Influence of astragalus polysaccharide on kidney status and fibrosis indices of a rat model of streptozotocin-induced diabetic nephropathy
Yue Ji, Xue-Rou Yan, Hong-Tao Yang*, Kang Yang, Qing-Yun Zhao, Shou-Ci Hu, Qi-Hang Su
1Tianjin University of Traditional Chinese Medicine, Tianjin, China. 2The First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China.
Background
Diabetic nephropathy (DN) is a common micrangium complication of diabetes with high morbidity.Approximately 25 - 50% diabetes patients suffer from DN. These patients exhibit progressive loss of renal function, which can result in end-stage renal disease such as renal failure [1]. The pathogenesis and mechanism of DN are not completely clear. Recent studies showed that the dysfunction of energy metabolism and dystrophia can trigger metabolic memory, which inhibits the development and secretion function of pancreatic β cells,finally resulting in DN [2].
Traditional Chinese medicine (TCM) has been widely used to treat DN and has shown good effects. According to TCM theory, the etiological factor of DN is blood stasis, which causes disorders in body function, inhibits the production of Qi and blood, and triggers a deficiency of Qi. Qi deficiency can induce DN progression. Studies have suggested that Shuxuetong injection [extract of Shuizhi (Hirudo) and Dilong (lumbricus)] dramatically improves the blood glucose levels and renal function of DN patients [3]. Huangkui Capsule [extract of Huangkui(abelmosk)] improves microinflammation in DN patients[4]. Bailing Pills relieve microinflammation and oxidative stress in patients suffering from DN-induced chronic renal failure [5]. Additionally, Tangweikang Pill,including Huangqi (Radix Astragali seu Hedysari),Shuizhi (Hirudo), and Wuweizhi (Fructus Schisandrae Chinensis), are widely used in clinical practice [6, 7].Researchers have demonstrated that Tangweikang Pill up-regulate matrix metalloprotease-9 expression and down-regulate tissue inhibitor of metalloproteinases-1 expression in a kidney of Diabetic nephropathy model(DNM) rats [8, 9].
Huangqi (Radix Astragali seu Hedysari), which supplements Qi, activates circulation, and reduces blood stasis, is among the most important herbs for treating DN in the clinic [10, 11]. As mentioned in some classical ancient books of TCM, includingTang Ye Ben Caocompiled by Wang Haogu [12] andYi Zong Jin Jiancompiled by Wu Qian [13], the symptoms of DN are similar to those of “dispersion-thirst disease”,“hydroncus”, “urine turbid”, and “consumptive disease”in Chinese medicine, and the main pathogenesis of DN is due to the abnormality of Sanjiao (upper, or middle, or lower) [14]. Qi and Yin deficiency are manifestations of dysfunctions of the viscera or bowels. Huangqi (Radix Astragali seu Hedysari) has been applied in DN [15] to treat lung, spleen, liver, and kidney injury as well as enrich Qi and Yin, increase blood flow, and clear network vessels. Astragalus polysaccharide (APS) (Approved No.Z20040085) is one of the main highly active components in Huangqi (Radix Astragali seu Hedysari). In recent years, APS was found to have a wide range of pharmacological effects on the expression of insulin signaling molecules in the renal tissue of type 2 diabetic rats [16]. However, little is known about the factors influencing the mechanisms of the middle and late stage of DN. The aim of this study was to investigate the influence of APS on kidney status and fibrosis indices of rats with DN.
Materials and reagents
Animals and reagent
The following reagents were used: APS (Kekang Medical Science, Leqing, China); Streptozotocin and Coomassie brilliant blue (Sigma, St. Louis, MO, USA); Creatinine test kit (Beihua Kangtai Clinical Reagent Company,Beijing, China); Mouse anti-human α-SMA antibody(Dako, Carpinteria, CA, USA); Mouse anti-human TGF-β1 antibody (Santa Cruz Biotechnology, Dallas, TX,USA); PV-6001 immunostaining test kit(Zhongshanjinqiao Biology Science, Beijing, China);Trizol (Invitrogen, Carlsbad, CA, USA); A3500 transcription kit (Promega, Madison, WI, USA); and SD rats (Weitonglihua Company, Beijing, China).
