Long-term effect of Chinese herbal medicine Tianqi Capsule on the incidence of diabetes: an 8-year cohort study protocol
Bing Pang, Chun-Yong Han, Qing-Hu He, Jing Liu, Qing-Wei Li, Yu-Jiao Zheng, Feng-Mei Lian*, Xiao-Lin Tong*
1Department of Endocrinology, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, China.
2Sinobioway Group Co., Ltd., Heilongjiang Tian Ren Pharmarceutical Co., Ltd, Heilongjiang, China. 3Hunan University of Traditional Chinese Medicine, Hunan, China.
Background
The pandemic of type 2 diabetes mellitus (T2DM) is becoming a major public health issue. In 2015, the total number of diabetic patients was estimated to be 415 million globally, which was predicted to increase to nearly 642 million by 2040 [1]. Effective interventions on impaired glucose tolerance (IGT) can prevent or delay the occurrence or development of T2DM [2]. Current guidelines recommend that IGT patients participate in lifestyle modifications, which have been shown to be effective for reducing the incidence of T2DM and increasing the regression towards normoglycemia [3-5].However, it was not easy to maintain rigorous and sustain lifestyle intervention for the long-term. Besides, although lifestyle intervention could reduce the incidence of T2DM during its limited period of use, but this action did not persist. The use of metformin and acarbose may also be considered alone with significant gastrointestinal side effects, while other oral anti-diabetic drugs and anti-obesity drugs are not currently recommended for diabetes prevention, although randomized controlled trials have shown effectiveness [6-7]. Traditional Chinese medicine (TCM) may bring a new choice for its management due to its characteristics of “preventive treatment of disease”. Some classical prescriptions and folk remedies with outstanding curative effect have been used for hundreds of years and some have been developed into modern medicinal preparations for the treatment of diabetes mellitus with evidence of therapeutic effects [8-9].
Tianqi capsule is an approved natural TCM compound in China (Approved No. Z20020089). It is composed of ten medicinal herbs, including Huangqi (Radix Astragali seu Hedysari), Huanglian (Rhizoma Picrorhizae),Tianhuafen (Radix Trichosanthis), Nüzhenzi (Fructus Ligustri Lucidi), Shihu (Herba Dendrobii), Renshen(Radix Ginseng), Gouqi (Fructus Lycii), Mohanlian(Herba Ecliptae), Wubeizi (Galla Chinensis), and Shanzhuyu (Fructus Corni). The Tianqi Capsule Diabetes Prevention Study (REDUCES Study) was a multicenter,randomized, placebo-controlled trial in people with IGT.Results revealed that Tianqi capsule reduced a 32.1%relative risk in the progression from IGT to T2DM and increased the likelihood of regression to normoglycemia during the active intervention phase [10]. Other studies also showed that Tianqi capsule improved insulin resistance [11-12].
However, whether the risk reduction achieved during the active intervention by Tianqi capsule will last after discontinuation of the intervention is not known. The 8-year follow-up of REDUCES Study is designed to assess the long-term effects of Tianqi capsule intervention on the diabetes incidence, weight change, and cardiovascular disease risk factors.
Methods/Study design
Details of the design and methods of REDUCES Study have been published previously [10]. Briefly, REDUCES Study investigated the effect of Tianqi capsule on preventing T2DM in subjects with IGT over the course of a twelve-month treatment. 420 adults with IGT were recruited and randomly allocated in a double-blind manner to receive Tianqi capsule or placebo for twelve months. 75 g oral glucose tolerance tests were conducted every three months to assess the development of T2DM or restoration to normal glucose tolerance, and all subjects received the same lifestyle education. After a one-year intervention, participants were informed of the final results.
Study design and objectives
Our trial is a longitudinal follow-up of REDUCES Study participants over 8 years. The design is a perspective observational study. The main objective is to assess the long-term effect of Tianqi capsule on the incidence of T2DM, weight change, and related cardiovascular disease risk factors after discontinuation of active intervention.The diabetes status will be defined by self-reported diagnosed diabetes plus evidence of raised glucose levels in the medical record, taking hypoglycemic medications,or Hemoglobin A1c, fasting glucose and a 75 g oral glucose tolerance tests, done every three months during the active intervention period and at the end of follow-up,interpreted using 1999 WHO criteria for diabetes. The trial protocol is registered at ClinicalTrials.gov, ID number: NCT02848053.
Study population
Four hundred and twenty subjects who have originally participated in REDUCES Study. Of this, 36 subjects in the Tianqi capsule group and 56 in the placebo group had developed diabetes at the end of the twelve-month trial.
