Study on alantolactone-induced differentiation of mesenchymal stem cells into vascular cells
Yan-Jiao Lu, Qiong Lu, Ruo-Ke Su, Gang Wang*, Rui Tan *
1School of Life Sciences and Engineering, Southwest Jiaotong University, Chengdu, China. 2National Engineering Research Center for Biomaterials, Sichuan University, Chengdu, China. 3College of Medicine, Southwest Jiaotong University, Chengdu, China.
Background
Traditional Chinese medicines (TCMs) have been long used for the treatment of ischemic lesions. Several monomers or the effective parts of TCMs and compound preparations play efficient role in the prevention and treatment of such lesions, such as tanshinone IIA derived from the root of Danshen (Salvia miltiorrhiza) [1, 2].Therefore, construction of a cell model for rapid screening may be helpful to find effective drugs for the treatment of ischemic lesions and may provide a preliminary basis for the study of the mechanism of TCMs.
Angiogenesis is a key phenomenon related to the improvement in ischemic disease and is characterized by the proliferation and migration of vascular endothelial cells, based on the existing capillaries or venules [3].Endothelial cells are ligated to form vascular rings and new basement membranes [4, 5], which are reconstructed to form new blood vessels. In the blood vessels, the endothelial cells and smooth muscle cells are the main constituents of the vascular wall [6]. Vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen [7], which may stimulate the migration and proliferation of vascular endothelial cells [8] and enhance vascular permeability [9]. α-Smooth muscle actin (α-SMA)is specifically expressed in the smooth muscles and serves as a marker of vascular arterialization and network [6, 10].
Stem cell transplantation is an advanced medical technology that may repair/replace damaged cells or tissues for the purpose of curing diseases. After transplantation in the body, the suitable drugs selected in vitro may be used to promote the differentiation of stem cells into vascular-related cells, resulting in the replacement of the damaged cells in the ischemic region.As a consequence, this process may promote vascular regeneration and rapidly alleviate ischemic lesions.Mesenchymal stem cells (MSCs) originate from the mesodermal pluripotent stem cells and are often isolated from the bone marrow. MSCs have the characteristics of attachment, self-renewal [11], low immunogenicity [12],and differentiation into all mesoderm cell-derived cells [13]and partial ectodermal cells (such as the differentiation into muscle cells, neurons, and epithelial cells) [14] in vitro and in vivo. These cells may release some growth factors or cytokines to promote endogenous angiogenesis through paracrine effects [15]. Many studies have shown that MSCs may be differentiated into endothelial cells or vascular smooth muscle cells following treatment with suitable stimulants [16-19].
Tumuxiang (Inula helenium) has traditionally been prescribed as a Chinese herb for chest and abdomen pain,which was first recorded inBencaogangmu Shiyi, which was published in 1765 A.D.Chinese medicinal compound preparations Ruyi Zhenbao pill and Ershiwei Chenxiang pill contain tumuxiang (Inula helenium) and exhibit excellent curative effects against cerebral ischemia [2].Alantolactone is one of the main active components derived from several medicinal plants such as tumuxiang(Inula helenium) and has been shown to possess strong anti-inflammatory and antioxidative properties [20].However, its role in the promotion of angiogenesis and the underlying mechanisms remain unclear. Previous studies have shown that simvastatin could significantly increase the transcriptional expression of the VEGF promoter in a stable cell line [21-25]. In addition, some studies have indicated that simvastatin has stimulatory effects on the proliferation, migration, and angiogenesis of umbilical vein endothelial cells in different environments [25] and may increase the proliferation of rat bone marrow mesenchymal stem cells (rMSCs) and secretion of VEGF[26]. Hence, simvastatin was used as a positive control in the present study to investigate the gene and protein levels of the angiogenic markers VEGF and α-SMA in rMSCs following the treatment with different concentrations of alantolactone.