Animals and treatment
Laboratory animals and feeding environment.Seventy-two healthy Sprague-Dawley male rats, with an average body weight of 180 ± 20 g at the age of 8 weeks,were housed at room temperature (23 ± 1°C) with a 12 h light and 12 h dark cycle (lights on from 6:00 am to 6:00 pm). Food and water were available ad libitum.Experiments were carried out according to the institutional regulations and national criteria for animal experimentation. The Institution Animal Ethics Committee reviewed the entire animal protocol prior to conducting the experiments. The experimental procedures conformed to directive 2010/63/EU of the European Parliament and all animals were handled in accordance with the guidelines of Tianjin University of TCM Animal Research Committee (TCMLAEC2014005).
Animal grouping and modeling.DN was induced by unilateral nephrectomy and single intraperitoneal administration of 65 mg/kg of streptozotocin (STZ) [17 -21], which was dissolved in sodium citrate buffer at pH 4.2 - 4.5. Control animals were injected with vehicle(sodium citrate buffer, pH 4.2 - 4.5). Rats were randomly divided into three groups: negative control group (NC, n= 24); DNM, n = 24; and DNM with APS group (DNM +APS, n = 24). DNM + APS group rats were administered APS (50 IU/kg/d) by subcutaneous injection from the first week after operation until death. NC and DNM group rats were subcutaneously injected with an identical volume of physiological saline.
Indicators and detection methods
General statement.At week 14 after operation, 6 rats from each group were randomly selected for measurement of body weight, kidney weight, and kidney index. Blood was collected by the inner canthus method for blood glucose biochemical testing (ACCU - CHEK,Indianapolis, IN, USA). The kidney index was calculated as follows: kidney index = kidney weight/body weight ×100%.
Kidney function and 24-h urinary protein.At weeks 3,8, and 13 after operation, 6 rats from each group were randomly sacrificed and blood was collected by the abdominal aortic method for serum creatinine (Scr) and blood urea nitrogen (BUN) biochemical testing. On the day before sacrifice, the rats were placed in metabolic cages for 24 h to collect urine, which was tested by the biuret reaction for 24 h urinary protein. Blood samples were centrifuged at 3000 rpm for 15 min and serum was collected and stored at -80 °C until analysis as described below. Scr, BUN, and urine total protein in 24 h were tested on a plate reader using an experimental reagent kit.
Pathological and immunohistochemical examination.As described above, after the blood and urine were obtained, all animals were sacrificed by cervical dislocation. The kidneys were fully exposed and separated carefully from the peripheral tissue and renal capsule. After making a longitudinal incision along the kidney tissues with a sharp blade and harvesting, 10%formalin fixation was conducted to detect pathological changes by hematoxylin and eosin (HE) staining and immunohistochemistry (IHC). Kidney tissues were then paraffin-embedded and cut into 5 μM sections for staining with HE, while expression of TGF-β1 and α-SMA was detected by IHC using the streptavidin-peroxidase method.
Statistical analysis
The data were analyzed with SPSS 13.0 software (SPSS,Inc., Chicago, IL, USA). All data were expressed as the mean ± standard deviation in the tables and indicated by vertical bars in the figures. Differences between groups were determined by analysis of variance. A probability value ofPless than 0.05 was considered significant.
Result
General characteristics
Body weight in the DNM group was significantly decreased compared to in the NC group (P= 0.001, Table 1). Body weight in the DNM + APS group was markedly increased compared to in the DNM group (P= 0.017,Table 1). The kidney index was higher in the DNM group than in the NC group (P= 0.001, Table 1). Moreover, the kidney index was decreased in the DNM + APS group compared to in the DNM group (P= 0.026, Table 1).Blood glucose was increased in the DNM group compared to in the NC group (P= 0.001, Table 1),whereas there was no obvious difference in blood glucose between the DNM + APS group and DNM group,indicating that APS alone to maintain stable blood sugar levels at week 14 is not effective.
