Jianpi Qingchang Decoction-containing serum regulates the autophagy of interstitial cells
Yan-Cheng Dai, Ya-Li Zhang, Lie Zheng, You-Lan Chen, Xuan Chen, De-Liang Chen, Zhi-Peng Tang*
1Institute of Digestive Disease, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai,China. 2Department of Gastroenterology, Traditional Chinese Medicine Hospital of Shanxi Province, Xi’an, Shanxi,China.
Background
Ulcerative colitis (UC) is an inflammatory bowel disease with unclear pathogenesis that causes inflammation and formation of ulcers in the colon and rectum. In the eastern countries, the incidence of UC is constantly rising [1, 2].The intestinal irritation symptoms, including belly pain,diarrhea, gas/bloating, and tenesmus, are all associated with the pathophysiological aspects of an intestinal motility disorder. Due to the disease’s long duration, and wide range of pathological changes and recurrences that can sometimes occur even during remission, it can seriously affect quality of life [3-5]. Improving bowel symptoms in patients with UC is one of the therapeutic options to achieve deep remission [6, 7].
In ancient times, UC was calledChangpi, which was first recorded inHuangdineijing, published during the Han Dinasty of China(in the third century B.C.).According to the theory of Chinese medicine, a deficiency of the spleen can affect the digestive function of the large intestine, leading to poor appetite, abdominal distension, weakness, and increased bowel movement frequency. In addition, damp-heat may impair the collateral vessels of the large intestine, leading the stool to contain pus and blood. Therefore, strengthening the spleen and eliminating damp-heat are considered the treatment principles of UC.
On the other hand, modern medicine has shown that the interstitial cells of Cajal (ICCs) act as the physiological pacemakers that create the bioelectrical slow wave potential that leads to smooth muscle contraction. ICCs have been accepted as useful targets for pharmacological intervention against the gastrointestinal motor component of UC [8, 9]. Further, defects in autophagy of interstitial cells may lead to impaired pathogenic bacteria clearance, which can contribute to persistent chronic inflammation; whereas excessive autophagy may lead to autoinflammatory disorders of the intestine [10, 11]. Therefore, targeting and inhibiting autophagy are also possible treatments for UC via mediating the host defense and limiting tissue damage.
Jianpi Qingchang Decoction (JQD) is a proven recipe established by Professor Tang Zhipeng and is used to treat UC patients with spleen deficiency and damp-heat syndrome, based on the syndrome differentiation used in Chinese medicine [12]. The herbal composition of JQD includes Huangqi (Hedysarum Multijugum Maxim, 30 g),Machixian (Portulacae Herba, 30 g), Dangshen(Codonopsis Radix, 15 g), Huanglian (Coptidis Rhizoma,3 g), Diyu (Radix Sanguisorbae, 15 g), Sanqi (Panax notoginseng, 6 g), Baiji (Bletillae Rhizoma, 3 g),Muxiang (Aucklandiae Radix, 6 g), and Gancao (licorice,6 g). Astragalus polysaccharide, a bioactive extract of Huangqi (Hedysarum Multijugum Maxim), has been shown to reduce NF-κВ DNA phosphorylation activity,downregulate TNF-α, IL-1β, IL-6, and IL-17 expression,restore the number of Treg cells, and inhibit IL-17 levels in Peyer’s patches in experimental colitis [13, 14]. Further,the ethanol extract from Machixian (Portulacae Herba),exhibited effective protection against dextran sulfate sodium-induced UC by inhibiting the oxidative stress response through modulating malondialdehyde, nitrogen monoxide, and superoxide dismutase activities, reducing the mRNA expressions of pro-inflammatory cytokines(TNF-α, IL-1β and IL-6), and reducing the protein expressions of TNF-α and NF-κB p65 [15]. Sanqi (Panax notoginseng) reversed the disordered ratio of VEGFA165/VEGFA121, and attenuated the impairment of microvessels in experimental colitis [16].