Inclusion criteria
1. All study participants or relatives who provide information about deceased participants should write consent informed forms;
2. Subjects’ laboratory sheets can be acquired in order to judge diabetes status;
3. Subjects are not disabled, and can take care of themselves;
4. Subjects did not suffer the infectious disease.
Exclusion criteria
1. Subjects did not participate the REDUCES Study originally;
2. Subjects suffered serious mental illness;
3. Subjects suffered severe mental or cognitive impairment;
4. Subjects had a history of drug or alcohol dependence;
5. Subjects have participated in other drug clinical trials in the past 1 month.
Study methods and process
The flow diagram for this trial is presented in Figure 1.
1. Personal interview will be applied to identify the living status of subjects whose final diabetes status was IGT or normal glucose tolerance at the end of the REDUCES Study, for subjects whose diabetes status was available,results will be directly recorded.
1.1 To identify the living status of subjects, a variety of ways will be used: ①medical diagnosis on death; ②subjects’ hospital records or medical records; ③inquiry of participants themselves or their relatives; ④searching based on the address. By the ways above, subjects will be divided into three groups: dying group, living group and unknown group.
1.2 For deceased subjects, data were obtained by proxy interview from a living relative (spouse, sibling, or child)and medical record review. The cause of death should be investigated to judge whether deceased subjects have been diagnosed with T2DM or not.
2. Follow-up data for living subjects will be collected by personal interview and clinical examination and medical record review to determine diabetes status and date of diagnosis of diabetes.
2.1 To subjects that have determined the level of blood glucose, a variety of ways will be used to find the diagnostic evidence of diabetes, such as laboratory sheets on blood glucose, medical records review, the use of hypoglycemic medications or insulin, etc.
2.2 To subjects that have not determined the level of blood glucose, Hemoglobin A1c, fasting glucose and a 75 g oral glucose tolerance tests will be performed to judge diabetes status.
2.3 Special cases: ①for subjects who have moved, the interview will be done by phone and subjects can choose the local hospitals for clinical examination; ②in some centers, for subjects who can not participate in blood test in hospital, we will visit their home for clinical examination.
3. Questionnaires will be given to all the subjects or relatives to investigate the factors of diet and exercise,and the use of hypoglycemic medications and insulin,which probably affect the diabetes status during the 8-year of discontinuation of intervention.
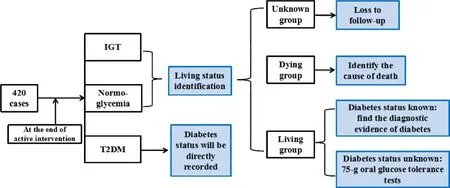
Figure 1 Flow diagram of study design
Outcome assessment
The exposure is set as participants taking Tianqi capsule during the active intervention, irrespective of the time and dosage. The primary outcomes are the incidence of T2DM between the Tianqi capsule group and the control group (refer to the guideline of the diagnosis of T2DM of WHO 1999). Numerical computation method: Incidence of diabetes mellitus = cases of diabetes mellitus / total cases of each group × 100%. The secondary outcomes are body mass index, blood glucose, blood lipid profiles including cholesterol, triglyceride, high-density lipoprotein and low density lipoprotein, and blood pressure.
Statistical analysis
The Cox proportional hazards regression model will be used to estimate the hazard ratio for the primary outcomes of diabetes incidence. Kaplan-Meier survival curves will be used to calculate to estimate the probability of remaining free of T2DM in the two groups.Participants who are lost during follow-up should be treated as censored observations. The difference between the survival curves will be tested with the log-rank test.Qualitative variables will be presented as frequencies, and quantitative variables will be summarized as means ±SD. Comparisons between groups will be done with the chi-square test of independence for qualitative variables,and t test for quantitative variables. The level of statistical significance was set atP< 0.05. Analyses will be done with SAS 8.2 software package.
Data collection and management
The data will be collected by the researchers using the paper version of the case report forms (CRFs), and then the data will be entered into the computer using Epidata software. Data entry will be completed twice to ensure accuracy. The source of any inconsistencies will be explored and resolved. During and after the research project, data will be locked up. Researchers should keep related information of participants strictly in this study.Capital forms, numbers or codes should be used rather than names of participants in CRFs or other documents.
Documents conservation
The documents such as CRFs, informed consent forms(ICFs), signature of participants and other cases are requested to conserve clearly according to Good Clinical Practice by every unit after the study.
