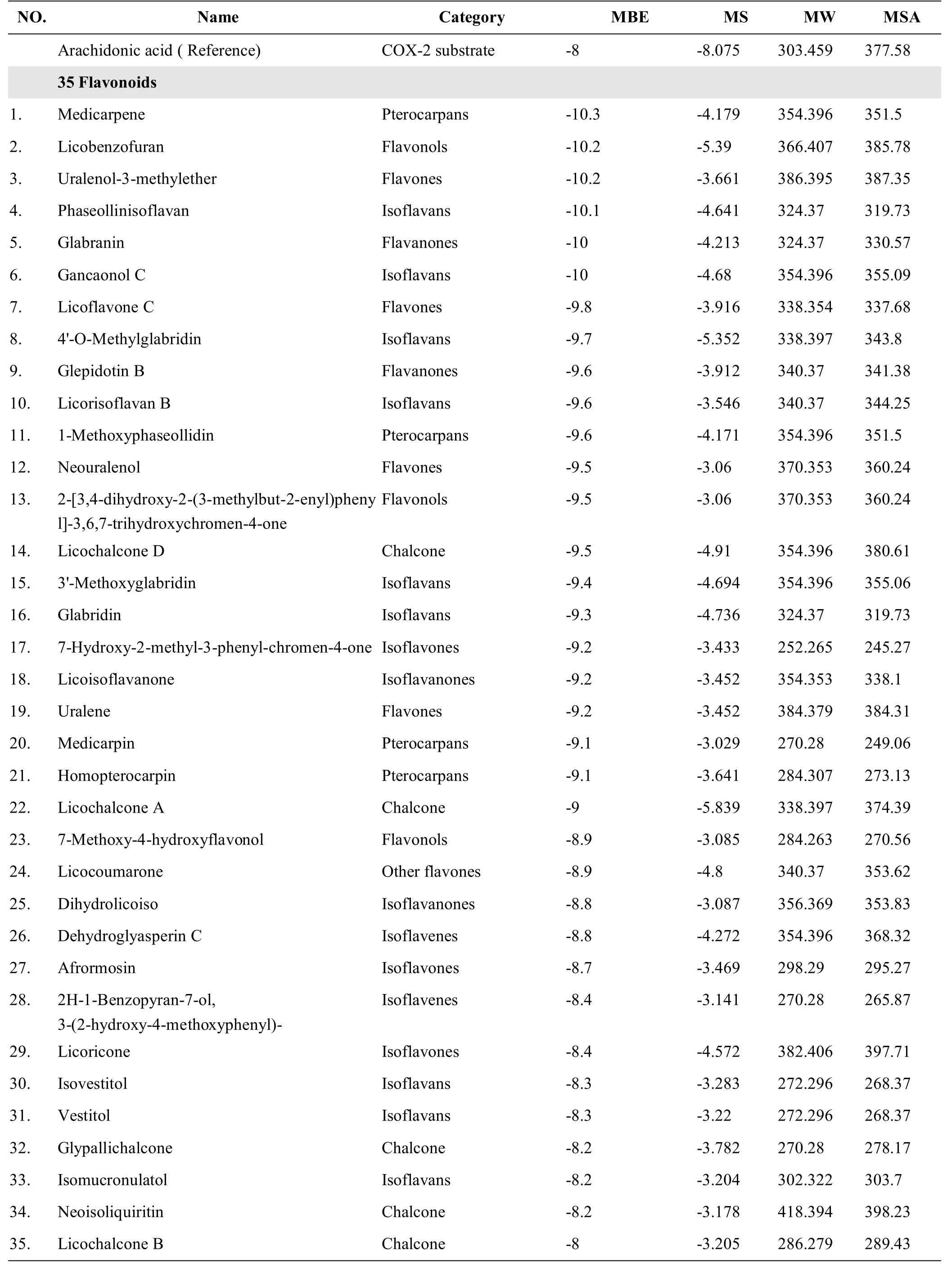
Table 5 The prediction of physicochem ical properties and binding energy with COX-2 of GC molecules including 35 flavone and 7 aromatic molecules with potential inhibitory effect on COX-2 enzyme
Continue
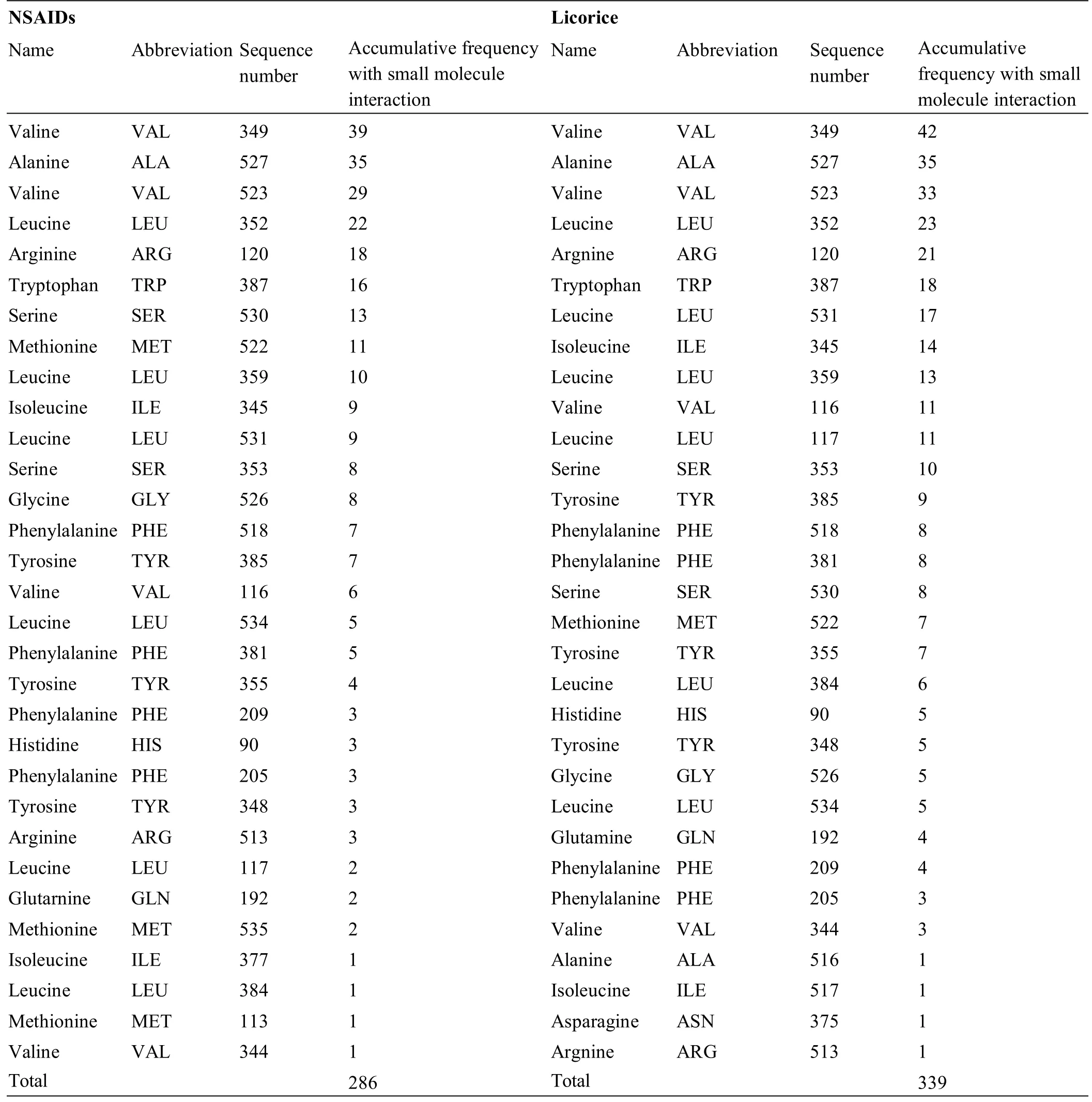
Table 6Am ino acid residues interacted with NSAIDsand GC compounds in COX-2 active site
We found that only 6 of the 42 small molecules(licochalcone A, glabridin, glycycoumarin,glypallichalcone,homopterocarpin,licochalcone B)were reported for the determination of their contents.The contentof licochalcone A was in the range of 0.01-14.47 mg/g,glabridin was 0.2-2.3 mg/g,glycycoumarin was 0.01-1.74 mg/g,glypallichalcone was 0.06-0.8 mg/g,homopterocarpin was 0.0006-0.09 mg/g,and licochalcone B was 0.08-2 mg/g.These data indicated that the role of GC was probably derived from the contents of these ingredients.