Biochemical indices
There was no significant difference in the level of Scr and BUN or in the level of 24 h urine protein at different time points in each group (Table 2 - 3). Compared to the NC group, DNM group rats showed obviously higher BUN levels at various time points (P= 0.021,P= 0.012,P=0.008, respectively, Table 2). Compared to the DNM group, DNM + APS rats showed obviously lower BUN levels at weeks 3 and 8 (P= 0.017 andP= 0.015,respectively, Table 2). Compared to the NC group, DNM group rats showed obviously higher Scr levels at various time points (P= 0.033,P= 0.026,P= 0.028, respectively,Table 3). Compared to the DNM group, DNM + APS rats showed obviously lower Scr levels at week 8 (P= 0.021,Table 3), while no difference was observed at weeks 3 and 13. Compared to the NC group, DNM group rats showed significantly higher 24 h urine protein levels (P=0.021,P= 0.037,P= 0.019, respectively, Table 4) and after week 8, urinary protein increased dramatically (P=0.017, Table 4). Compared to the DNM group, DNM +APS rats showed significantly lower 24 h urine protein levels at week 13 (P= 0.011, Table 4).
Pathological findings
The glomerular structure was clear in the NC group and no obvious anomaly mesenchymal hyperplasia or thickening of the basement membrane was observed. The cortical and medullary renal tubular structures were normal, arranged in order, without inflammation and fibrosis (Figure 1A). Compared to the NC group, HE staining in the DNM group (Figure 1B) revealed hyperplasia of the glomerular mesangial matrix, atrophia of the kidney tubules, and thickening of the basement membrane. In some areas, focal glomerulosclerosis,Bowman’s capsule thickening and renal interstitial inflammatory cell infiltration, and fibrous proliferation were observed. These pathological changes compared to the DNM group were relieved to varying degrees in the DNM + APS group (Figure 1C). Moreover, the DNM +APS group showed that kidney tubule atrophia was decreased dramatically and renal interstitial fibrosis was not observed clearly in HE staining.
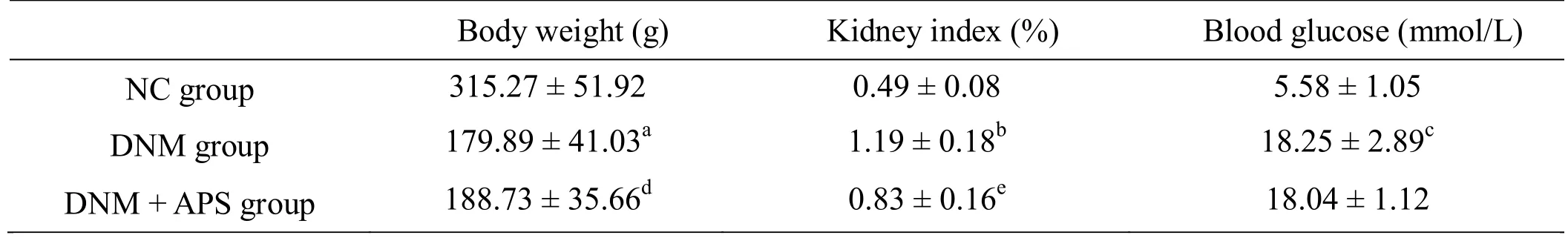
Table 1 General statement

Table 2 Blood urea nitrogen changes (mmol/L)
Table 3 Serum creatinine changes (μmol/L)
d 确认是和DNM还是DN比较?