Our previous studies have found that JQD can be used to treat initial or mild UC, improving the local intestinal symptoms and general body state of patients with UC [17,18]. In addition, JQD has been shown to regulate intestinal motility in an UC mouse model by inhibiting the intestinal inflammatory cascade, reducing ICC autophagy, and regulating the ICC/smooth muscle cell network [19, 20]. Less than 20% of JQD-containing serum (JQD-CS) has been found to promote the proliferation and differentiation of ICCs [21]. Yet, the specific mechanism through which JQD regulates ICC autophagy remains unknown. The aim of this study was to investigate the effects of JQD-CS on the autophagy and the underlying mechanism.
Materials and methods
Preparation of JQD-CS
Specific pathogen-free Balb/c 6 to 8-week-old male mice,weighting 20 ± 2 g, were purchased from Shanghai SLAC Laboratory Anima Co. Ltd. [certificate No. SCXK (Hu)2012-0012]. All animals were randomly divided into two groups (n = 50 for each group): the normal control group and the JQD-treated group. The JQD-treated group received 0.02 mL/g body weight/day of JQD (0.4275 g JQD/mL) by gavage twice every day for 7 days. Mice in the normal control group received an equivalent volume of CMC solution. On the seventh day, one hour after intragastric administration, blood was sampled from the abdominal aorta, stored at 4oC for 2 hours, and then centrifuged at 3000 rpm for 10 min. The supernatant was collected, sterilized and inactivated at 56oC for 30 min,and stored at -80oC.
Cells culture and identification
Cells were obtained from specific pathogen-free Balb/c mice (9-12 days old) as described previously, and were cultured at 37oC in a 95% O2/5% CO2incubator in smooth muscle growth medium (SMGM; Gibco, Grand Island, NY, USA) supplemented with 2%antibiotics/antimycotics (Gibco, Grand Island, NY, USA)and murine stem cell factor (SCF; 5 ng/mL;Sigma-Aldrich, St. Louis, MO, USA) [22].
ICCs were identified using monoclonal rat anti-c-Kit monoclonal antibody (1:100, Abcam, Cambridge, MA,USA) and fluorescein isothiocyanate goat-anti-rat antibody (1:200, Invitrogen, Carlsbad, CA, USA), and observed under fluorescence microscope as described previously [23].
Cells viability assay
The viability of the ICCs was determined using the MTT assay as described previously [24]. The effect of different concentrations of JQD-CS on cell viability was assessed as the cell viability of treated cells compared with that of untreated control cells, which were arbitrarily assigned a viability of 100%.
Cell treatment
After 72 h in culture, the ICCs were divided into five groups: the blank group, culture solution; the rapamycin group, culture solution with 20 nmol/L of rapamycin(Selleck, Houston, TX, USA); the 5% and 20% JQD-CS groups, culture solution with rapamycin and 5% or 20%JQD-CS; and the 3-Methyladenine (3-MA) group, culture solution with rapamycin and 10 nmol/L of 3-MA (Selleck,Houston, TX, USA). The cells were treated for 48 hours before analysis.
Transmission electron microscopy
The cells were collected, washed with PBS, centrifuged at 1000 rpm for 10 min, fixed with 2.5% glutaraldehyde and 1% osmic acid, dehydrated with a gradient of ethyl alcohol and acetone, embedded and saturated in 50%ethyl alcohol for 15 min, 70% ethyl alcohol for 15 min,90% ethyl alcohol for 15 min, 90% ethyl alcohol and 90% acetone (1:1) for 15 min, 90% acetone for 15 min,then sliced into ultrathin sections (50 nm), and stained with 3% uranyl acetate and lead citrate. Transmission electron microscopy (TEM; Philips, Eindhoven,Netherlands) was used to observe the structure of ICCs and autophagosomes.
Western blotting analysis
Western blot analyses were performed as previously described [25]. The corresponding primary antibodies[c-Kit, microtubule-associated protein 1 light chain 3(LC3-II)], Beclin-1, phosphatidylinositol 3-kinase (PI3K),p-PI3K, protein kinase B (AKt), pAKt, mammalian target of rapamycin (mTOR), p-mTOR; used at a concentration of 1:1000) were purchased from Abcam (Cambridge, MA,United States).