Materials and methods
Culture of HEK 293-pVEGFp-Luc cell line
HEK 293 cells were purchased from the Cellbank of Chinese Academy of Science (Shanghai, China), grown in high-glucose Dulbecco's Modified Eagle's Medium(DMEM) (Hyclone, Logan, Utah, USA) containing 10%fetal bovine serum (FBS, Hyclone, Logan, Utah, USA)and 1% penicillin-streptomycin (Gibco, New York, USA),and maintained at 37 °C in a 5% CO2humidified incubator (Sanyo, Japan). Cells were transfected with the plasmid of pVEGFp-Luc in a 6-well plate using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)according to the manufacturer's protocol. Two days later,neomycin (Gibco, New York, USA) was added into the medium with the final concentration of 800 μg/mL to kill the untransfected cells. Seven days later, cells were diluted to 10 cm culture dishes so that they were completely separated to be single cells and sustained with 800 μg/mL neomycin. About 7 days later, clones derived from the single cells were gradually proliferated and the concentration of neomycin was decreased to 200 μg/mL thereafter. Six single-cell-derived clones were carefully picked out from the dish after trypsinization and cultured in independent dishes, generating the stable cell lines of HEK 293-pVEGFp-Luc. Chromosomes of the 6 stable cell lines were extracted and used as the templates for PCR to confirm the integration of the plasmid pVEGFp-Luc into the chromosomes. Consequently, expression of luciferase gene in the 6 stable cell lines were measured and compared using fluorescently quantitative RT-PCR as well as luciferase activity evaluation. The cell line with the highest expression level of luciferase was picked out for all the following experiments.
Cell proliferation-toxicity assay
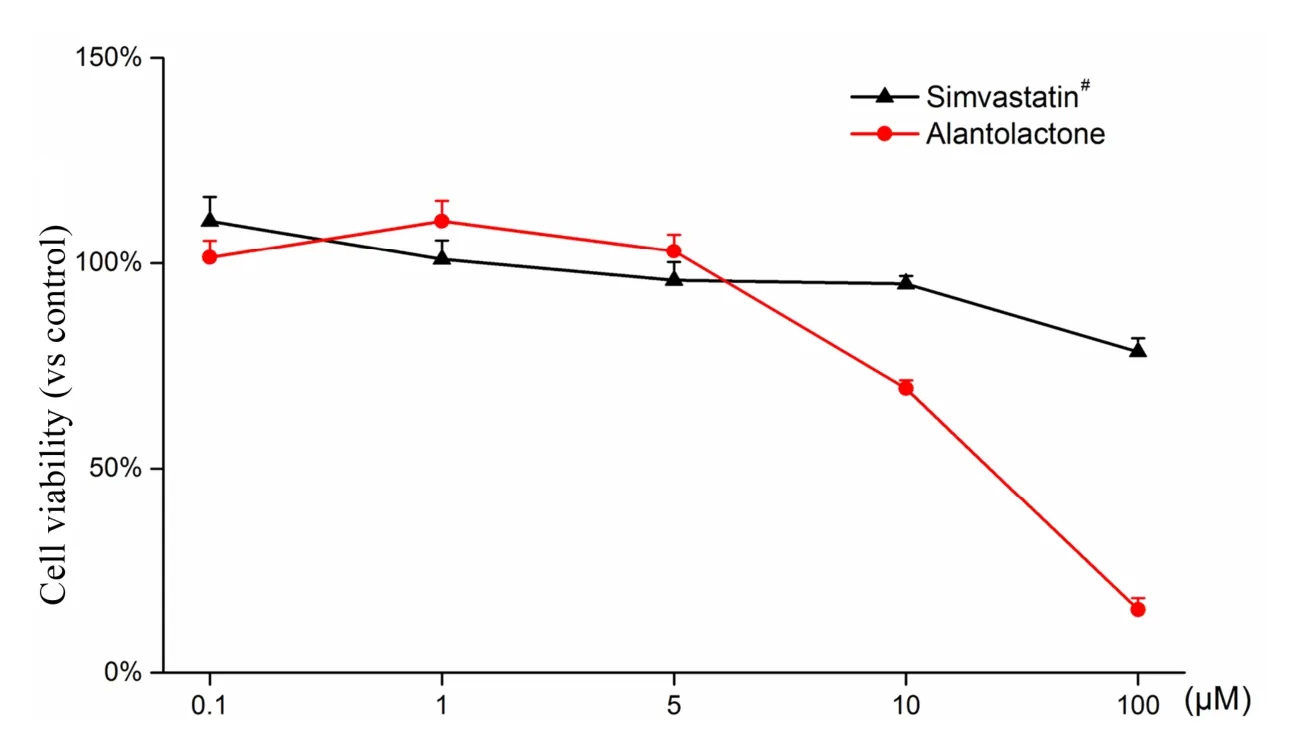
Cell counting kit-8 (CCK-8, Dojindo Laboratories,Kumamoto, Japan) was used to evaluate the toxicity of alantolactone in vitro. HEK-293-pVEGFp-Luc cells were seeded into 96-well plates. After cell attachment,high-glucose DMEM containing 10% FBS and 1%penicillin-streptomycin was used to dilute alantolactone(purity: high-performance liquid chromatography ≥ 98%,Shanghai Jinsui Biotech Co., Ltd. China) and simvastatin(positive control; purity: high-performance liquid chromatography ≥ 98%, Shanghai Jinsui Biotech Co., Ltd.China) to obtain final concentrations of 0.1, 1, 5, 10, and 100 μM. The experimental groups were treated with the drug-containing culture medium and the control group was treated with 0.1% dimethyl sulfoxide (DMSO, Biosharp,HeFei, China) in cell culture medium. The cells were incubated (37 °C, 5% CO2) for 24 h. Following incubation,the culture medium was aspirated and cells were treated with 100 μL of a reagent containing 10 μL of CCK-8 and 90 μL of high-glucose DMEM in the dark. The blank wells were treated with 100 μL of the mixture without cells.Cells were incubated (37 °C, 5% CO2) for 2 h in the dark.The multifunctional microplate reader (Varioskan Flash;Thermo Fisher, Waltham, MA, USA) was used to measure the absorbance (A) at a wavelength of 450 nm. Cell survival was determined as follows: Cell survival rate (%)= (Adrug−Ablank)/(Acontrol−Ablank) × 100%.
Luciferase transcriptional activity assay
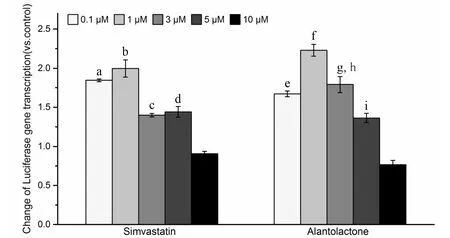
