Pharmacological intervention of traditional Chinese medicine for the quality of life in patients with colorectal cancer: a systematic review and meta-analysis
Wen-Qi Huang, Zhu Yang, Dong-Xin Tang, Feng-Xi Long, Li Luo, Bing Yang, Juan Li, Jie Chen
1Guiyang College of Traditional Chinese Medicine, Guiyang, Guizhou, China. 2The First Affiliated Hospital of Guiyang College of Traditional Chinese Medicine, Guiyang, Guizhou, China.
Background
Colorectal cancer (CRC) is the third most common cancer in adults, as well as the third leading cause of cancer-related deaths. It causes over 600 thousands deaths worldwide, and approximately 123 million new cases are diagnosed every year with CRC [1, 2]. Currently, surgical treatment alone can achieve efficacy in curing patients with early-stage CRC. However, most patients with CRC were at an advanced stage or metastasis at the time of diagnosis. Thus, surgery may not be an appropriate treatment option and focus should be given to palliative treatment. Under such circumstances, the main objective of palliative treatment is to delay cancer progression,prolong lifetime, and improve the quality of life of patients [3]. For these patients, chemotherapy can be considered as the first-line therapy. Usually, the first-line chemotherapy regimen includes FOLFIRI or FOLFOX(irinotecan or oxaliplatin combined with fluorouracil and leucovorin), but it would bring some non-negligible toxicities that can affect the quality of life of patients [4,5].
As early as the third century B.C., the pathogeny of cancer was recorded in Huangdineijing, which is related to emotion and cold-evil. It was reported in the Sanguozhi that Hua Tuo treated cancer with surgical operation. Over the past few decades, the use of traditional Chinese medicine (TCM) has been paid increasing attention in the treatment of cancer. As one of the palliative and adjuvant therapies, TCM not only aims for tumor eradication, but also focuses on improving the vital-Qi of the human body,and reducing the possibility of recurrence and metastasis[6]. In addition, TCM plays an important role in reducing toxicity and enhancing the efficacy of radiotherapy and chemotherapy, thereby improving post-operative recovery,as well as quality of life in patients with CRC. This meta-analysis aimed to determine the efficacy of TCM in improving the quality of life in patients with CRC through a systematic evaluation.
Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Publication search strategy
Relevant randomized controlled trials (RCT) were searched from electronic databases, such as Cochrane Library, Pubmed, Embase, Medline, and Web of Science,from the time of establishment till July 2016, with no language restriction. The search keywords included TCM or Chinese herbal medicine and colorectal cancer,colorectal tumor, colon cancer or colon tumor and quality of life or life quality.
We performed screening by reading the abstracts of the selected literature, and then by reading the full texts to determine whether they meet the inclusion criteria.
Inclusion criteria
(1) Study design: RCT. (2) Study objective: The patients who were clinically diagnosed with CRC, regardless of gender, tumor stage, and means of tumor treatment. (3)Intervention: The experimental group used TCM combined with modern anticancer therapy (e.g.chemotherapy and surgery) or TCM alone. The control group used modern anticancer therapy alone as mentioned above or combined with placebo. (4) Outcome measures: Data including clinical efficacy, Karnofsky Performance Status (KPS) score of the quality of life,toxicities, and immunological test were compared between the experimental and control groups.
Exclusion criteria
(1) Patients who received non-drug treatment by means of TCM including acupuncture, Tai chi, and TCM concept.(2) The literatures that had incomplete information and unavailable outcome measures. Moreover, the literatures that had unclear outcomes and trial activities were not applied for data information.
Literature selection
Two authors performed preliminary screening based on the title and abstract of the obtained literature excluding repetitive literature. Full texts of the literature that may meet the inclusion criteria were downloaded, and further screening was performed by reading. The studies that met the inclusion criteria were adopted eventually.Disagreements were discussed and resolved with consensus or a third reviewer was asked to provide additional advice.
Literature data extraction
Data analysis was performed for literature data: (1) Basic information: author, title, publisher, publication year,sample size, and treatment methods (regimen) of the experimental and control groups. (2) Efficiency indicators:number of effective rate, KPS scores of quality of life,comparison of toxicities, and immunological tests.
Evaluation of risk of bias
The Cochrane risk of bias tool was used to evaluate the quality of the literature based on the following six aspects:selection bias (random sequence generation and allocation concealment), implementation bias (blinding method was implemented for the patients and the physicians), detection bias (blinding method was performed for evaluating the results, attrition bias(incomplete data), reporting bias (selective outcome reporting), and other biases (calculation methods of sample size, inclusion/exclusion criteria, comparability of baseline data, sources of funding, and other potential defects that may impact the overall assessment).Evaluation of risk of bias was performed based on the criteria mentioned above, including low, high, and unclear risks, and evaluation was performed for each criterion. If the literature met all the seven aspects discussed above, then it was considered a low risk of bias.If the literature did not meet even one of the seven aspects mentioned above, then it was considered a high risk of bias.
