A systematic summary of natural compounds in Radix Glycyrrhizae
Ming Yang, Yi Jin, Li-Ping Yang
1Department of Pharmacy of Beijing Hospital, National Center of Gerontology, Assessment of Clinical Drugs Risk and Individual Application Key Laboratory; National Clinical Research Center of Respiratory Diseases, Beijing, China.2School of Life Sciences and Biopharmaceuticals, Shenyang Pharmaceutical University, Shenyang, China.
Background
Gancao (Radix glycyrrhizae, GC), also known as honey grass, is famous for its sweet taste. GC comprises the dry roots and rhizomes of the perennial herb licorice,Guangguogancao (Glycyrrhiza glabra l), and Zhangguogancao (Glycyrrhiza inflata bat), which is mainly found in regions spanning from northern of China to inner Mongolia, Gansu. The book of Shennongbencaojing, from the Donghan Dynasty of China (the third century A.D.), pointed out that GC can not only strengthen bones and long muscles, but also possesses detoxification abilities. According to the Chinese Pharmacopoeia, the dry roots and rhizomes of GC have the ability to replenish Qi, tonify spleen, along with heat-clearing, toxicity-preventing, phlegm-dispelling,and cough-, spasm- and pain-relieving abilities, thereby harmonizing the effects of other medicines [1]. In recent years, pharmacological studies have shown that GC has anti-ulcer, anti-inflammatory, anticonvulsant, anti-tumor,anti-HIV, anti-allergic, antitussive, analgesic, and antispasmodic properties, along with the ability to lower blood cholesterol, increase bile secretion, and other pharmacological effects. In addition to its traditional use,clinically, components of GC, such as glycyrrhizic acid,glycyrrhetinic acid, liquiritigenin, isoliquiritigenin, and other natural compounds, have been used to make drugs and pharmaceutical preparations commonly used in the treatment of peptic ulcers, hyperlipidemia, depression,tuberculosis, bronchial asthma, viral hepatitis, thrombotic vasculitis, contact dermatitis, allergic dermatitis, eczema,and so on. In traditional Chinese medicine, it is the specific natural ingredient that plays an important role in the pharmacological aspects and treatment effect.Recently, a variety of flavonoids, terpenoids, and saponins have been isolated from GC; some coumarins and other ingredients have also been found. Although there are many studies about the composition and structures of the components of GC, there is a lack of comprehensive and systematic classification and review,especially about its main bioactive ingredients. This article systematically collates reports about the chemical constituents of GC, and summarizes them according to the frequency of their reports.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria: Research articles on various chemical constituents of GC, such as structural identification,chemical composition analysis, and fingerprint mapping.Exclusion criteria: Review articles on GC contained in Chinese traditional patent formulation or GC preparations,extraction methods, or pharmacological effects.
Document retrieval
CNKI, Wanfang Data, VIP, CBM, and Pubmed database were used to collect articles about the chemical constituents from GC. The retrieval period was from the time of inception of the respective database to March 2016. For title-based searches, the English search terms were as follows: glycyrrhizae, component, ingredient,composition and constituent. In case of Pubmed, for example, the specific search strategy is shown in Table 1.
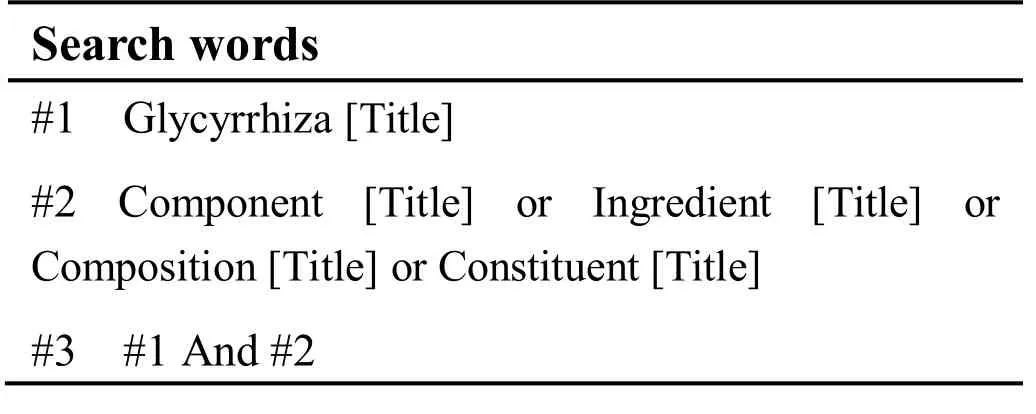
Table 1 The search strategy
Data extraction
Excel software was used to develop the data extraction table, including the Chinese and English name, chemical name, molecular formula, molecular weight, and structure of each component. The extracted information was classified and aggregated. Information on similar chemical compositions was collated, and the number of studies reported was counted. The compositions were then classified into several categories according to the structural characteristics, and the number of components in various categories was counted.