HEK-293-pVEGFp-Luc cells were seeded in a 96-well plate (Corning, New York, USA). The cells were allowed to attach. According to the drug safety concentration range,high-glucose DMEM containing 10% FBS and 1%penicillin-streptomycin was used to dilute simvastatin and alantolactone to obtain concentrations of 0.1, 1, 3, 5, and 10 μM. The experimental groups were treated with the drug-containing culture medium, while the control group was incubated with the culture medium containing 0.1%DMSO. The cells were incubated (37 °C, 5% CO2) for 24 h. Drug-activated VEGF promoter regulated the luciferase activity and the protein level was detected by the luciferase assay system with Reporter Lysis Buffer(Promega, Madison, WI, USA) and Pierce® BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The rate of change in the luciferase activity of the drug-treated group versus that of the control group (Udrug/Ucontrol× 100%, whereUdrugandUcontrolare relative luciferase activities with and without drug treatment, respectively) was compared.
Culture of rMSCs
Cells isolated from the tibial bone marrow cavity of SPF grade male SD rats (3-4 weeks; from Experimental Animal Centre of Sichuan Province, China) were used.Low-glucose DMEM containing 15% FBS and 1%xxxxxx penicillin-streptomycin was used to culture freshly isolated cells. After 48 h, the cells were rinsed once or twice with 1 mL of sterile 1× PBS and the culture solution was replaced with low-glucose DMEM containing 10% FBS and 1% penicillin-streptomycin.Cells were passaged once the fusion was between 80%and 90%. The third generation of rMSCs was used for experimental purpose.
Detection of the mRNA expression of vascular-related genes by RT-PCR and qPCR
The third generation of rMSCs with good morphologies were used for these experiments. Once the fusion reached between 80% and 90%, cells were inoculated into six-well plates and allowed to adhere. High-glucose DMEM supplemented with 10% FBS and 1%penicillin-streptomycin was used to dilute simvastatin(the positive control) and alantolactone at final concentrations of 0.1, 1, and 5 μM. The experimental groups were treated with the drug-containing culture medium, while the control group was incubated with the medium containing 0.1% DMSO. Cells were incubated(37 °C, 5% CO2) for 2 or 4 days. Following incubation,cells were lysed and the RNA extracted with Trizol(Invitrogen, Carlsbad, CA, USA). About 3 μg RNA was reverse transcribed and the first-strand cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kit (#K1622, Thermo Fisher, Waltham, MA,USA). Using cDNA as the template,β-actin(ACTB,reference gene),VEGF, andα-SMAtarget gene fragments were amplified by RT-PCR. PCR conditions were as follows: 98 °C for 2 min; 25 cycles of 98 °C for 10 s,62 °C for 10 s, and 72 °C for 10 s; and 72 °C for 5 min.We prepared 1.5% agarose gel and 4 μL of DL 1000 DNA marker and 5 μL of RT-PCR amplification products were subjected to electrophoresis at 80 V for 45 min. Gel analysis was performed to evaluate the specific amplification of the reference gene and the target gene fragment. Using cDNA as the template, PCR was performed on a real-time qPCR instrument and the relative expression of the mRNA of each gene was calculated. PCR program used was as follows: 95 °C for 10 min; 39 cycles of 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s; melt curve from 72 °C to 95 °C at an increment of 0.5 °C.ACTB,VEGF, andα-SMAgene primer sequences are shown in Table 1.