Study quality control
Before the study, the trial protocol has been reviewed and revised by experts on diabetes, epidemiology,methodology and statistics several times. All the researchers belonging to the trial are asked to participate in a series of training to ensure that the personnel involved fully understand the study protocol and standard procedures for the study. This clinical trial is independent from sponsors and competing interests. During the study,the researchers will be responsible for ensuring the facticity, integrity and accuracy of all data when data are filled in CRFs. Researchers should check the uniformity and logic of data. Outcome assessment and data analysis will be independently performed without access to subjects’ group information (Peking University Health Science Center and Center of Endocrinology and Cardiovascular Disease, National Center of Cardiology &Fuwai Hospital, Beijing, China).
Ethics and dissemination
The protocol has been approved by the Medical Ethics Committee of Guang’ anmen Hospital of China Academy of Chinese Medical Sciences (approval number:2016-046-KY-01). The study will be conducted according to the principles of the Declaration of Helsinki.
ICFs have been reviewed and approved by ethics committees. We inform subjects relevant information in words and writing meanwhile by understandable language. All subjects will provide voluntary written ICFs after a full discussion about the potential benefits and risks before the study. ICFs will be signed by the subjects or their relatives with date. Signed ICF will be preserved by researchers and subjects independently.
The results will be disseminated through peer-reviewed journal articles and presented abstracts and posters at scientific conferences in the field of diabetes and TCM,as well as the general public through internet and newspaper.
Discussion
Diabetes is the major cause of kidney failure, blindness,and non-traumatic leg amputations in the world and a leading cause of cardiovascular disease and stroke,therefore, it represents a critical public health problem.Early interventions with lifestyle modification and/or anti-diabetic drugs on prediabetes can prevent or delay the occurrence or development of T2DM. After the studies ended and the interventions were stopped or reduced, there remained less participants that developed diabetes in the previously treated groups than that in the untreated control groups. Moreover, achieving normal glucose levels even transiently during the studies was associated with a persistent reduction in subsequent development of diabetes [2, 13-16].
Chinese herbal medicines have long been used in the prevention of T2DM. IGT is categorized as “spleen pyretic abundance” in TCM [17]. Tianqi capsule is a kind of traditional Chinese patent medicine (TCPM) widely used in China. Several large-scale clinical trials have been designed to assess the effects of Tianqi capsule on the prevention of T2DM in patients with IGT covered different population characteristics [10, 18-19]. Results indicated that Tianqi capsule reduced the risk of progression to T2DM, and increased the possibility of regression toward normoglycemia, but the evidence to support the long-term validity is not enough. Valuable information about the long-term effect of Tianqi capsule on the effect of cumulative development of diabetes between two groups will be collected. Furthermore, the extensive outcome assessment on cardiovascular disease risk factors will make it possible to explore the effect of Tianqi capsule on providing multiple therapeutic effects on multiple targets. In brief, this study will investigate the long-term effect of Chinese herbal medicine on preventing T2DM. The results should be helpful in increasing the cognition degree of TCPM in the field of preventing T2DM and should be generalisable to IGT population, especially in China. Many patients with IGT will be more likely to choose TCPMs because they are convenient to be administrated and taken along, as well as possessing the favorable effect on preventing or delaying the progression from IGT towards T2DM.
Our study has some limitations. One limitation is the absence of early diabetic microvascular complications as outcomes, such as microalbuminuria. Because we consider if patients are diagnosed with T2DM, the treatment will be complex, which makes it difficult for efficacy evaluation. Moreover, the intervention duration is one year, maybe which are not enough to lead to the effect on microvascular complications. Another limitation is that we will not investigate the effect of Tianqi capsule on all cause and cardiovascular mortality, incidence of cardiovascular event and stroke in these participants. In future studies, we are possible to address these issues.
Questionnaire
1. Which therapies do you use to treat IGT after the studies ended?
1) Lifestyle modification (diet or exercise);
2) Oral hypoglycemic drugs or insulin;
3) Continue to take Tianqi capsule;
4) Traditional Chinese medicine(Name:_________________)
5) None
2. Which ingredients three meals a day include? (two or more option)
1) Breakfast: □meat □egg □milk or soybean milk □vegetable or fruit □starchy foods (total quantitative intake: _________________);
2) Lunch: □meat □egg □milk or soybean milk □vegetable or fruit □starchy foods (total quantitative intake: _________________);
3) Supper: □meat □egg □milk or soybean milk □vegetable or fruit □starchy foods (total quantitative intake: _________________);
3. Which way do you frequently use for cooking?
□ steamed □saute □fried□stew □cooking;
4. How often do you eat fried food, meat or sweets in the past 12 months?
1) One to three times a week;
2) One to six times a month;
3) One to two times a day;
4) Almost every meal;
5) Seldomly eat;
6) Never eat;