Frequency of interaction of small molecules with am ino acid residues at the activity center of COX-2
The selected 42 GC components and the 40 NSAIDs interacted with 31 amino acid residues in the active site of COX-2,and these amino acid residues were sorted according to the number of times of action(Table 6).The results showed that the names and sequences of the top 6 amino acid residues were the same between the 42 licorice components and the 40 NSAIDs;they were in the order of valine acid 349,alanine 527,valine 523,leucine 352,arginine 120,and tryptophan 387.Starting from the beginning of the seventh amino acid residue,the order of interaction between the small molecules and the amino acid residues used in both Chinese andWestern medicine was slightly different,but the roles of the amino acid residues were basically the same.These results implied that the 42 GC components had a potent inhibitory effect on COX-2.
Discussion
GC is one of the most commonly used Chinese herbal medicines in clinical practice,and our previous studies have shown that its main constituents are flavonoids,terpenoids/saponins(glycyrrhizic acid -glycyrrhizin,glycyrrhetinic acid),aliphatics,and aromatic compounds[17].Clinically,GC is widely used in the treatment of liver disease,gastrointestinal disease,enteritis,exogenous fever,cough,sore throat,chronic skin diseases,and food poisoning[18].
The COX enzyme has two isoenzymes,COX-1 and COX-2.COX-1 is a structural enzyme,which exists in many normal tissues and maintains the integrity of normal gastrointestinal mucosa.When it is blocked,it leads to serious adverse effects in the gastrointestinal tract.COX-2 is an inducible enzyme in most organs,and its level is significantly increased in inflammatory tissues[19,20].The expression of COX-2 increased when it was stimulated with both intracellularand extracellular stimuli,such as lipopolysaccharide,TNF-α,and interleukin-1β.COX-2 can promote the transformation of AA to prostaglandin G2.prostaglandin G2 is further converted to prostaglandin H2,followed by the formation of a series of prostaglandins including prostaglandin E2(PGE2),prostaglandin D2,prostaglandin F2α,and thromboxane,through the actions of synthetase and reductase,and through the processes of dehydration and isomerization[21].COX-2 is sustained and abundantly expressed in the acute phases and paracmasis of the entire inflammation process,and AA is converted to different prostaglandins at different stages of the inflammation process,from PGE2 in acute stage to prostaglandin D2 in paracmasis.The COX enzymes are prostaglandin H2-generated rate-limiting enzymes,having a direct impact on the role of prostaglandins and thromboxane in inflammation[22].Selective COX-2 inhibitors,such as rofecoxib,celecoxib,parecoxib,valdecoxib,and etherside,predominantly inhibit the production of COX-2,buthave little effects on COX-1, resulting in less side effects, such as gastrointestinal bleeding.In this study,we used software simulation systems(DS 4.5 and AutoDock Vina)to target COX-2,using NSAIDs as references and the crystal structure of COX-2 and AA(PDB ID:1CVU)as a template,to screen all the known active ingredients of GC;the active ingredients of GC obtained from our previous study.
First,we calculated the MW,MSA,and MS of the 40 NSAIDs and those of the 513 GC smallmolecules at the same time,and compared the physicochemical properties of the two groups.The results showed that aromatics were close to NSAIDs in terms of the three properties,followed by flavonoids,whereas the aliphatics and terpenoids/saponins differed from the NSAIDs in terms of these three properties.We then screened 118 GC small molecules that could bind to COX-2.We found that the binding energy values of half of the GC small molecules,obtained from their binding with the COX-2 protein,were lower than those obtained from their binding with the substrate,AA.Moreover,for the flavonoids and aromatics,the binding energy values obtained from their binding with the COX-2 protein were lower than those obtained from their binding with the AA.For the aliphatics and terpenoids,the binding energy values obtained from their binding with the COX-2 protein were higher than those obtained from their binding with the AA.
To screen accurately for the possible COX-2 inhibitor GC components,we compared the differences between the 118 GC small molecules and the 40 NSAIDs in terms of binding energy and physicochemical properties.The results showed that the three physicochemical properties of the 35 flavonoids and the 7 aromatics were close to those of the NSAIDs,and the binding energy value obtained from their binding with COX-2 was also lower than that obtained from their binding with the substrate,AA;the binding energy values were close to those of most NSAIDs,particularly those of the selective COX-2 inhibitors.Therefore,we predicted that the 35 flavonoids and the 7 aromatics in GC may have a better inhibitory effecton COX-2.