Data analysis
Meta-analysis was performed using Review Manager 5.3.Heterogeneity was performed for the literature obtained finally using I2and P. If I2< 50% and P > 0.1, then fixed effects model was used and if I2≥ 50% and P ≤ 0.1, then random effects model was used. Binary data were measured using odds ratio (OR) and 95% confidence interval (CI). Continuous data were measured using weighted mean difference (WMD) and 95%CI. If the evaluation tools were different, then standardized mean difference (SMD) was used to perform the measurement.The judgment of bias was by visual inspection of funnel plot asymmetry. Sensitivity analysis was used to investigate the impact of analysis of the fixed or random effects models on the results of heterogeneity and any other assumptions. The analysis involved scientific calculation for combination of effects and CI through different data models, followed by testing whether a significant difference was observed between the combined effects, and subsequent determination of sensitivity.
Results
Search results
A total of 222 articles were obtained through literature search, and of these, 35 duplicates were excluded. Based on the titles and abstracts, 162 articles were excluded, and a total of 25 articles were obtained. After reading the full texts and based on the strict evaluation criteria, 7 articles were excluded. Finally, 18 qualified articles were included in the meta-analysis (Figure 1) [7-24].
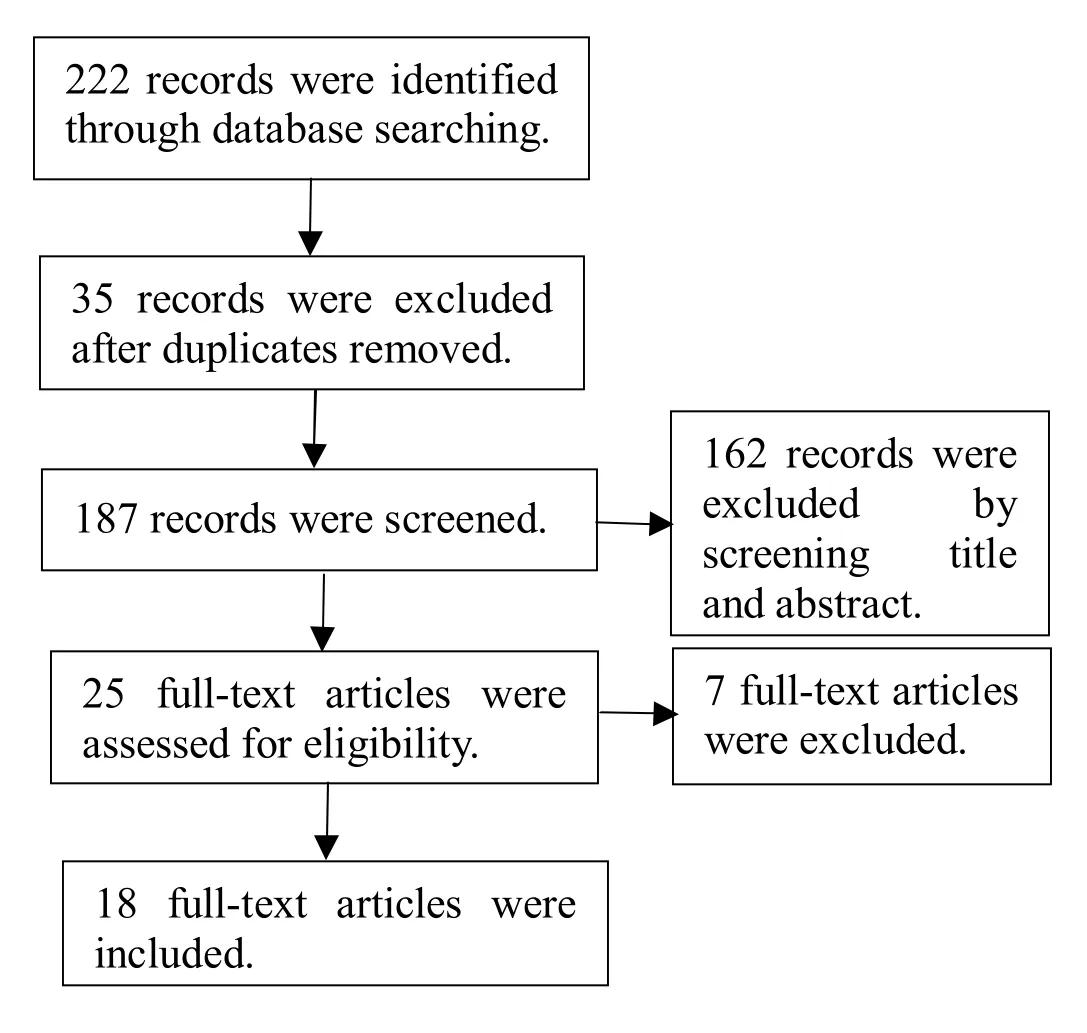
Figure 1 Flow chart
All the 18 articles were implemented and planned in China. Among them, 17 [8-24] were published in the Chinese journal, while one article [7] was published in the Indian journal. All the studies were parallel, two-arm trials. The basic information of the included literature is shown in Table 1.
Characteristics of the included literatures
In the 18 eligible articles, all patients were clinically diagnosed with CRC, and the patients were aged between 18 - 80 years. The patients were of Asian origin, and most of them had middle and advanced stages of cancer. Three articles used Ducks staging [8-9, 22] and six articles used TNM staging [11-12, 16, 18, 20, 24], while one article used both the types [19], but the other articles did not mention the staging status of the patients. Formulations of TCM used in the 18 articles included decoctions,injections, capsules, and pills. Among them, 8 articles used decoctions [9-11, 17-18, 21, 23-24], 4 articles used injections [7-8, 12-13], 5 articles used capsules [14, 16,19-20, 22], and one article used pills [15]. All the articles introduced the major components of these medicines.