Patch-clamp experiments
The conventional whole-cell patch-clamp technique was used to record L-type calcium currents. Patch-clamp pipettes were constructed from borosilicate glass capillaries (GC 150T-7.5, Clark Electromedical Instruments, London, United Kingdom) using a 2-stage puller (PP-83, Narishige, Tokyo, Japan). The resistance of the patch pipette filled with pipette solution (containing(mmol/L): KCl, 140; Na2ATP, 2.7; Na2GTP, 0.1; dis-salt,2.5; HEPES, 5; EGTA, 0.1; MgCl2•6H2O, 5; adjusted to pH 7.2 with Tris) was 3-5 MΩ. The isolated myocytes were transferred to a small chamber on the stage of an inverted microscope (IX-71 Olympus, Japan) for 10-15 min and were well-attached to the bottom of the chamber.Then, the chamber was continuously superfused with Ca2+-free physiological saline solution (containing(mmol/L): NaCl, 135; KCl, 5; HEPES, 10; CaCl2,2;MgCl2, 1.2; Glucose, 10; adjusted to pH 7.4 with Tris).An 8-channel perfusion system (L/M-sps-8, List Electronics, Germany) was used to exchange the perfusate. Current-Voltage curves were generated using voltage-clamp protocols consisting of a V hold at -80 mV,followed by a 3 s long pre-pulse at -50 mV to inactivate T-type Ca2+channels, and then by a series of 600 ms depolarizations from -70 mV to 60 mV in 10 mV increments. An EPC-10 amplifier (EPC Instrument,Germany) was used to record the whole-cell currents. All experiments were controlled by PatchMaster 2.52(Yushen Bio-Technique Co. Ltd., Shanghai, China) and performed at 25oC [26].
Cell cycle analysis
Cells (5 × 105) were seeded on a 60-mm dish and grown overnight. Then they were collected (1 × 106) and centrifuged at 1000 g for 5 min at 4oC. Pellets were washed with ice-cold PBS and fixed with 75% ethanol at-20oC for 24 h. The cells were washed twice with PBS to remove trace ethanol and resuspended in 1 mL PBS. They were then incubated with 5μL of ribonuclease A (RNase A; 10mg/mL) at 37 °C for 45min, and subsequently stained with 5 uL propidium iodide (10 mg/mL) for 30 min at 37 °C in the dark. Samples were analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA)[27].
Statistical analysis
The results are expressed as means ± standard deviations(± s) and analyzed using the SPSS18 software (SPSS Inc., IL, United States). APvalue < 0.05 was considered to indicate statistical significance. Histograms were generated using GraphPad Prism 5.0 (GraphPad Software Inc., CA, United States).
Results
Culture and identification of ICCs
As shown in Figure 1, clear morphological characteristics of ICCs were observed 48 h after cell collection. Briefly,a large number of cells were observed attached to the cell dish wall. Furthermore, c-Kit protein expression was found in most cells along with DAPI-stained nuclei. The cell bodies were triangular or spindle shaped, the nuclei were large, and adjacent cells formed a network-like structure with their processes.
JQD-CS promoted ICC proliferation
When the serum concentration was less than 20%, the OD value of ICCs increased over time and with increasing serum concentration. However, when the serum concentration was higher than 20%, the OD value decreased. Especially when the serum concentration was 60%, the growth of ICCs was severely inhibited (Figure 2). Hence, 5% and 20% treatment groups were chosen for future experiments.
JQD-CS suppressed ICC autophagy
The morphology of ICCs was uniform and a small number of autophagic vacuoles were observed in the blank group, whereas the presence of huge vacuoles,reducing organelles, chromatin margination, and many autophagic vacuoles were found in the rapamycin group.Significantly lower amounts of autophagic vacuoles were visible in the 5% JQD-CS and the 20% JQD-CS group than those in the rapamycin group; a more integrated ICC structure and significantly lower numbers of autophagic vacuoles were observed in the 20% JQD-CS group than those in the 5% JQD-CS group. The morphology of ICCs in the 3-MA group was similar to that of the blank group;a small number of autophagic vacuoles were observed(Figure 3).