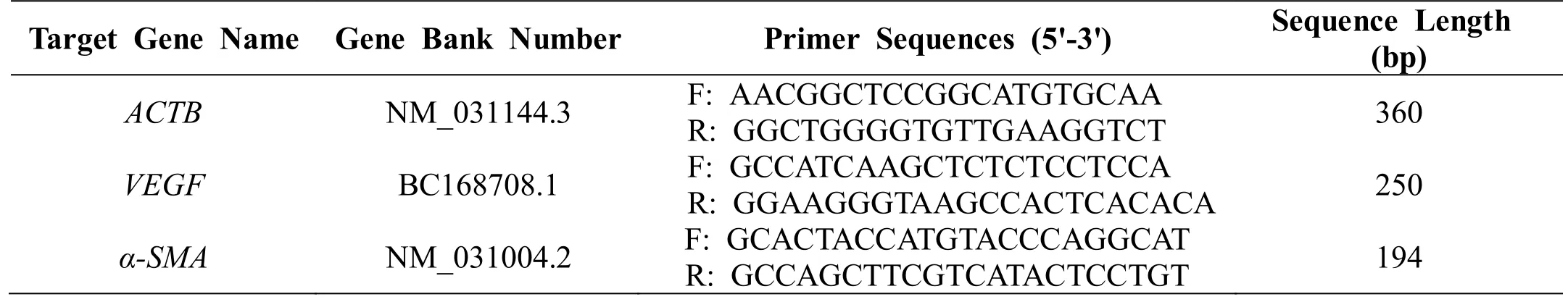
Table 1 ACTB, VEGF and α-SMA gene primer sequences
Immunofluorescence technique
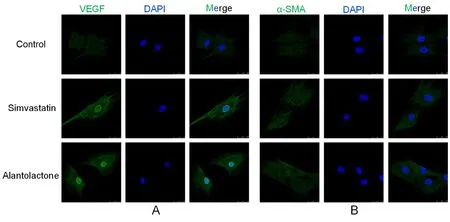
The third generation of rMSCs with good morphologies were used for the immunofluorescence study. Once the fusion reached between 80% and 90%, cells were seeded into 35-mm glass-bottomed dishes (Φ = 15 mm, NEST,Wuxi, China) and allowed to attach. Simvastatin and alantolactone were diluted to 5 and 1 μM, respectively,with low-glucose DMEM containing 10% FBS and 1%penicillin-streptomycin. The experimental group was treated with the drug-containing culture solution, while the control group was incubated with the medium containing 0.1% DMSO. After 3 days of induction, cells were fixed with 4% paraformaldehyde for 30 min, followed by their treatment with 0.2% Triton X-100 (Amersco, Ladner,PA,USA) for 15 min. The cells were blocked with 5% bovine serum albumin (Amersco, Ladner,PA, USA) blocking solution at 25 ℃ for 1 h and overnight incubated with a suitable primary antibody at 4 °C. Following incubation,cells were treated with appropriate secondary antibody(Table 2) at 37 °C in the dark for 30 min and incubated with 4¢,6-diamidino-2-phenylindole (DAPI; 100 ng/mL,Biofroxx, Germany) for 20 min. The samples were placed in 1 mL of 1× PBS in glass-bottomed dishes and the expressions of α-SMA and VEGF proteins were detected by confocal laser scanning microscope (DMIL 4000B,Leica, Germany).