The treatment method included the regimen of the control and experimental groups of 16 articles [7-13,15-19, 21-24] combined with TCM therapy. Treatment method of one article [14] was listed as follows: the experimental group used TCM therapy alone and the control group used chemotherapy alone. The experimental group in one article [20] used TCM therapy after surgery and the control group used placebo treatment. Chemotherapy for most of the articles [7, 9,11-12, 13, 16-18, 22-24] was FOLFOX4, while some used 5-FU+CDDP [8], MeF(v) [10], 5-FU [14], CF+5-FU[15], XELOX [18], and L-OHP+CF+5-FU [21].
The outcome measures observed in this study were the number of effective rate, KPS scores of the quality of life,comparison of toxicities, and immunological tests.Among them, the effective rate was mentioned in 7 articles [7, 9, 12, 14, 16-17, 21], and its calculation was based on the Response Evaluation Criteria in Solid Tumors published by the World Health Organization(WHO). Tumor responses included complete remission(CR), partial remission (PR), stable disease (SD), and progressive disease. The effective rate was represented by CR+PR. KPS score was used in 14 articles [7-12, 15-16,18-21, 23-24]. Among them, the efficacy manifestation of 11 articles [7-10, 15-16, 18-21, 23] was represented as Mean±SD and that for three articles [11-12, 24] was represented by the number of people. Because of few literatures, judgment was based on the data of the 11 articles. There were 13 articles [7, 9-13, 15-16, 18, 21-24]that referred to toxicity and reported toxicities such as leukopenia, thrombocytopenia, nausea and vomiting,diarrhea, and neurotoxicity. Moreover, the grades of adverse reactions were determined by the standards of acute and subacute toxicity of anticancer medicines of WHO, and all grades were included except grade 0. There were 12 articles [8-11, 13-14, 16-17, 20-23] that referred to immunological test, while the immunological parameters observed in this study were CD3, CD4, and CD4/CD8.
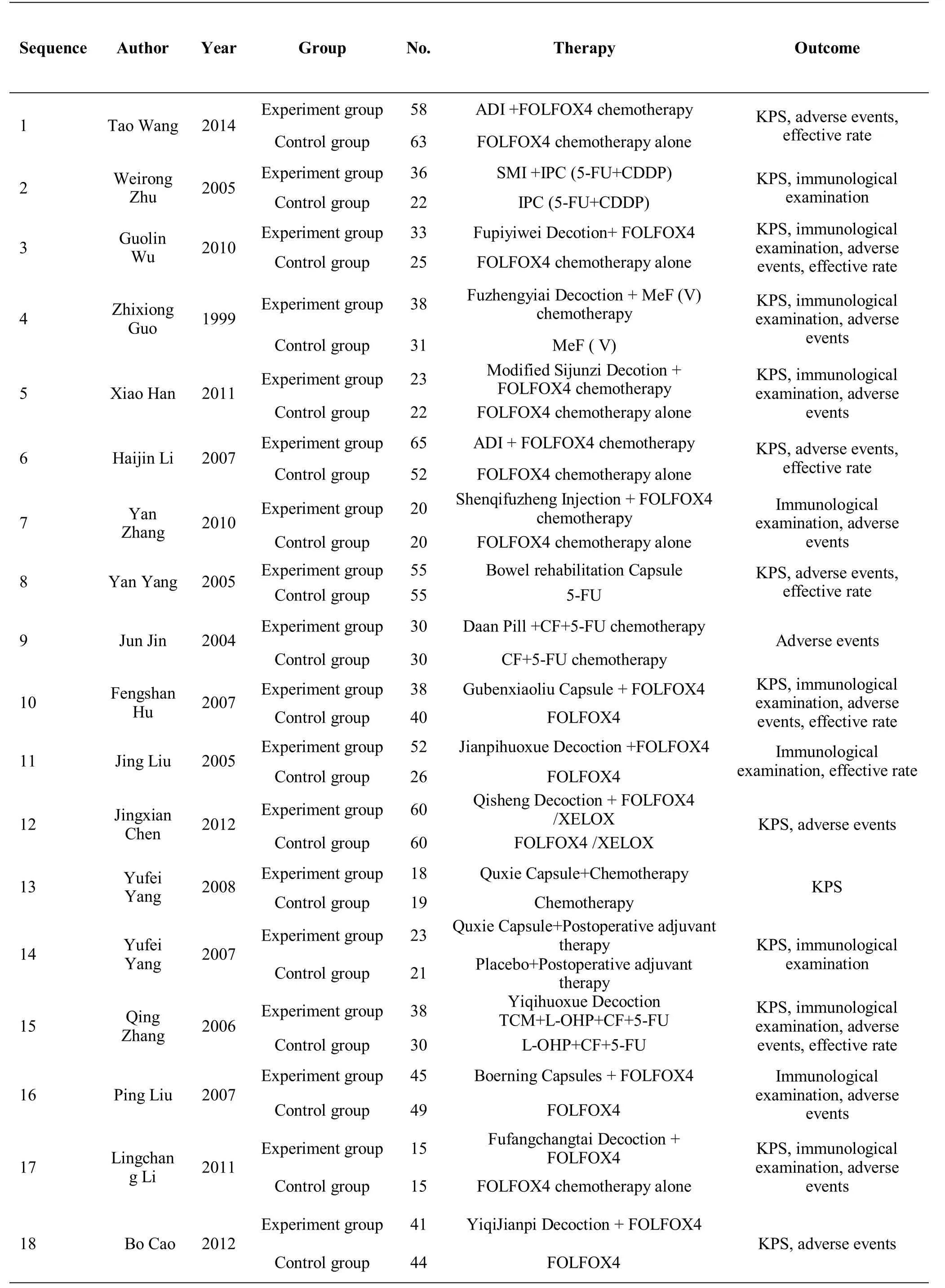
Table 1 Basic information of the included literatures
Evaluation of the risk of bias
Based on the above items of risk assessment, Review Manager 5.3 software was used to conduct risk assessment for the included articles, and the results showed that all the included articles had higher risk of bias (Figure 2).