JQD-CS regulated the PI3K/AKt/mTOR signal pathway
No significant differences in the expression of PI3K, AKt,mTOR, p-mTOR were found among groups (P> 0.05).The expression of LC-3II and Beclin-1 was higher (AllP< 0.001) and the expression of c-Kit, p-PI3K, and p-AKt was lower (P< 0.001,P< 0.001 andP= 0.003 respectively) in the rapamycin group than that in the control group. The expression of LC-3II and Beclin-1 was decreased (P =0.001,P =0.006) and the expression of c-Kit, p-PI3K, and p-AKt was increased (P =0.035,P =0.018,P =0.045) in the 20% JQD-CS group compared to that in the rapamycin group (Figure 4).
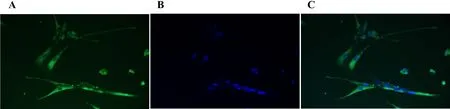
Figure 1 ICCs stained with c-Kit antibody (630/HP)
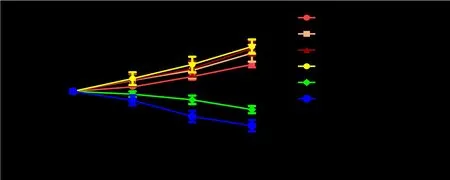
Figure 2 Effect of different concentrations of JQD-CS on ICCs proliferation
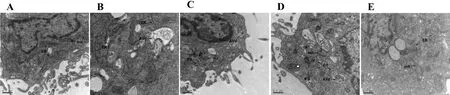
Figure 3 Transmission electron micrographs of ICCs and autophagosomes
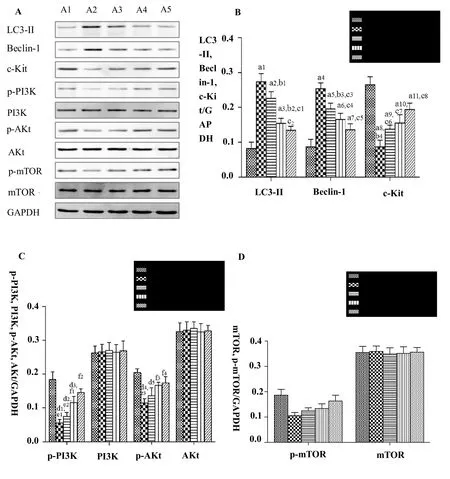
Figure 4 The protein expression levels of LC3-II, Beclin-1, c-Kit, PI3K, p-PI3K, AKt, p-AKt, mTOR,p-mTOR
JQD-CS inhibited the inflow of Ca2+
An inward current was induced by stimulation. The data from each group of cells were recorded, the original current densities were calculated, the I-V curves were drawn, and the maximum current density values (pA/pF)of Ca2+in the ICCs were analyzed. The pA/pF of Ca2+in the ICCs increased (P =0.001) in the rapamycin group compared to that in the control group. However,compared to that in the rapamycin group, the pA/pF was significantly decreased in the 20% JQD-CS group (P =0.032). Further, the pA/pF was significantly lower in the 5% and the 20% JQD-CS groups than that in the 3-MA group (P =0.009,P =0.003; Figure 5).
JQD-CS regulated the cell cycle
The number of cells in the G1 phase decreased (P= 0.015)and the number in the G2/M phase increased (P< 0.001)in the rapamycin group compared to those in the blank control group. However, compared to that in the rapamycin group, the number of cells in the G1 phase increased (P= 0.009) and the number in the G2/M phase decreased (P= 0.018) in the 20% JQD-CS group. Further,the number of cells in the G2/M phase was higher in the 20% JQD-CS group than that in the 3-MA group (P=0.001; Figure 6).