The components of GC, whose composition was reported only once were listed in the table, whereas those whose composition was reported in more than two reports were included in the figure. According to the numbers reported in the literature, the order of the licorice components in the chart was determined.
Results
Literature search results and basic information
A total of 1756 studies were acquired, among which 1701 were retrieved from five databases, including CNKI,Wanfang Data, VIP, CBM, and Pubmed database. The remining 55 articles were obtained from other resources.In total, 743 articles were obtained after ticking. 403 articles were obtained by reading the essays and abstracts,and 252 articles about natural components of GC were obtained by reading the full text. The article screening process and the results are shown in Figure 1.
Finally, a total of 653 natural components were collated from the 252 articles, and according to their chemical structure, they were classified into flavonoids(201 species, 200 articles), terpenoids and saponins (167 species, 153 articles), coumarins (30 species, 29 articles),aliphatic compounds (206 species, 27 articles), aromatic compounds (35 species, 14 articles), and other compounds (14 species, 17 articles). Five categories of natural components are described below.
Flavonoids
In the 252 studies on the constituents of GC, 201 flavonoids were reported in 200 articles. The basic skeleton of flavonoids is C6-C3-C6, which can be divided into nine motifs, flavanones (38 components, 133 articles), isoflavones (49 components, 60 articles),flavones (25 components, 44 articles), chalcone (24 components, 100 articles), flavonols (23 components, 23 articles), isoflavans (19 components, 31 articles),pterocarpans (6 components, 13 articles), isoflavanones(4 components, 9 articles), and isoflavenes (4 components,7 articles). The remaining was classified into other flavones (9 components, 8 articles). The details are listed as follows.
Flavanones.There were 38 types of flavanones, with their structures being reported in 133 articles, of which seven components were reported by more than two articles, including liquiritin in 94 articles, liquiritigenin in 56 articles, liquiritigenin-4’-apiosyl (1-2)-glucoside in four articles, isogrolrol in four articles,liquiritigenin-7,4’-diglucoside in three articles, glabranin in two articles, and pinocembrin in two articles. Their structures are shown in Figure 2. The other 31 flavanones were reported only in one article, and are listed in Table 2S.
Isoflavones.We collated 49 isoflavones, and their structures were reported by 60 articles, of which 15 components were reported by more than two articles, and their structures are shown in Figure 3. There were twenty-three studies about formononetin, fourteen articles on ononin, eight articles on glabrone, seven articles on licoisoflavone A, four articles on 4’,7-dimethoxyisoflavone, licoricone, isoononin, and licisoflavone B,three articles on calycosin, prunetin, and lupiwighteone,and two articles on 5,7,4’-trihydroxy-6,8-diisoprenylisoflavone, semilicoisoflavone B, gancaonin H, and gancaonin G. The other 34 isoflavones were reported by only one study, and are listed in Table 3S.
Flavones. The types of flavones and their structures were reported in 44 studies; 10 compounds were reported by more than two articles, and the structures are shown in Figure 4. Among them, fourteen articles were on 4’,7-Dihydroxy-flavone; eleven articles on liquiritinapioside; nine articles on licoflavone C; seven articles on licoflavone; four articles on isoviolanthin; four articles on licoflavone B; three articles on kanzonol E;two articles about schaftoside, violanthin, and genkwanin.The other 17 components were reported only once, and are listed in Table 4S.