There were 7 articles [11, 13-14, 16, 18, 21, 24] that divided the groups by random number table, and 3 articles [17, 19, 20] that divided the groups by random number generated by the software, while one article [9]randomly divided the groups by tossing a coin. However,the included articles did not have specific description of the allocation concealment.
Of all the articles, only one article [20] used placebo in the control group, while others did not use placebo in the control group and compared with TCM in the experimental group. Therefore, these experimental group articles were evaluated to be at high risk when the blinding method was implemented. Meanwhile, none of the articles specifically explained the performance bias that was caused by lack of blinding of the statistician and outcome assessors.
Except 2 articles [10, 20], none of the other articles explained the data changes of immunological test for the patients in the two groups, and the included data in the other 16 articles were consistent.
There was only one article [15] that had a high risk of selectively reporting the outcomes due to single and simple data, but all the other articles had equivalent evaluation for the treatment of patients in the two groups.No significant problems of funds and calculation of sample size were found in any articles. At the same time,it was hard to determine the authenticity of the experimental results and other bias was defined as unclear risk.
Data analysis
Comparison of the recent clinical response rates between the experimental and control groups
A total of 7 articles [7, 9, 12, 14, 16, 17, 21] reported the comparison of recent clinical response rates (CR+PR)between the experimental and control groups. There were a total of 630 patients, and the results showed no statistical difference in the clinical response rates between the two groups [P = 0.06, OR = 1.38, 95%CI = (0.98,1.95)]. The reported clinical response rates in the experimental group were not higher than those in the control group, and the obtained forest plot of the analysis is shown in Figure 3. The funnel plot of the comparison of clinical response rates between the two groups was symmetrically distributed, suggesting that there were fewer biases published in the included studies (Figure 4).
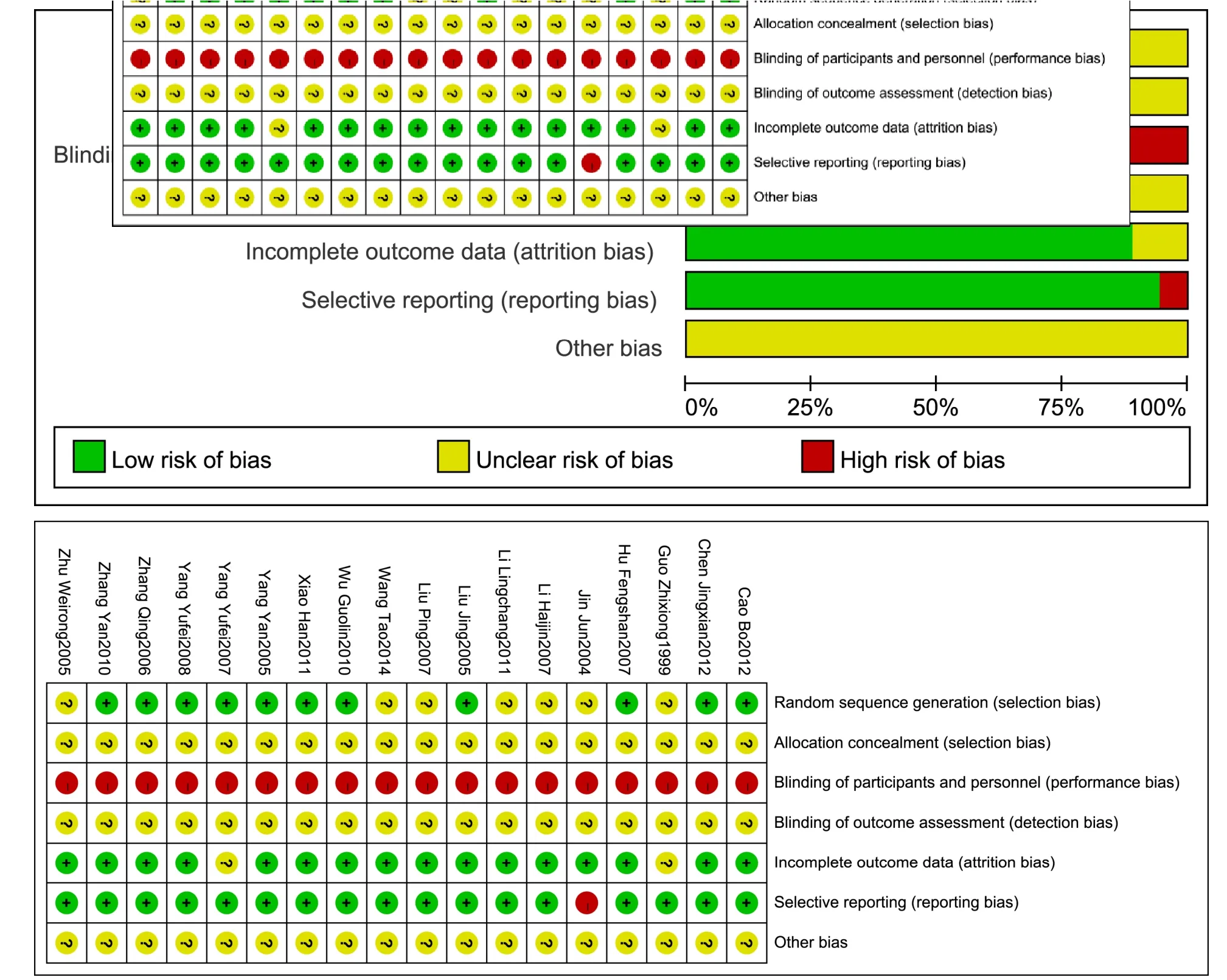
Figure 2 Summary of the evaluation of risk of bias of the included literatures
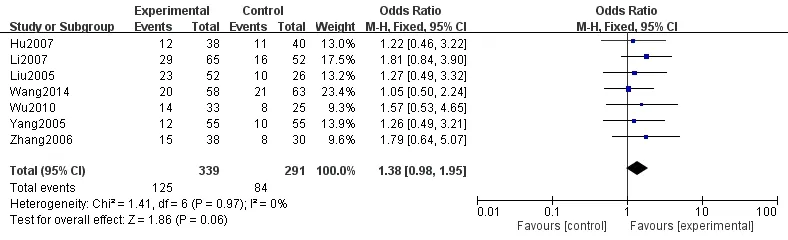
Figure 3 Comparison of the clinical response rate between the experiment and control groups
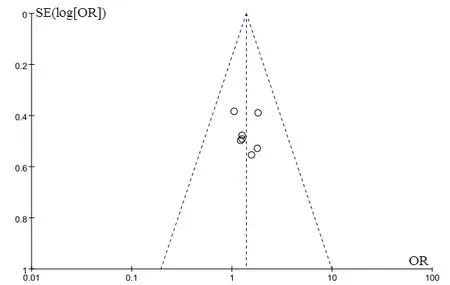
Figure 4 Funnel plots of the comparison of the clinical response rates between the experimental control groups
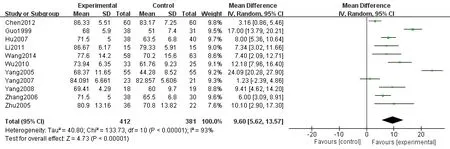
Figure 5 Comparison of post-treatment KPS scores between the experiment control groups