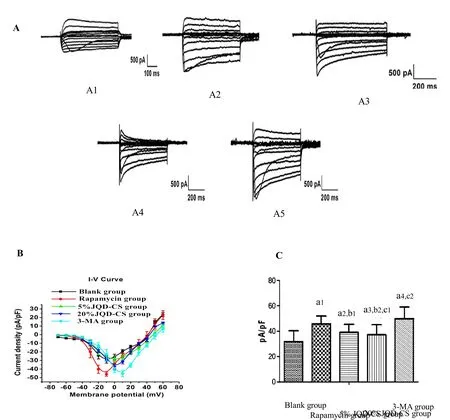
Figure 5 Whole-cell patch clamp recordings L-type calcium currents
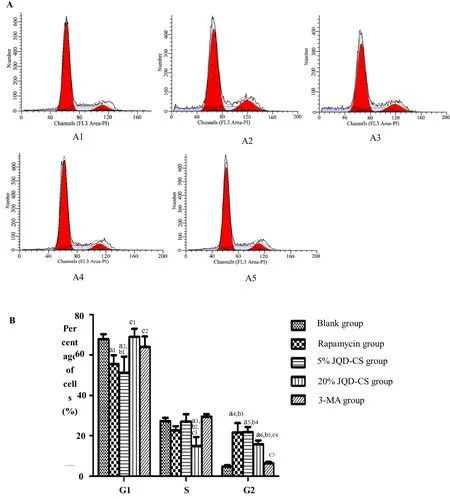
Figure 6 Flow cytometry analysis of the cell cycle in each group
Discussion
Autophagy is a conserved cell process indispensable for maintaining homeostasis of the intestinal microenvironment [27]. Many of the contents found in autophagosomes, such as protein aggregates and intracellular pathogens, threaten cell viability [28].Autophagosome formation is mediated by autophagy-associated genes (ATGs). ATGs are modulated by the PI3K/AKt/mTOR signaling pathway. The AKt protein combines with the corresponding structural domain of the activated PI3K. After being phosphorylated,the activated AKt promotes a series of biological activities; therefore, AKt phosphorylation is one of the main indicators for measuring the activation level of PI3K. mTOR is the protein factor downstream of PI3K/AKt, and is a negative regulator of autophagy. The PI3K/AKt/mTOR signal pathway plays an important role in intestinal epithelial cell metabolism, proliferation, and differentiation processes, and it also participates in repairing intestinal damage [29, 30].
Rapamycin is a lipophilic macrolide antibiotic which has the ability to arrest the growth of certain cells by inhibiting mTOR. In this study, the effect of JQD-CS on rapamycin-induced autophagy in ICCs was examinedin vitro. Our results show that, compared to the expression in the blank group, the expression of LC-3II and Beclin-1 increased and the expression of c-Kit, p-PI3K, p-AKt decreased in the rapamycin group, whereas the addition of 20% JQD-CS decreased the expression of LC-3II and Beclin-1 and increased the expression of c-Kit, p-PI3K and p-AKt. These data suggest that JQD-CS can antagonize the autophagy-inducing effect of rapamycin in ICCsin vitroby promoting phosphorylation of the PI3K/Akt pathway. Moreover, this antagonistic effect was dose-dependent to a certain extent.
Ca2+is one of the important regulators of the autophagy pathway, and its levels are controlled by the endoplasmic reticulum. An increase in stress on the endoplasmic reticulum can promote the expression of LC3-II on the autophagic vacuole membrane surface, and can promote protein degradation as well as other mechanisms that induce autophagy. Ca2+promotes autophagy through a Ca2+/Calmodulin-dependent protein kinase, which activates adenosine monophosphateactivated protein kinase (AMPK). Hence, AMPK induces autophagy by inhibiting of mTORC1 via the CaMKKβ-AMPK-mTOR pathway.
Raising Ca2+currents can activate autophagy, which can also be achieved through mechanisms involving CaMKKβ and AMPK [31-33]. The Ca2+flow signal in the blank group was probably that of the stable state of ICCs, as these ICCs were healthy and showed little sign of activated autophagy. However, the inflow of Ca2+increased in the rapamycin group. This might be one of the mechanisms involved in the induction of autophagy.The maximum Ca2+current density of the ICCs in the 20% JQD-CS group was lower after intervention with JQD-CS than that in the rapamycin group, indicating that JQD-CS can inhibit Ca2+inflow, and thereby regulate rapamycin-induced autophagyin vitro. The maximum Ca2+current density in the 3-MA group was higher than that in the rapamycin group. 3-MA is an autophagy inhibitor; however, the results suggest that its major role in inhibiting the formation of autophagic bodies does not depend on the regulation of the Ca2+inflow.