Table 4 24 h proteinuria (mg/24 h)
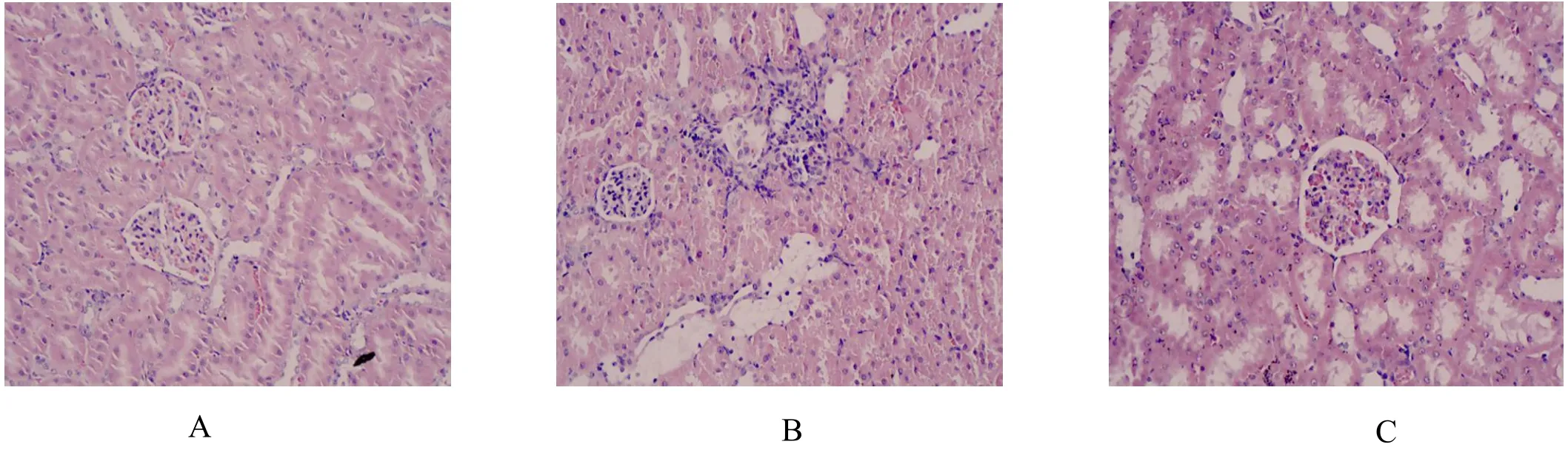
Figure 1 HE staining
Immunostaining effect of TGF-β1 and α-SMA in kidney tissue
The immunostaining result of TGF-β1 in the NC group(Figure 2A) revealed slightly positive expression in the renal tubular epithelial cells appearing as diffuse brown-yellow or brown particles and most glomeruli showed negative expression. Compared to the NC group,numerous brown-yellow positive granules were observed in the renal tubular and renal interstitial regions in the DNM group (Figure 2B), indicating that TGF-β1 expression in the kidney was higher than that in the NC group. Moreover, in the DNM + APS group (Figure 2C)the level of yellow granular positive reactants showed a slight loss of color and were decreased compared to those in the DNM group. In the NC group (Figure 2D), the positive expression of α-SMA appeared as a few of brown-yellow or brown particles mainly localized in the renal arterial vascular smooth muscle cells and occasionally in the renal tubules and renal interstitial.Compared to the NC group, more renal interstitial area was covered by brown-yellow or brown particles,indicating that the expression of α-SMA was enhanced in the DNM group (Figure 2E). Moreover, in the DNM +APS group (Figure 2F) the level of yellow granular positive reactants showed a slight loss of color and were decreased compared to in the DNM group.