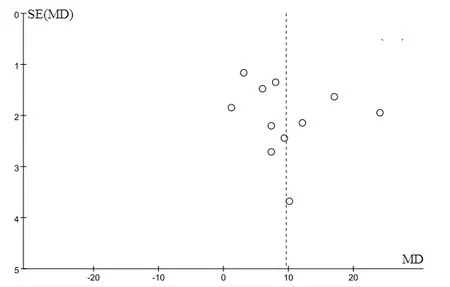
Figure 6 Funnel plot of post-treatment KPS scores between the experiment control groups
Comparison of KPS scores between the experimental and control groups
A total of 11 articles [7-10, 15-16, 18-21, 23] reported the comparison of KPS scores (Mean±SD) between the experimental and control groups, and 793 patients were included in these studies. The results demonstrated that post-treatment KPS scores were statistically significant in the patients in the two groups [P < 0.001, WMD = 9.60,95%CI = (5.62, 13.57)]. Also, KPS scores of the experimental group were higher than those in the control group, and the obtained forest plot is shown in Figure 5.The funnel plot of comparison of KPS scores was asymmetrically distributed, suggesting that there were publication biases in the included studies (Figure 6).
Toxicity
In all the articles, the control groups were given chemotherapy, and the experimental groups were given TCM combined with chemotherapy, except in one article[14], which included TCM alone. Therefore, the articles reported various toxicities that appeared after the use of chemotherapy, such as nausea and vomiting, leukopenia,thrombocytopenia, diarrhea, constipation, liver and kidney dysfunction, and neurotoxicity. After sorting out the data, the main toxicities included were leukopenia,thrombocytopenia, nausea and vomiting, diarrhea, and neurotoxicity. There were 10 articles [7, 9-12, 16, 21-24]that analyzed leukopenia, 8 articles [7, 9-10, 12, 16, 21,23-24] that analyzed thrombocytopenia, 10 articles [7,9-12, 15-16, 21, 23-24] that analyzed nausea and vomiting, 8 articles [7, 9, 11-12, 15-16, 21, 24] that analyzed diarrhea, and 8 articles [7, 9, 12, 16, 21-24] that analyzed neurotoxicity. The results showed that the occurrence of leukopenia in the two groups was statistically significant [P < 0.001, OR = 0.31, 95%CI =(0.19, 0.50)]. The occurrence of thrombocytopenia in the two groups was statistically significant [P = 0.003, OR =0.49, 95%CI = (0.31, 0.78)]. Statistical differences were observed in the occurrence of nausea and vomiting between the two groups [P < 0.001, OR = 0.30, 95%CI =(0.16, 0.54)]. Comparison of the occurrence of diarrhea between the two groups was also statistically significant[P < 0.001, OR = 0.40, 95%CI = (0.27, 0.58)].Comparison of the occurrence of neurotoxicity between the two groups was statistically significant [P < 0.001,OR = 0.43, 95%CI = (0.30, 0.61)]. However, the occurrence of toxicities, such as leucopenia,thrombocytopenia, nausea and vomiting, diarrhea, and neurotoxicity was lower in the experimental group than in the control group. The obtained funnel plot was symmetrically distributed, suggesting that publication biases were fewer in the included studies.