During immunofluorescence staining, cells were rinsed thrice with 1× PBS buffer at each step except after the treatment with the blocking solution. Finally, 1 mL of 1×PBS buffer was added to the bottom of the glass-bottomed plate to prevent the cells from dehydration.
Statistical analysis
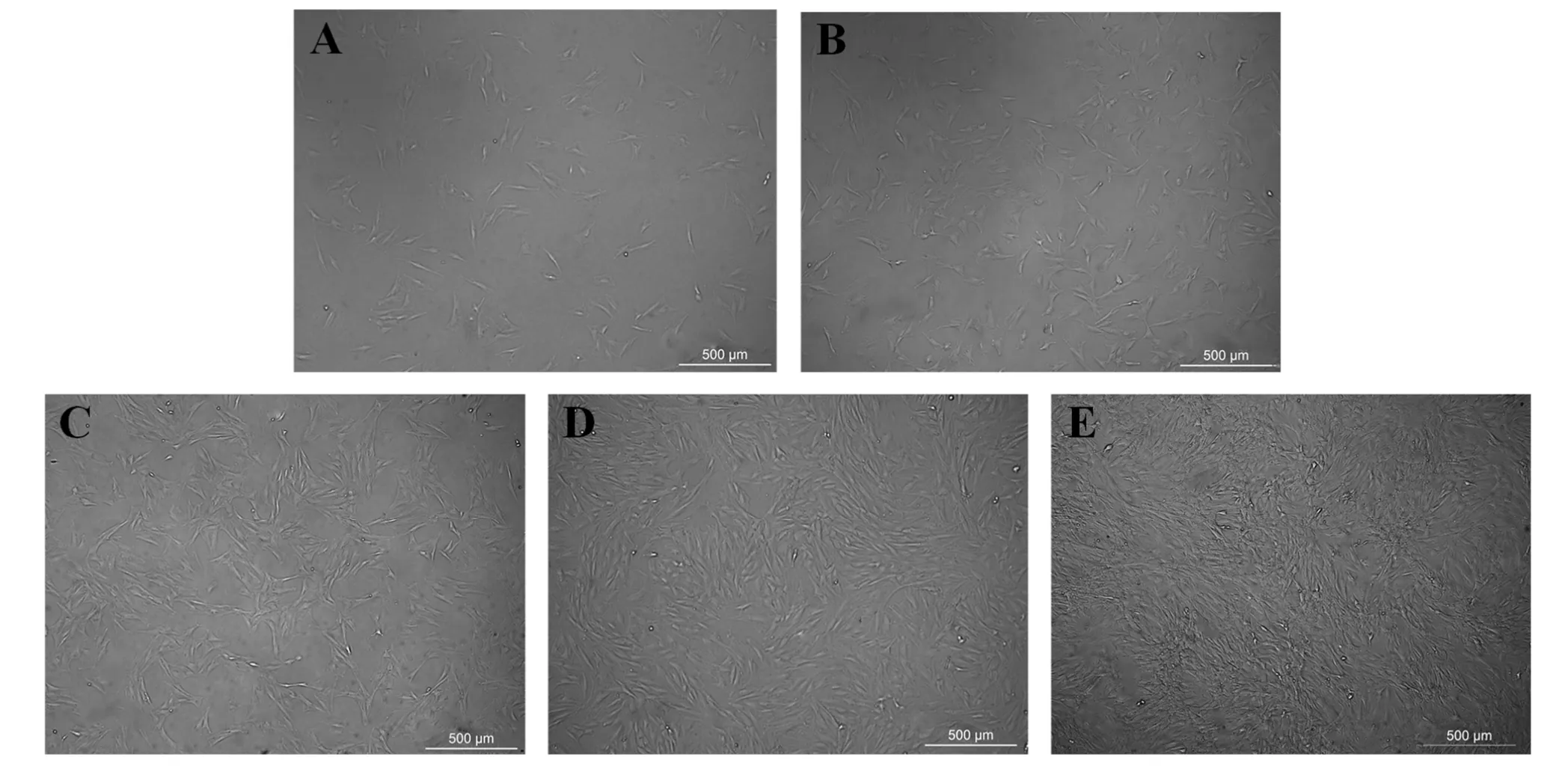
Three parallel samples were set up for each experiment.The qPCR data were analyzed by Bio-Rad CFX Manager 3.0 software. Experimental data were measured in the MS Excel 2016 software and are expressed as mean ±standard deviation. A value of 0.01 We used the CCK-8 method to evaluate the safety of different concentrations of alantolactone in vitro. The effects of different concentrations of simvastatin and alantolactone on the survival rate of HEK-293-pVEGFp-Luc cells are shown in Figure 1.Following stimulation with 10 μM alantolactone, the survival rate of cells was 69.49% ± 1.985% (n = 3). In comparison with the cells from the control group, the survival of those induced with 10 μM simvastatin was inhibited to a certain extent. The survival rate of cells was only 15.38% ± 1.280% (n = 3) after treatment with 100 μM alantolactone. However, 0.1, 1, and 5 μM of alantolactone and simvastatin at less than 10 μM concentrations had no inhibitory effects on the survival rate of cells. In summary, alantolactone at concentrations as high as 10 μM had no effect on the transcriptional activity of the reporter gene underVEGFpromoter in cells. Table 2 Information and dilution ratio of primary and secondary antibody Figure 1 Different concentrations of alantolactone on the survival rate of HEK 293-pVEGFp-Luc cell line#, Positive control, n = 3. In comparison with the cells from the control group, those treated with alantolactone and simvastatin at 0.1, 1, 3, and 5 μM concentrations showed a significant increase in the transcription of theVEGFpromoter that regulated luciferase expression in cells versus those in the control group, especially with the treatment with 1 and 3 μM of alantolactone and 0.1 and 1 μM concentrations of simvastatin (alantolactone,P= 0.001,P= 0.010 and simvastatin,P< 0.001,P= 0.010, respectively). (Figure 2).Moreover, 3μM of alantolactone showed better effect than that of 3 μM of simvastatin (P= 0.036). After 24 h of incubation, the primary rMSCs isolated from rats showed very few adherent cells. These adherent cells were mostly short, spindle-shaped, and slightly round in morphology. After 48 h of culture, the cells started showing small colony distribution and became longer and larger. With the prolongation of the culture time, the colonies gradually enlarged and the cells showed several forms such as long spindles, circles, and triangles. Once the cell density reached between 80% and 90%, the cell morphology gradually unified after subculture (Figure 3).The third generation of rMSCs showed spindle-like shapes.The cell density gradually increased and the cells gradually filled up the gaps with a further increase in the incubation time, followed by overlapping growth states(Figure 3E). Figure 2 Effects of different concentrations of alantolactone on the expression of VEGF promoter transcription Figure 3 morphology of rMSCs Figure 4 