Discussion
DN is the serious diabetic complication with clinical features of urine protein excretory rate increasing over time and renal hypofunction progressing to nephrosis in the terminal stage. Many diabetic pathological products,including catecholamines, endothelin, insulin-like growth factor-1, advanced glycation end-products, and TGF-β1,have been correlated with organ fibrosis. Previous studies indicated that the TGF-β1-induced signaling pathway exerts an important participatory role in renal fibrosis under certain renal pathological conditions [22]. In this study, we prepared an animal model of DN by uninephrectomy and streptozotocin injection [23]. Rats in the DNM group exhibited severe renal injury including increased levels of blood glucose and serum BUN and increased urine protein levels at 24 h, as well as a significant decrease in body weight. Additionally,pathology studies in the kidney revealed hyperplasia of the glomerular mesangial matrix, atrophia of the kidney tubules, and infiltration of inflammatory cells, indicating that DN in rats results in a large volume of proteinuria.The results of the current study indicated that APS, a component of TCM, down-regulates the expression of α-SMA and TGF-β1 in diabetic kidneys and plays an important role in the development of renal fibrosis associated with DN in mice.
Accumulation of extracellular matrix is a cause of glomerulosclerosis and tubulointerstitial fibrosis in the pathological process of DN, and TGF-β1 plays an important role in tubulointerstitial fibrosis. TGF-β1 may induce the adhesion and sedimentation of the intercellular matrix and inhibit cellular matrix degradation,contributing to tubulointerstitial fibrosis [24, 25]. Thus,inhibiting TGF-β1 may relieve DN progression. Jeremiah[26] conducted UUO rat experiments and showed that after removing an obstruction, renal tubular interstitial fibrosis was relieved and TGF-β1 expression was downregulated, supporting that inhibiting the high expression of TGF-β1 can relieve or delay renal tubule interstitial fibrosis.
In recent years, most studies of TGF-β have explored its signal transduction pathway. TGF-β can induce activation of a variety of signaling molecules, such as Smad proteins, mitogen-activated protein kinases, and phosphatidyl inositol 3 kinase/protein kinase B, etc., and the TGF-β1/Smad signaling pathway is a major inducer[27] of renal fibrosis. α-SMA is a cytoskeleton protein in eukaryotic cells that affects the microfilament structure of cells [28]. In normal renal tissue, the α-SMA is only expressed in the middle of the renal vessel and is rarely expressed in the glomeruli and renal interstitium. Under pathological conditions in the kidney, α-SMA can be expressed and proliferated around the glomerular mesenchymal region, surrounding area of the glomerulus,surrounding fibrosis area, and surrounding the blood vessels [29].
Fibroblasts can be transformed into myofibroblasts under some conditions, and the changes in the microenvironment may affect the transformation of fibroblasts into myofibroblasts. Not only transdifferentiation of renal tubular epithelial cell function, but also activation of renal interstitial fibroblasts leads to renal interstitial fibrosis, resulting in muscle fibroblast proliferation of activation. Many studies of kidney disease have confirmed that the number of muscle fibroblasts, α-SMA expression level and the degree of renal interstitial fibrosis are positively correlated with kidney disease progression. The marker protein α-SMA is an important indicator of renal interstitial fibrosis progression [30].
Other studies have reported that TCM have beneficial effects in treating DN. Chenet al[31] indicated that a mixture of Gegen (Radix Puerariae) and Shanzha(Fructus Crataegi) inhibits renal injury in type 2 diabetes via decreasing PI3K/AKt. Zhanget al[32] indicated that Shenkang granules ameliorate renal injury in a rat model of DN by preventing the accumulation of extracellular matrix, decreasing the expression of collagen I and III,and inhibiting the expression of matrix metalloproteinases-2 and -9 in the renal tissue.Particularly relevant to the present study are the recent findings that a combination of APS and another TCM extract, rhein, alleviate the pathologies of chronic renal failure, including functional damage to the glomeruli,interstitial inflammation, and apoptosis of renal tubular cells [33].
In the present study, we found that APS reduced the expression of α-SMA and TGF-β1in vivoand alleviated renal tissue injury. Compared to previous studies of TCM in DN, the protective effect occurred via a different mechanism, suggesting that a TCM compound therapeutic strategy for DN may be effective.