Immunological analysis
Among all the included articles, 12 articles [8-11, 13-14,16-17, 20-23] reported immunological test, which included CD3, CD4, C8, CD4/CD8, NK, IgA, IgG, and IgM. After sorting out the data, the major contents of this study included the tests for CD3, CD4, and CD4/CD8.Eight articles analyzed CD3, eight articles [9-11, 14, 17,20, 22-23] analyzed CD4, while another eight articles analyzed CD4/CD8 [8, 11, 14, 16, 17, 20, 22, 23].Comparison of post-treatment CD3 between the two groups was statistically significant [P < 0.001, MD = 5.55,95%CI = (4.83, 6.28)]. Comparison of post-treatment CD4 between the two groups was statistically significant[P < 0.001, MD = 6.75, 95%CI = (5.25, 8.26)]. The comparison of post-treatment CD4/CD8 between the two groups was also statistically significant [P = 0.001, MD =0.26, 95% CI = (0.10, 0.41)]. All the immunological parameters (CD3, CD4, and CD4/CD8) showed higher values in the experimental group than in the control group.However, funnel plots of the above analysis were all asymmetrically distributed, suggesting that publication bias of the included studies was strong.
Sensitivity analysis
This study performed sensitivity analysis by converting the comparison of different models. The results showed that a minor difference was observed without essential change, except for KPS score, suggesting that the rest of results of the meta-analysis were stable and strongly reliable (Table 2).
Discussion
Due to extensive changes in lifestyle and eating habits,and increased work pressure, the number of patients with CRC is increasing steadily. Approximately 1 million new cases of CRC and more than 500 thousands cases of death are identified worldwide every year [25]. Currently,surgery is the mainstay of treatment for patients with early stage CRC. However, by the time of diagnosis,metastases was reported to occur in around 25% of patients with CRC, and another 30-50% of patients diagnosed with early stage CRC developed metastatic disease during the clinical follow-up period [26].Operation cannot be performed during metastasis, and other treatment methods such as chemotherapy and biological therapy should be implemented. However,these treatment methods either alone or in combination with others show various limitations and shortcomings such as recurrence of the disease after the operation,metastasis, side effects of radiotherapy and chemotherapy(bone marrow suppression, gastrointestinal reactions,heart damage, liver and kidney dysfunction, local radiation damage), and high costs [27]. Therefore, these treatment therapies may lead to discomfort caused by either the cancer itself or by the toxicities induced by these treatment therapies, which altogether impact the quality of life of patients with CRC.
The WHO has classified cancer as a chronic disease[27]. Thus, the objective of treatment for patients with tumor has switched to a comprehensive medical model involving biological, physical, and social factors from simple biomedical model, aiming to prolong the survival of the patients with cancer, as well as to improve their quality of life.
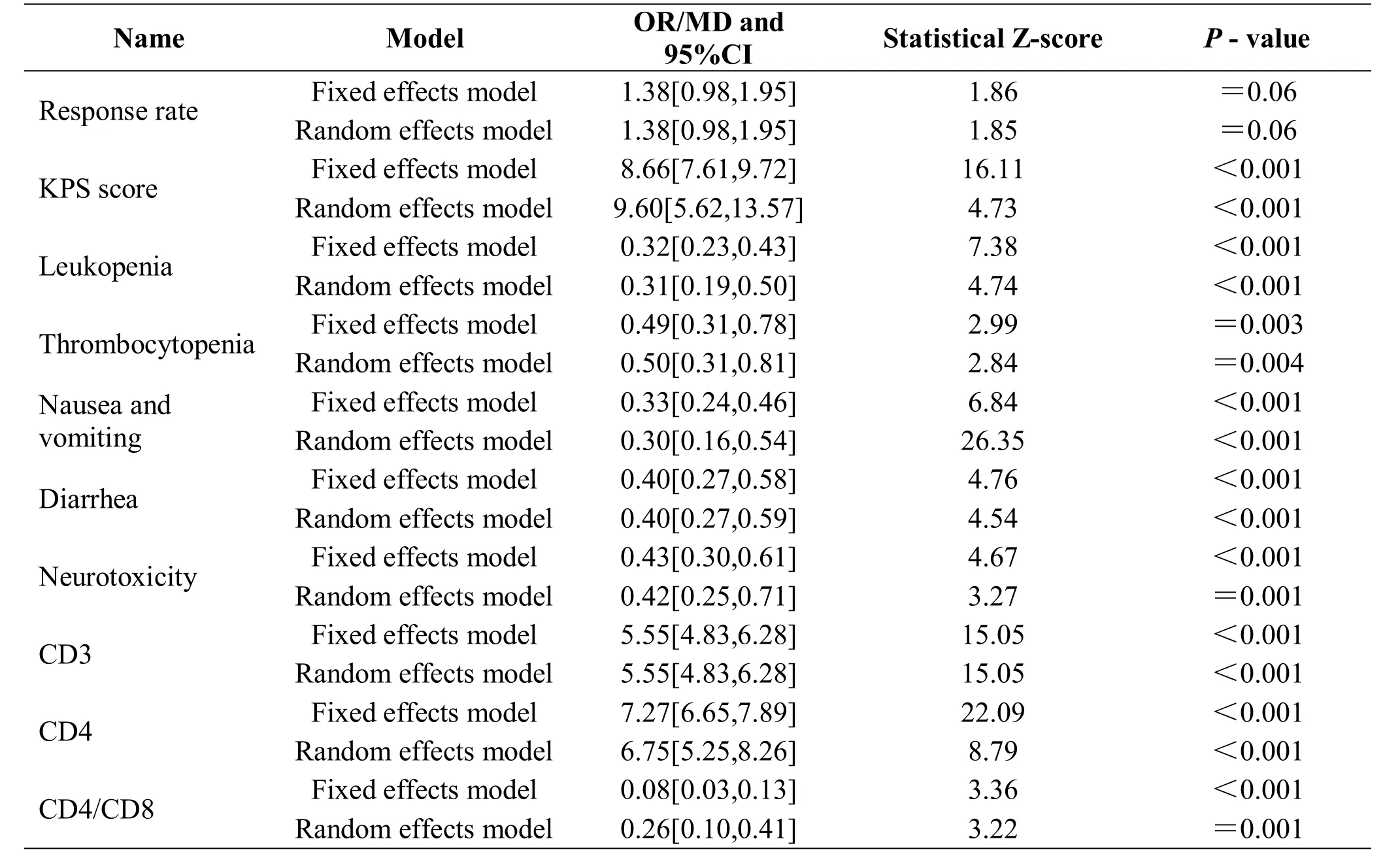
Table 2 Sensitivity among different effect models of each evaluating indicator
In modern studies, TCM has been proved to induce apoptosis and reverse multidrug resistance, and it has a comprehensive role in angiogenesis, signal transduction pathways, and metastasis. In addition, TCM can regulate immune function, improve efficacy, reduce toxicity, and improve the quality of life of patients [28]. In China,TCM has already been involved throughout the process of prevention and treatment of tumor, and in turn can improve the quality of life of patients.