Electrophoretic bands of RT-PCR amplified products of ACTB, VEGF, α-SMA genes of rMSCs Figure 5 Immunofluorescence assay of rMSCs expressing The brightness of all the RT-PCR amplicons of the reference geneACTB(360 bp) was identical (Figure 4A-a and 4B-a). The band was located between 300 and 400 bp,as observed using the DL 1000 marker, and no miscellaneous band was observed. The target amplicons of angiogenic marker genesVEGF(250 bp) andα-SMA(194 bp) could be specifically amplified. The band was located between 200 and 300 bp (Figure 4A-b and 4B-b) and had an approximate size of about 200 bp (Figure 4A-c and 4B-c) without any miscellaneous bands. Thus, the designed primer sequences for the internal reference geneACTBand the angiogenic marker genesVEGFandα-SMAwere specific. Furthermore, the complete sequence information was available for the first strand of the synthesized cDNA. We detected the relative expression of the mRNAs of the angiogenic marker genesVEGF(Figure 4C) andα-SMA(Figure 4D) by qRCR following treatment of rMSCs with 0.1, 1, and 5 μM simvastatin and alantolactone at 1, 3, 5, and 7 days. rMSCs induced with 5 μM simvastatin or 1 μM alantolactone for 3 days showed a significant increase in the relative mRNA expressions ofVEGFandα-SMAgenes as compared to the cells from the control group. However, VEGF mRNA expression in 5 μM of simvastatin group showed higher level than that of 5 μM of alantolactone group at 3, 5, 7 day. α-SMA mRNA expression in 5 μM of simvastatin group also showed higher level than that of 5 μM of alantolactone at 1, 7 day. We induced rMSCs with 5 μM simvastatin and 1 μM alantolactone for 3 days. The in situ protein expression of the angiogenic markers VEGF (Figure 5A) and α-SMA(Figure 5B) was detected by laser scanning confocal microscopy. The results showed that the treatment of cells with 5 μM simvastatin or 1 μM alantolactone induced rMSC expression for 3 days showed similar intensity of green fluorescence, the FITC marker of rMSCs expressing VEGF and α-SMA proteins, but stronger intensity than that of the cells from the control group. In this study, we constructed an HEK-293 cell model usingVEGFgene promoter as the drug target. The cell model allows rapid and efficient screening of the vascular-induced active components from a large number of drug monomer molecules. We further used rMSCs to conduct a preliminary study evaluating the activity of alantolactone. We found 3μM of alantolactone has better effect on upregulating the transcriptional Luc gene activity ofVEGFpromoter than that of 3 μM of simvastatin. rMSCs induced with 1 μM of alantolactone or simvastatin showed similar upregulated expression ofα-SMAandVEGFmRNA. Moreover, the treatment of rMSC cells with 5 μM simvastatin or 1 μM alantolactone showed similar upregulated expression of α-SMA and VEGF protein. However, our result showed the effect of 5 μM of alantolactone on upregulating the mRNA expression ofα-SMAandVEGFgenes was weaker than that of 5 μM of simvastatin, which may be evaluated in the further vivo experiments. In summury, these results highlight the reliability of the highly efficient system for the screening of active drug molecules and confirmed the similar