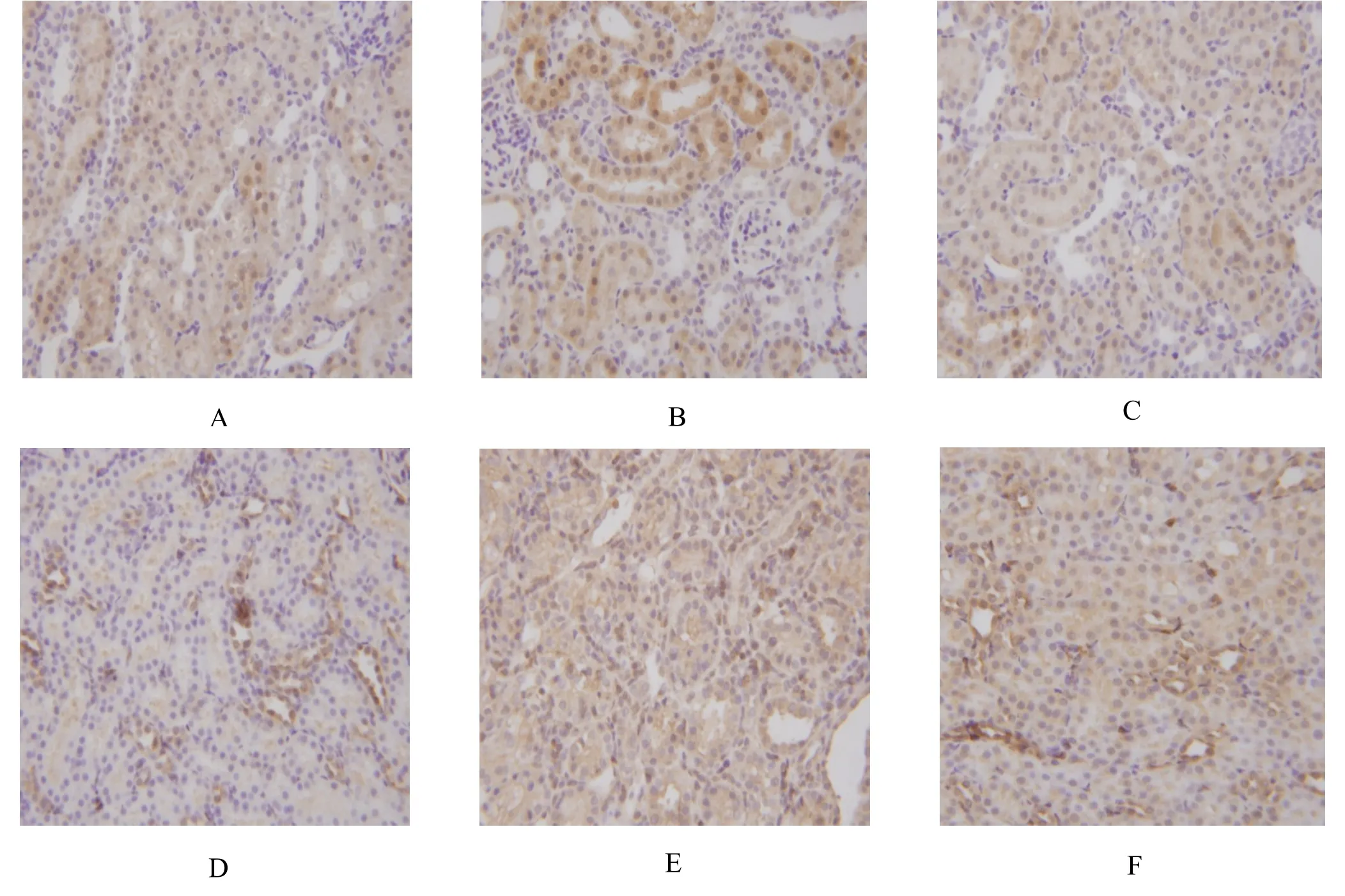
Figure 2 Immunostaining effect of TGF-β1 and α-SMA in kidney
However, the down regulation effect on the blood sugar were not observed after APS treatment. We predict that as the disease progressed (observation period: 13 weeks), using APS alone to maintain stable blood sugar levels over time is not effective. Previous experiments showed similar results [34, 35], however, further confirmation is required. In some clinical trials, TCM agents are prescribed as single agents, and coadministration of two or more agents (APS +metformin or APS + insulin) may be necessary in most patients.