In this study, meta-analysis was used to perform the combined analysis of 18 studies (total 1312 cases). The results suggested that there was no statistical significance in the recent clinical response rate between the experimental group [TCM alone or TCM combined with Western Medicine (WM)] and control group (WM alone),which might be due to the long-term process associated with TCM and its slow and long-lasting effects. TCM aims to regulate Yin and Yang of the human body, thereby strengthening the body resistance to eliminate pathogenic factors and achieve dynamic equilibrium, leading to improvement of the overall quality of life of patients.Therefore, TCM plays an important role in long-term efficacy. However, TCM has its own benefits in improving KPS scores, alleviating toxicities induced by chemotherapy, and improving immunity. All these factors in the experimental group were higher than those in the control group, and the difference was statistically significant, suggesting that TCM has a role in reducing toxicities and enhancing efficacy for chemotherapy. At the same time, TCM can improve the activity of immune cells that help to fight tumors. The results of this study demonstrate that TCM combined with modern medical therapy shows better efficacy for patients with CRC than modern medical treatment alone. We also observed an improvement in the quality of life of patients who received the combined treatment. These findings suggest that the treatment of TCM combined with WM has significant efficacy compared to WM treatment alone.
Although sensitivity analysis showed that the stability of most of the results was strong and reliability was high,there were still some shortcomings in this study, which included low quality of the included articles, small sample size, less than 100 cases in each group, indefinite randomized methods, no allocation concealment, no blinding methods, and no specific illustration for clinical staging of the included patients. Thus, all the included articles were at a high risk of bias. Especially, the funnel plots of KPS scores and immunological test were asymmetrical, suggesting high publication bias, which would impact the reliability of the results. The sensitivity analysis of KPS scores demonstrated obvious differences, which showed that its results are unstable.Moreover, the evaluation indicators were not comprehensive. For example, there was no comparison of long-term survival that can better evaluate the efficacy of TCM. Therefore, multi-center randomized controlled trials with high quality and larger sample size are needed in order to obtain reliable evidence to confirm that TCM can significantly improve the quality of life in patients with CRC.
Conclusion
TCM does not show significant efficacy in improving the recent clinical response rate for patients with CRC, but it can significantly improve KPS scores of the patients,reduce the toxicity after chemotherapy, regulate immune function, and improve the overall quality of life in patients with CRC. Thus, TCM has certain advantages,but rigorously designed multi-center randomized controlled trials with larger sample sizes should be conducted. Full explanation of the randomization,blinding method, and reasons for shedding cases will help in reducing the risk of bias as far as possible and in improving the reliability of the results.
1. Siegel RL, Naishadham D, Jemal A. Cancer statistics.CA Cancer J Clin 2012, 62: 10-29.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015, 65: 5-29.
3. Moriarity A, O’Sullivan J, Kennedy J, et al. Current targeted therapies in the treatment of advanced colorectal cancer: a review. Ther Adv Med Oncol 2016, 8: 276-293.