vascular induction function of alantolactone with simvastatin at the gene and protein levels. Angiogenesis plays an important role in physiological and pathological processes. Therefore, the regulation of angiogenesis plays an important role in the treatment of diseases caused by abnormal angiogenesis. TCMs exert significant effects on angiogenesis-related diseases such as cerebral ischemia. Of these, compounds such as caffeic acid from the root ofFusarium solanimay promote the formation of capillary buds in zebrafish SIV [27].Calycosin from Huangqin (Scutellaria baicalensis Georgi)may promote angiogenesis in human umbilical vein endothelial cells and zebrafish embryo and upregulate the expressions of VEGF, VEGF receptor-1, andVEGFreceptor-2 mRNAs [28]. Fibroblasts induced by the combination of small molecule compounds may be directly reprogrammed into functional neurons [29]. Therefore, the combination of TCMs and selection and screening of potential compounds may allow the use of two or several compounds for the induction of rMSCs,resulting in significant upregulation of vascular marker expression and promotion of vascular phenotype transition. This cell screening model and verification experiment may provide methods for the rapid screening and quantitative judgment of more drugs and verifying the potential of rMSCs to differentiate into blood vessels.The present report provides a theoretical basis for the study of rMSC transformation into vascular phenotype and for screening drugs that promote angiogenesis. 1. Zhou L, Bondy SC, Jian L,et al. Tanshinone IIA attenuates the cerebral ischemic injury-induced increase in levels of GFAP and of caspases-3 and -8.Neuroscience 2015, 288: 105-111. 2. Kang XL. Analysis of chemical constituents of three Tibetan medicine. Southwest Jiaotong university 2016. 3. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6: 389. 4. Hillen F, Griffioen AW. Tumour vascularization:sprouting angiogenesis and beyond. Cancer Metast Rev 2007, 26: 489-502. 5. Makanya AN, Hlushchuk R, Djonov VG.Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling.Angiogenesis 2009, 12: 113. 6. Wo BB. The effect of Copper Needle on the expression of α-SMA and NF-k Bp65 in Hemangioma and Histopathological Changes.Central South University 2009. 7. Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res 2006, 312: 527. 8. Olsson AK, Dimberg A, Kreuger J,et al. VEGF receptor signalling - in control of vascular function.Nat Rev Mol Cell Bio 2006, 7: 359-371. 9. Cao R, Bråkenhielm E, Pawliuk R,et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med 2003, 9: 604-613. 10. Liu R, Leslie KL,Martin KA. Epigenetic regulation of smooth muscle cell plasticity. Biochim Biophys Acta 2015, 1849: 448-453. 11. Dominici M, Le BK, Mueller I,et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2009, 8:315. 12. Ryan JM, Barry FP, Murphy JM,et al. Mesenchymal stem cells avoid allogeneic rejection. J inflamm 2005,2: 8. 13. Zhao W, Sarkar D, Ankrum J,et al. Therapeutic Applications of Mesenchymal Stem/Multipotent Stromal Cells. Humana Press 2011: 195-218. 14. Phinney, Donald G, Prockop,et al. Concise review:mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells 2007, 25:2896-2902. 