Conclusion
In conclusion, we demonstrated that APS has significant effects on DN. APS not only significantly decreased the renal function damage in the initial stage and urinary albumin in the middle and late stage, but also improved renal tubular interstitial injury in DN rats. The mechanism may be related to downregulation of the expression of TGF-β1 and α-SMA and accordingly a delay in the progression of renal interstitial fibrosis in DN rats.
1. Pang GM, Yan Y, Zhu P,et al.Clinic draft specification of traditional Chinese medicine about diabetic peripheral neuropathy. Chin J Tradit Chin Med Pharm 2010, 2: 260-264.
2. Wang YZ, Zhang ZH. Mechanism of diabetic Nephropathy. Chin Rem Clin 2008, 8: 58-60.
3. Xiang HD. Pathological in diabetic nephropathy. Int J Endocrinol 2004, 24: 125-136.
4. Min XL, Lan LG. Effect of Shu-Tong injection on diabetic nephropathy. Shanxi J Tradit Chin Med 2008,29: 407-409.
5. Gao ZT, Wang G, .Effect of Huangkui capsule on micro-inflammation in diabetes patients. Chin J Integr Tradit West Nephrol 2011, 12: 1104-1105.
6. L Lin, Ni Q, Liu XM. Tang-Wei-Kang capsule reverse diabetic nephropathy in 132 patients. J Med Res 2008, 37: 46-50.
7. Wang HW, Qing N, Pang JL,et al. Study of the Rule about Chinese Medicine in Treating Diabetic Nephropathy. Chin Arch Tradit Chin Med 2008, 26:2365-2368.
8. Lin L, Guo L. Effect of TWK on Expression of MMP-9 in Renal Cortex in Streptozotocin - Induced Diabetic Rats. J Shanxi Coll Tradit Chin Med 2003, 4:8-18.
9. Lin L, Qing Q, Liu XM. Effects and mechanisms of Tang-Wei-Kang capsule protecting kidney function in diabetes rat. Chin J Chin Mat Med 2003, 28: 62-66.
10. Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kid-ney diseases.Nephrol 2005, 10: 48-56.
11. Alexopoulos E, Gionanlis L, Papayianni E,et al.Predictors of outcome in idiopathic rapi-dly progressive glomerulonephritis. BMC Nephrol 2006,7: 1-13.
12. Wang HG. Decoction and Material Medica. People’s Medical Publishing House, 1986.
13. Wu Q. The Golden Mirror Of Medicine. Chinese Medicine Press, 2002.
14. Ji Y, Li JC, Meng JY,et al.Study of dual-directional regulatory effect of Banxia (Pinellia ternata) and Huanglian (Coptis chinensis) drug pair on gastrointestinal movement of mice. Tradit Med Res 2018, 3: 148-156.
15. Ji Y, Wang TR, Zhang KX,et al.Literature analysis of the regularity and adverse reaction of cinnamon and its prescription preparations. Chin J Pharmacovigil 2018, 15: 163-168.
16. Zhou Y, Wu Y. Effects of astragalus polysaccharide on insulin signal transduction in renal tissue of type 2 diabetic rats. J Hubei Med Univ 2005, 18: 242-247.
17. Xu Y, Zhou SW, Tang JL,et al. Optimal selection of rat models of experimental diabetic nephropathy.Acta Acad Med Milita 2006, 28: 2247-2249.
18. Li ZJ, Zhang Y. The advances in research on animal model of diabetic nephropathy. Chin B Life Sci 2011,23: 90-95.
19. Yang F, Tang LQ, Wang FL,et al. Influential factors on the establishment of experimental diabetic nephropathy in rats. Anhui Med Pharma J 2012, 16:735-738.