ICCs were arrested by rapamycin mostly in the G2 phase, which can disturb cell proliferation and differentiation. After JQD-CS intervention (especially with 20% JQD-CS), the ratio of ICCs within the G2 phase was reduced and the ratio of ICCs within the G1 phase increased. This shows that JQD-CS can oppose the cell cycle arrest initiated by rapamycin and promote the proliferation and differentiation of ICCs. However, the effect on the cell cycle was weaker than 3-MA.
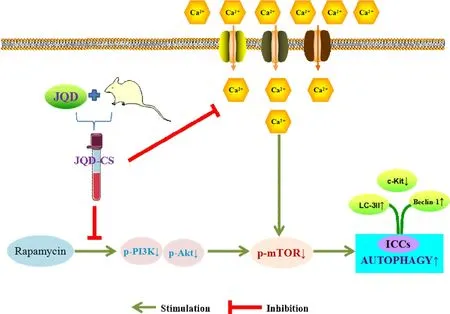
Figure 7 The protective mechanism of JQD on ICCs autophagy
Conclusion
JQD-CS can antagonize rapamycin-induced autophagy in ICCsin vitroby promoting phosphorylation of the PI3K/AKt pathway, regulating the cell cycle, and inhibiting Ca2+inflow. This may be a mechanism through which JQD inhibits the excessive autophagy of ICCs,thereby regulating the intestinal motilityin vivo(Figure 7).
1. Ng SC, Shi HY, Hamidi N,et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018, 390:2769-2778.
2. Hodson R. Inflammatory bowel disease. Nature 2016,540: S97.
3. Alrubaiy L, Cheung WY, Dodds P,et al.Development of a short questionnaire to assess the quality of life in Crohn’s disease and ulcerative colitis. J Crohns Colitis 2015, 9: 66-76.
4. Barbara G, Cremon C, Stanghellini V. Inflammatory bowel disease and irritable bowel syndrome:similarities and differences. Curr Opin Gastroenterol 2014, 30: 352-358.
5. Jonefjäll B, Öhman L, Simrén M,et al. IBS-like Symptoms in Patients with Ulcerative Colitis in Deep Remission Are Associated with Increased Levels of Serum Cytokines and Poor Psychological Well-being.Inflamm Bowel Dis 2016, 22: 2630-2640.
6. Hibi T, Panaccione R, Katafuchi M,et al. The 5C Concept and 5S Principles in Inflammatory Bowel Disease Management. J Crohns Colitis 2017, 11:1302-1308.
7. Harbord M, Eliakim R, Bettenworth D,et al.European Crohn’s and Colitis Organisation [ECCO].Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis.Part 2: Current Management. J Crohns Colitis 2017,11: 769-784.
8. Dai YC, Zhang YL, Tang ZP. Regulation of ICC Autophagy-New Target for Treatment of Intestinal Dysmotility in Ulcerative Colitis. Wei Chang Bing Xue 2015, 20: 377-379.
9. Dai YC, Tang ZP, Li K,et al. Research progress in intestinal motility during ulcerative colitis. Shijie Huaren Xiaohua Zazhi 2007, 15: 721-724.
10. Shen T, Li S, Cai LD,et al. Erbin exerts a protective effect against inflammatory bowel disease by suppressing autophagic cell death. Oncotarget 2018,9: 12035-12049.
11. Angelidou I, Chrysanthopoulou A, Mitsios A,et al.REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J Immunol 2018, pii:ji1701643. [Epub ahead of print]
12. Wang ZM, Dai YC, Zhang YL,et al. Data mining analysis on the medication rules of Ding’S Internal Medicine HUANG Wendong Schools on ulcerative colitis. Zhong Guo Zhong Yi Ji Zhen 2016, 25:1652-1655.
13. Lv J, Zhang Y, Tian Z,et al. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κВ activation. Int J Biol Macromol 2017, 98: 723-729.