15. Gnecchi M, Zhang Z, Ni A,et al. Paracrine mechanisms in adult stem cell signaling and therapy.Circ Res 2008, 103: 1204-1219. 16. Jianguo W, Tianhang L, Hong Z,et al. Optimization of culture conditions for endothelial progenitor cells from porcine bone marrow in vitro. Cell Prolif 2010,43: 418-426. 17. Reyes M, Dudek A, Jahagirdar B,et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Investig 2002, 109: 337-346. 18. Oswald J, Boxberger S, Jørgensen B,et al.Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22: 377. 19. Tang YL, Zhao Q, Qin X,et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 2005, 80: 236-237. 20. Wang X, Lan YL, Xing JS,et al. Alantolactone plays neuroprotective roles in traumatic brain injury in rats via anti-inflammatory, anti-oxidative and anti-apoptosis pathways. Am J Transl Res 2018, 10:368-380. 21. Lu Q. Construction and application of drug screening cell model for cerebral ischemia. Southwest Jiaotong university 2014. 22. Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci 2006, 101: 189-198. 23. Yamaji R, Fujita K, Nakanishi I,et al. Hypoxic up-regulation of triosephosphate isomerase expression in mouse brain capillary endothelial cells.Arch Biochem Biophys 2004, 423: 332-342. 24. Urbich C, Dernbach E, Zeiher AM,et al.Double-edged role of statins in angiogenesis signaling. Circ Res 2002, 90: 737-744. 25. Zhu XY, Daghini E, Chade AR,et al. Disparate effects of simvastatin on angiogenesis during hypoxia and inflammation. Life Sci 2008, 83: 801-809. 26. Chen JM, Zhang FM, Wang LS. Influence of simvastatin on the proliferation and paracrine functions of bone marrow-derived mesenchymal stem cells. Acta Univ Med Nanjing (Natural Science)2010: 20-24. 27. Liu CL, Cheng L, Kwok HF,et al. Bioassay-guided isolation of norviburtinal from the root of Rehmannia glutinosa, exhibited angiogenesis effect in zebrafish embryo model. J Ethnopharmacol 2011, 137:1323-1327. 28. Jing YT, Shang L, Zhen HL,et al. Calycosin Promotes Angiogenesis Involving Estrogen Receptor and Mitogen-Activated Protein Kinase (MAPK)Signaling Pathway in Zebrafish and HUVEC. Plos One 2010, 5: e11822. 29. Li X, Zuo X, Jing J,et al. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell Stem Cell 2015, 17:195-203.Results
Safety evaluation of alantolactone in vitro

Effects of different concentrations of alantolactone on the transcription of VEGF promoter
rMSC cell culture
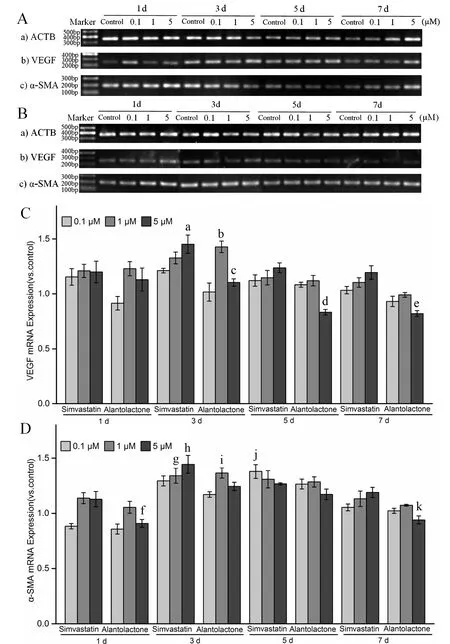
Detection of the relative expression of the mRNAs of vascular marker genes in alantolactone-induced rMSCs by RT-PCR and qPCR
In situ immunofluorescence detection in rMSCs expressing angiogenic marker molecules
Discussion
 Traditional Medicine Research2018年4期
Traditional Medicine Research2018年4期
- Traditional Medicine Research的其它文章
- Long-term effect of Chinese herbal medicine Tianqi Capsule on the incidence of diabetes: an 8-year cohort study protocol
- Influence of astragalus polysaccharide on kidney status and fibrosis indices of a rat model of streptozotocin-induced diabetic nephropathy
- Jianpi Qingchang Decoction-containing serum regulates the autophagy of interstitial cells
- Research progress on anti-tumor properties of Marsdenia tenacissima