5. Number of staple food__________;
6. Which kind of the following physical activity you do daily?
1) Very little physical activity;
2) Slight physical activity;
3) Moderate physical activity;
4) Severe physical activity;
7. Do you conduct regular physical exercise?
1) None;
2) Slight physical exercise (walking, tai chi);
3) Moderate physical exercise (jogging, dancing,badminton, bowling);
4) Severe physical exercise (running, swimming,gymnastics, basketball, football)
8. How often do you do exercise?
1) Less than 3 times a month;
2) Once a week;
3) Twice to three times a week;
4) More than four times a week;
9. How long do your physical exercise last?
1) Less than 15 minutes;
2) 15 to 30 minutes;
3) 30 to 60 minutes;
4) More than one hour;
10. Do you smoking?
1) Yes
2) No
3) Quit smoking (___years)
11. How many cigarettes do you smoke every day?
1) Less than 5 a day;
2) 5 to 10 a day;
3) 10 to 20 a day;
4) More than 20 a day;
12.Do you drinking alchohol?
1) Yes
2) No
3) Quit drinking alchohol (___years)
13. How much alchokol do you intake every day?
1) One tael a day;
2) Two to five tael a day;
3) Six to eight tael a day;
4) One catty or more a day.
1. International Diabetes Federation, IDF Diabetes Altas Globally, International Diabetes Federation, 7th edition 2015, http://www.idf.org/diabetesatlas.
2. Phillips LS, Ratner RE, Buse JB,et al. We can change the natural history of type 2 diabetes.Diabetes Care 2014, 37: 2668-2676.
3. Pan XR, Li GW, Hu YH,et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20: 537-544.
4. Tuomilehto J, Lindström J, Eriksson JG,et al.Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001, 344: 1343-1350.
5. Knowler WC, Barrett-Connor E, Fowler SE,et al.Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002, 346: 393-403.
6. American Diabetes Association. Standards of Medical Care in Diabetes-2016: Prevention or Delay of Type 2 Diabetes. Diabetes Care 2016, 39:S36-S38.
7. Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies.Diabetes Obes Metab 2014, 16: 719-727.
8. Ning G, Hong J, Bi Y,et al. Progress in diabetes research in China. J Diabetes 2009, 1: 163-172.
9. Grant, SJ, Bensoussan A, Chang D,et al. Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose.Cochrane Database Syst Rev 2009: CD006690.
10. Lian F, Li G, Chen X,et al. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: A double-blind, randomized,placebo-controlled, multicenter Trial. J Clin Endocrinol Metab 2014, 99: 648-655.
11. Zhong HF, Liang QL, Luo GA,et al.Simultaneous LC–UV–MS–MS analysis of nine pivotal metabolites in human serum: Application to studies of impaired glucose tolerance. Chromatographia 2011, 73:149-155.
12. Zhang SX, Sun H, Sun WJ,et al.Proteomic Study of Serum Proteins in a Type 2 Diabetes Mellitus Rat Model by Chinese Traditional Medicine Tianqi Jiangtang Capsule Administration. J Pharm Biomed Anal 2010, 53: 1011-1014.
13. Li G, Zhang P, Wang J,et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008, 371: 1783-1789.
14. Lindstr¨om J, Ilanne-Parikka P, Peltonen M,et al.Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention:follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368:1673-1679.
15. Knowler WC, Fowler SE, Hamman RF,et al.Diabetes Prevention Program Research Group.10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374: 1677-1686.
16. Gerstein HC, Mohan V, Avezum A,et al. DREAM On (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Ongoing Follow-up)Investigators. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes.Diabetologia 2011, 54: 487-495.
17. China Association of Traditional Chinese Medicine.Guideline for TCM Diabetes Prevention and Treatment. China, Beijing: Traditional Chinese Medicine Press of China 2007.
18. Sun XF, Qu KY, Huang HT,et al. Tianqi Jiang Tang capsule for prevention of type 2 diabetes mellitus: a randomized,double-blind,placebocontrolled study in Chinese individuals with impaired glucose tolerance.Chin J Diabetes 2011, 19: 433-436.
19. Wang YR, Tong XL, Xiao XH,et al. The therapeutic efficacy of Tianqi capsule intervention of patients with impaired glucose tolerance. Chin J Diabetes 2011, 19: 525-528.
 Traditional Medicine Research2018年4期
Traditional Medicine Research2018年4期
- Traditional Medicine Research的其它文章
- Influence of astragalus polysaccharide on kidney status and fibrosis indices of a rat model of streptozotocin-induced diabetic nephropathy
- Study on alantolactone-induced differentiation of mesenchymal stem cells into vascular cells
- Jianpi Qingchang Decoction-containing serum regulates the autophagy of interstitial cells
- Research progress on anti-tumor properties of Marsdenia tenacissima