To verify the accuracy of our predictions,we checked the existing literature for research on GC ingredients and found a number of reports about licoflavone,licochalcone A,and epoxidizing enzymes.The study by Cui Yet al.showed that licochalcone A significantly reduced paw edema induced by carrageenan;licochalcone A could significantly inhibit both COX-2 activity and expression induced by lipopolysaccharide in murine macrophages[23].The study by Kwon HSetal.showed that licochalcone A could inhibit inflammatory reactions in macrophages and protectmice from endotoxin shock by suppressing the generation of nitric oxide and prostaglandin E2.In addition,it could inhibit the expression of inducible nitric oxide synthase and cyclooxygenase[24].The study by Furuhashi Ietal.showed that licochalcone A induces an anti-inflammatory effect through the inhibition of COX-2-dependent PGE2 production,but it had no effect on COX-1-dependent PGE2 production [25].Other reports showed the relationship between glabridin and COX-2.The study by Chandrasekaran CVetal.revealed that glabridin significantly inhibited PGE2,lipoxygenase,and COX of human neutrophils(HL-60),whereas isoliquiritigenin exerted inhibitory effect against only COX expression,but failed to suppress lipoxygenase expression[26].These reports verified our results,which were predicted with the computer simulation software obtained from third parties,indicating that the above predictions are credible.Based on our prediction,licochalcone A and glabridin were both among the 35 flavonoidswhich could inhibit COX-2.
The MBE,MW,MSA,and MS of licochalcone A and glabridin were very close to those of the following NSAIDs:zaltoprofen,oxaprozin,florfenic acid,and flurbiprofen.GC ingredients,licorice coumarin and homopterocarpin,were close to licochalcone A and glabridin in terms of the four properties indicated above.In addition,glypallichalcone and licochalcone B were close to the following NSAIDs,naproxen and ibuprofen,in terms of the four properties indicated above.The contents of the six GC ingredients mentioned above have been reported.The anti-inflammatory effects of most GC components are derived from these ingredients.Previous studies on the anti-inflammatory effects of GC components focused on steroids,such as glycyrrhizic acid(also named glycyrrhizin)and glycyrrhetinic acid[11-13].This study provides a material basis for the development of non-steroidalGC drugs.
Conclusion
From 513 natural components of GC,we identified 35 flavonoid molecules and 7 aromaticmolecules that could inhibitCOX-2 activity.Among these,licochalcone A and glabridin have inhibitory effects on COX-2,and glycyrrhizic acid(a terpenoid)has no inhibitory effect on COX-2.This conclusion has been verified by other studies. The three-step program, pharmacophore screening,molecular docking,and physicochemical properties analysis,is feasible.It can be used to screen for potential inhibitors of known targets from natural ingredients of traditional Chinesemedicine.
1.China Pharmacopoeia Comm ittee.Pharmacopoeia of the People's Republic of China,2015 edition.Medicine Science and Technology Press of China 2015.
2.Zhang MF,Shen YQ.Advances in studies on Glycyrrhizae Radix et Rhizoma and its active components in anti-inflammation and mechanism.Drugs Clinic 2011,26:261-267.
3.Pu JY,He L,Wu SY,et al.Anti-Virus Research of Triterpenoids in Licorice.Chin J Virol 2013,29:673-678.
4.Chen YH,Huang MJ,Wang WQ,et al.Comparative Study on Major Ingredient Contents and Effect of Depressing Transaminase of Licorice from Different Sources.Chin J Exp Tradit Med Formulae 2013,19:113-116.
5.Yu TF,Tian XD,Li R,et al.Antitussive and expectorant effects of licorice flavonoids,Extractum Glycyrrhizae and glycyrrhetinic acid.Chin patent drug 1993,15:32-33.
6.He D,Liu FQ,Li HD.Research Progress on detoxification of Glycyrrhiza uralensis.Central South Pharm 2009,7:927-931.
7.Gao XY,Wang WQ,Wei SL,et al.Review of pharmacological effects of Glycyrrhiza Radixand itsbioactive compounds.Zhongguo Zhong Yao Za Zhi2009,34:2696-2700.
8.Dong FY,Wang JJ.Anti-inflammatory mechanism of Glycyrrhetinic acid and its derivatives.J Dalian Med Univ 2014,36:195-197.
9.Guo HY.Clinical application of Radix glycyrrhizin.Clin Med 2010,30:109-111.