4. Goldberg R, Sargent D, Morton R, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004, 22: 23-30.
5. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004, 22: 229-237.
6. Cao HJ, Mu YJ, Li X, et al. A systematic review of randomized controlled trials on oral Chinese herbal medicine for prostate cancer. PLoS One 2016, 11:e0160253.
7. Wang T, Nan HL, Zhang CZ, et al. Aidi injection combined with FOLFOX4 chemotherapy regimen in the treatment of advanced colorectal carcinoma. J Cancer Res Ther 2014, 10: 52-55.
8. Zhu WR, Zheng L, Guo YB, et al. Clinical research of intraperitoneal chemotherapy plus Shenmai Injection intreating advanced colorectal cancer. Chin J Integr Med 2005, 3: 266-269.
9. Wu G, Yu G, Li J, et al. Short term therapeutic effect on treatment of postoperational large intestinecarcinoma by Fupiyiwei decoction combined with chemotherapy and it’s effecton immune function.Zhongguo Zhong Yao Za Zhi 2010, 35: 782-785.
10. Guo Z. Clinical observation on treatment of 38 cases of postoperational large intestinal cancer by fuzheng yiai decoction combined with chemotherapy. Chin J Integr Med 1999, 19: 20-22.
11. Xiao H, Yang J. Immune enhancing effect of modifid Sijunzi Decoction on patients with colorectal cancer undergoing chemotherapy. Chin J Integr Med 2011,31: 164-167.
12. Li HJ, Dong L, Fu SY. Comparative study on treatment of advanced colorectal cancer by Aidi injection combined with FOLFOX4 regimen and by FOLFOX4 regimen alone. Chin J Integr Med 2007,27: 1086-1089.
13. Zhang Y, Guo LL, Zhao SP. Effect of shenqi fuzheng injection combined with chemotherapy in treating colorectal cancer. Chin J Integr Med 2010, 30:280-282.
14. Yan Y, Liu BQ, Du QX. The clinical study of bowel rehabilitation capsule for treating middle-late colorectal cancer in 55 cases. Jiangsu J Tradit Chin Med 2005, 26: 18-20.
15. Jin J, Zhang MX. Curative effect observation relieving the gastrointestinal reaction of postoperative chemotherapy for colorectal cancer with Da An Wan.Chin J Inf Tradit Chin Med 2004, 11: 823-824.
16. Hu FS, Zhang Q, Wang XM, et al. The effects of Gubenxiaoliu Capsules in combination with FOLFOX4 regimen to treat advanced colorectal cancer. Chin J Inf Tradit Chin Med 2007, 14: 13-14.
17. Liu J, Wang WP, Zhou YY, et al. The influence of Jianpi Huoxue Recipe combined with chemotherapy on immunology and hemorheology of postoperative patients with colorectal cancer. Jiangsu J Tradit Chin Med 2005, 26: 13-14.
18. Chen JX, Shen XH. Effects of Qisheng Mixture on chemotherapy induced myelosuppression in patients with colorectal cancer. Chin J Integr Med 2012, 32:1161-1165.
19. Yang YF, Chen ZX, Xu Y, et al. Randomized controlled study on effect of Quxie Capsule on the median survival time and qualify of life in patients with advanced colorectal carcinoma. Chin J Integr Med 2008, 28: 111-114.
20. Yang YF, Xu Y, Wu Y, et al. Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer. Chin J Integr Med 2007, 27:879-882.
21. Zhang Q, Zhao WS, Yu J, et al. Clinical study on advanced colorectal cancer treated by Yiqi Huoxue TCM combined with chemotherapy. Chin J Inf Tradit Chin Med 2006, 13: 17-18.
22. Liu P, Liu JH, Zhu DQ, et al. Clinical observation of colon carcinoma treated with Boerning capsules plus FOLFOX4 regimen. Chin Ger J Clin Oncol 2007, 6:328-330.
23. Li LC, Fang MZ, Wang XN, et al. Clinical observation of Fufangchangtai decoction combined with FOLFOX4 regimen for postoperative colorectal cancers. Chin Ger J Clin Oncol 2011, 10: 225-227.
24. Cao B, Den WL. Clinical observation of treatment with Yiqi Jianpi decoction combined with FOLFOX4 for the postoperation patients of colorectal cancer.Chin Ger J Clin Oncol 2012, 11: 605-608.
25. De Vries NL, Swets M, Vahrmeijer AL, et al. The immunogenicity of colorectal cancer in relation to tumor development and treatment. Int J Mol Sci 2016,17: 1030.
26. Schmoll HJ, van Cutsem E, Stein A, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 2012, 23:2479-2516.
27. Qi FH, Zhao L, Zhou AY, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Bio Science Trends 2015, 9: 16-34.
28. Nie J, Zhao CL, Deng L, et al. Efficacy of traditional Chinese medicine in treating cancer. Biomed Rep 2016, 4: 3-14.
 Traditional Medicine Research2018年2期
Traditional Medicine Research2018年2期
- Traditional Medicine Research的其它文章
- History of recurrent miscarriage in traditional Chinese medicine literature
- Clinical evidence and potential mechanisms of Chinese medicines for the treatment of diabetic retinopathy
- A systematic summary of natural compounds in Radix Glycyrrhizae
- Meta-analysis of neostigmine injections given at the Zusanli (ST 36) acupoint in the treatment of postpartum urinary retention