14. Zhao HM, Wang Y, Huang XY,et al. Astragalus polysaccharide attenuates rat experimental colitis by inducing regulatory T cells in intestinal Peyer’s patches. World J Gastroenterol 2016, 22: 3175-3185.
15. Yang X, Yan Y, Li J,et al. Protective effects of ethanol extract from Portulaca oleracea L on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am J Transl Res 2016, 8: 2138-2148.
16. Wang SY, Tao P, Hu HY,et al. Effects of initiating time and dosage of Panax notoginseng on mucosal microvascular injury in experimental colitis. World J Gastroenterol 2017, 23: 8308-8320.
17. Dai YC, Zhang YL, Wang LJ,et al. Clinical presentation and treatment strategies for ulcerative colitis: A retrospective study of 247 inpatients. Chin J Integr Med 2016, 22: 811-816.
18. Dai YC, Zheng L, Zhang YL,et al. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis: A randomized controlled trial.Medicine (Baltimore) 2017, 96(16): e6651.
19. Zheng L, Zhang YL, Dai YC,et al. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol 2017, 23: 1180-1188.
20. Dai YC, Zheng L, Zhang YL,et al. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol 2017, 23: 4724-4734.
21. Dai YC, Zhang YL, Zheng L,et al. Effect of Jianpi Qingchang Decoction containing serum on proliferation of mice intestinal interstitial cells of Cajal in vitro. Yun Nan Zhong Yi Xue Yuan Xue Bao 2017, 40: 1-4.
22. Huang L, Du B, Gong Y,et al. Effects of Exogenous Interleukin-9 on the Growth, Proliferation and Activity of Interstitial Cells of Cajal Cultured in vitro.Digestion 2016, 94: 154-165.
23. Xu WD, Jiang X, Lan L,et al. Long-term culture and cryopreservation of interstitial cells of Cajal. Scand J Gastroenterol 2012, 47: 89-98.
24. Guo C, Yang M, Jing L,et al. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int J Nanomedicine 2016, 11: 5257-5276.
25. Zheng YY, Wang M, Shu XB,et al. Autophagy activation by Jiang Zhi Granule protects against metabolic stress-induced hepatocyte injury. World J Gastroenterol 2018, 24: 992-1003.
26. Cai ZX, Tang XD, Wang FY,et al. Effect of gingerol on colonic motility via inhibition of calcium channel currents in rats. World J Gastroenterol 2015, 21:13466-13472.
27. Wang H, Ye Y, Chui JH,et al. Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep 2010, 24: 647-651.
28. Lu XC, Tao Y, Wu C,et al. Association between variants of the autophagy related gene--IRGM and susceptibility to Crohn’s disease and ulcerative colitis:a meta-analysis. PLoS One 2013, 8: e80602.
29. Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell 2014, 54: 224-233.
30. Liu M, Kay JC, Shen S,et al. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 2015, 12: 151.
31. Noda T. Regulation of Autophagy through TORC1 and mTORC1. Biomolecules 2017, 7: E52.
32. Cui X, Luo Y, Li C,et al. Changes of intracellular Ca2+in quercetin-induced autophagy progression.Acta Biochim Biophys Sin (Shanghai) 2015, 47:908-914.
33. Grotemeier A, Alers S, Pfisterer SG,et al.AMPK-independent induction of autophagy by cytosolic Ca2+increase. Cell Signal 2010, 22:914-925.
34. East DA, Campanella M. Ca2+in quality control: an unresolved riddle critical to autophagy and mitophagy. Autophagy 2013, 9: 1710-1719.
 Traditional Medicine Research2018年4期
Traditional Medicine Research2018年4期
- Traditional Medicine Research的其它文章
- Long-term effect of Chinese herbal medicine Tianqi Capsule on the incidence of diabetes: an 8-year cohort study protocol
- Influence of astragalus polysaccharide on kidney status and fibrosis indices of a rat model of streptozotocin-induced diabetic nephropathy
- Study on alantolactone-induced differentiation of mesenchymal stem cells into vascular cells
- Research progress on anti-tumor properties of Marsdenia tenacissima