10.Wang CY,Kao TC,Lo WH,et al.Glycyrrhizic acid and 18beta-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-kappaB through PI3K p110delta and p110gamma inhibitions.J Agric Food Chem 2011,59:7726-7733.
11.Yu JY,Ha JY,Kim KM,et al.Anti-Inflammatory Activities of Licorice Extract and Its Active Compounds,Glycyrrhizic Acid,Liquiritin and Liquiritigenin,in BV2 Cells and Mice Liver.Molecules 2015,20:13041-13054.
12.Gujral ML,Sareen K,Phukan DP,et al.Antiarthritic activity of glycyrrhizin in adrenalectomised rats.Indian J Med Sci1961,15:624-629.
13.Huang QC,Wang MJ,Chen XM,et al.Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis?Oncotarget2016,7:1193-1202.
14.Tsao SM, Yin MC. Antioxidative and antiinflammatory activities of asiatic acid,glycyrrhizic acid,and oleanolic acid in human bronchial epithelial cells.J Agric Food Chem 2015,63:3196-3204.
15.Jia T,Qiao J,Guan D,et al.Anti-Inflammatory Effects of Licochalcone A on IL-1β-Stimulated Human Osteoarthritis Chondrocytes.Inflammation 2017,40:1894-1902.
16.Wang R,Zhang CY,Bai LP,et al.Flavonoids derived from liquorice suppress murine macrophage activation by up-regulating heme oxygenase-1 independent of Nrf2 activation. IntImmunopharmacol2015,28:917-924.
17.Yang M,Jin Y,Yang LP.A systematic summary of natural compounds in Radix Glycyrrhizae.Tradit Med Res 2018,3:82-94.
18.Wang Y,Qu CY,Peng XJ.Research progress in pharmacological studies of Glycyrrhiza uralensis Fisch and its derivatives.Gansu Med J 2011,30:398-401.
19.Li SJ,Fu ST,Wei W.Application of COX-1 and COX-2,5-LOX inhibitors in prevention and treatment of thrombotic diseases and vascular inflammation.Central South Pharmacy 2005,3:111-112.
20.Chen KL,Chen G.Distribution and Action of Natural Cycloxygenase and Lypoxygenase Inhibitor in Traditional Chinese Materia Medica.J South Central Univ Nationalities2009,28:42-46.
21.Wu CY,Chi PL,Hsieh HL,et al.TLR4-dependent induction of vascular adhesion molecule-1 in rheumatoid arthritis synovial fibroblasts:Roles of cytosolic phospholipase A(2)α/cyclooxygenase-2.J Cellular Physiol2010,223:480-491
22.Yin HY,Zhou YH,Zhu MJ,et al.Role of mitochondria in programmed cell deathmediated by arachidonic acid-derived eicosanoids.Mitochondrion,2013,13:209-224.
23.Cui YM,Ao MZ,Li W,et al.Anti-inflammatory activity oflicochalcone A isolated from Glycyrrhiza inflate.Z Naturforsch C 2008,63:361-365.
24.Kwon HS,Park JH,Kim DH,et al.Licochalcone Aisolated licorice suppresses lipopolysaccharidestimulated inflammatory reactions in RAW 264.7 cell and endotoxinshock in mice.Mol Med 2008,86:1287-1295.
25.Furuhashi I,Iwata S,Shibata S,et al.Inhibition by licochalcone A,a novel flavonoid isolated from liquorice root,of IL-1β-induced PGE2 production in human skin fibroblasts.Pharm Pharmacol 2005,57:1661-1666.
26.Chandrasekaran CV,Deepak HB,Thiyagarajan P,et al.Dual inhibitory effect of Glycyrrhiza glabra(GutGardTM) on COX and LOX products.Phytomedicine 2011,18:278-284.
 Traditional Medicine Research2018年3期
Traditional Medicine Research2018年3期
- Traditional Medicine Research的其它文章
- The protective effect of Dendrobium officinale polysaccharides on photoaging fibroblasts by scavenging reactive oxygen species and promoting the expression of TGF-β1
- Polysaccharide extracts of Cirsium japonicum protect rat H 9c2 m yocardial cells from oxidative stress induced by hydrogen peroxide
- Study of dual-directional regulatory effect of Banxia(Pinellia ternata)and Huanglian(Coptis chinensis)drug pair on gastrointestinal movement of m ice
- Effect of alternate-day-fasting combined with Lingguizhugan Decoction on blood lipid profiles of hyperlipidem ic rats