20. Yaribeygi H, Mohammadi MT, Rezaee R,et al.Fenofibrate improves renal function by amelioration of NOX-4, IL-18, and p53 expression in an experimental model of diabetic nephropathy. J Cell Biochem 2018.
21. Zhao Y, Huang W, Wang J,et al. Taxifolin attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Am J Transl Res 2018, 10: 1205-1210.
22. Boukhalfa G, Desmouliere A, Rondeau E,et al.Relationship between alpha-smooth mu-scle actin expression and fibrotic changes in human kidney.Exp Nephrol 1996, 4: 241-247.
23. Hewitson TD, Becker GJ. Interstitial myofibroblasts in IgA Glomerulonephritis. Am J Nephrol 1995, 15:111-117.
24. Tang WW, Van GY, Qi M. Myofibroblast and alpha 1(III) collagen in experimental tub-ulointerstitial nephritis. Kidney Int 1997, 51: 926-931.
25. Iwano M, Plieth D, Danoff TM,et al.Evidence that fibroblasts derive from epithelium d-uring tissue fibrosis. J Clin Invest 2002, 110: 341-350.
26. Grupp C, Lottermoser J, Cohen DI,et al.Transformation of rat inner medullary fibroblasts to myofibroblasts in vitro. Kidney Int 1997, 52:1279-1290.
27. Ina K, Kitamura H, Tatsukawa S,et al.Significance of α-SMA in myofibroblasts emerge-ng in renal tubulointerstitial fibrosis. Histol Histopathol 2011, 26:855-866.
28. Murphy JT, Duffy SL, Hybki DL,et al.Thrombin-mediated permeability of human micr-ovascular pulmo-nary endothelial cells is calcium dependent. J Trauma 2001, 50: 213-222.
29. Satpathy M, Gallagher P, Lizotte-Waniewski M,et al.Thrombin-induced phosphorylati-on of the regulatorylight chain of myosin II in cultured bovine corneal en-dothelial cells. Exp Eye Res 2004, 79:477-486.
30. Birukova AA, Smurova K, Birukov KG,et al.Role of Rho GT pases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004, 67: 64-77.
31. Z Chen,Y Yuan,X Zou,et al. Radix Puerariae and Fructus Crataegi mixture inhibits renal injury in type 2 diabetes via decreasing of AKT/PI3K. Bmc Complementary & Alternative Medicine 2017, 17:454-458.
32. YU Zhang, N Zhou, H Wang, S Wang,et al. Effect of Shenkang granules on the progression of chronic renal failure in 5/6 nephrectomized rats.Experimental & Therapeutic Medicine 2015, 9:2034-2042.
33. Y Lian, L Xie, M Chen,et al. Effects of an astragalus polysaccharide, and rhein combination on apoptosis in rats with chronic renal failure [J]. Evid Based Complement Alternat Med 2014, 27:1862-1868.
34. Lai YN, Yu MH, Zhu QY,et al. Effect of astragalus polysoccharidde on TGF-β1 in renal tissue of diabetic rats. Fudan Univ J Med Sci 2002, 29:255-257.
35. Li ZJ, Zhang Y, Liu YM,et al. Effect of astragalus polysaccharin on expression of nephrin and podocin in podocytes of early diabetic nephropathy rats. Chin J Pathophysiol 2011, 27: 1772-1776.
 Traditional Medicine Research2018年4期
Traditional Medicine Research2018年4期
- Traditional Medicine Research的其它文章
- Long-term effect of Chinese herbal medicine Tianqi Capsule on the incidence of diabetes: an 8-year cohort study protocol
- Study on alantolactone-induced differentiation of mesenchymal stem cells into vascular cells
- Jianpi Qingchang Decoction-containing serum regulates the autophagy of interstitial cells
- Research progress on anti-tumor properties of Marsdenia tenacissima