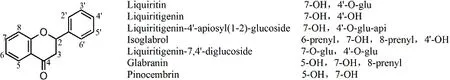
Figure 2 Chemical structures of main flavanones
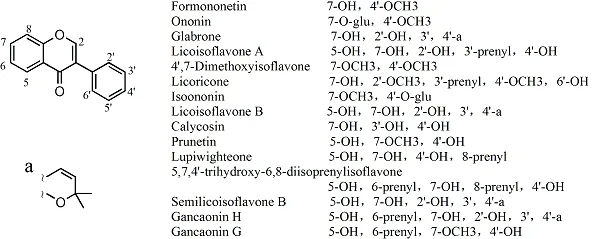
Figure 3 Chemical structures of main isoflavones
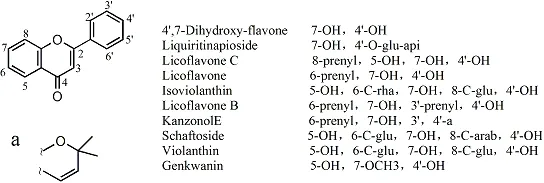
Figure 4 Chemical structures of main flavones
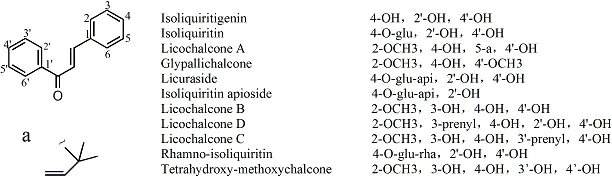
Figure 5 Chemical structures of main chalcone
Chalcone.There were 24 components of chalcone, and their structures were reported in 100 papers. There were eleven kinds of ingredients reported at least twice, of which there were 56 studies on isoliquiritigenin, 35 articles on isoliquiritin, 29 articles on licochalcone A, 12 articles on glypallichalcone, 11 articles on isoliquiritin apioside, 9 articles on licuraside, 7 articles on licochalcone B, 4 articles on licumalcone D, 3 articles on licochalcone C, 3 articles on rhamno-isoliquiritin, and 2 articles on tetrahydroxy-methoxychalcone. Their structures are shown in Figure 5. The other 13 components were reported only once, and are listed in Table 5S.
Flavonols.There were 23 articles that reported 23 kinds of ingredients and the structure of flavonols, of which there were five articles on licoflavonol and isoquercitrin,4 articles on rutin, 3 articles on glycyrrhiza-flavonol A and isolicoflavonol, and 2 articles on uralenol,neouralenol, licobenzofuran, and astragulin. The other 14 components were reported by only one study, and are listed in Table 6S. The nine aforementioned components were reported by more than two articles, and their structures are shown in Figure 6.
Isoflavans.There were 31 articles that reported 19 components of the isoflavans and their structures, of which 9 components were reported by two or more than two articles (Figure 7), including glabridin in 11 articles,licoricidin in 8 articles, phaseollinisoflavane and vestitol in 3 articles, and 4’-O-methylglabridin, hispaglabinein A,hispaglabridin B, licorisoflavan A, and glyasperin D in 2 articles. The other 10 components were reported only once (Table 7S).
Pterocarpans.There were 13 articles that reported six components of pterocarpans and their structures, of which 3 components were reported by two or more articles(Figure 8), including homopterocarpin in 7 articles,medicarpin in 6 articles, medicarpin-3-O-glucoside in 3 articles. 10-methoxy medicarpin, mei dexualine, and 1-methoxyphaseollidin were reported in only one article.
Isoflavanones.The four components of isoflavanones and their structures were reported in nine studies. Among them, there were 4 articles on glisoflavanone, 3 articles on licoisoflavanone, 2 articles on 3’-(γ,γdimethylallyl)-kievitone, and 1 article on dihydrolicoiso.Three components were reported by two or more than two articles (Figure 9).

Figure 6 Chemical structures of main flavonols

Figure 7 Chemical structures of main isoflavans

Figure 8 Chemical structures of main pterocarpans
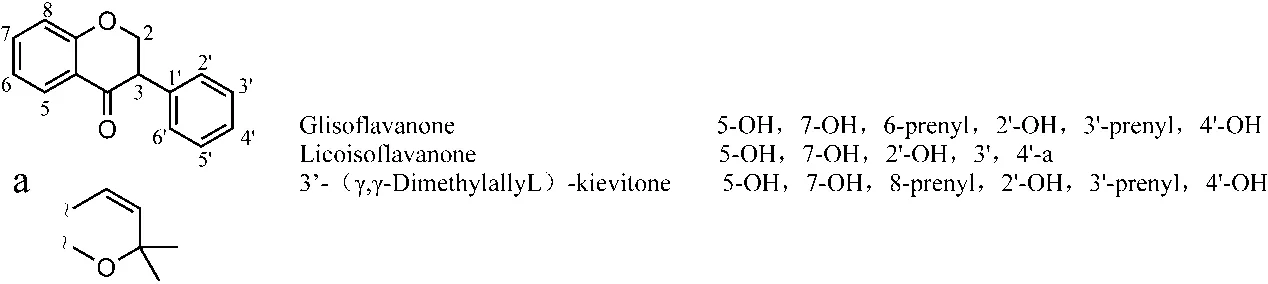
Figure 9 Chemical structures of main isoflavanones

Figure 10 Chemical structures of main isoflavenes
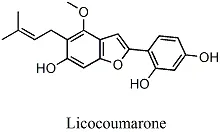
Figure 11 Chemical structures of licocoumarone
Isoflavenes.There were seven papers that reported on four components of isoflavenes and their structures.Among them, there were 2 studies on dehydroglyasperin D and 1 article on light licorice, licorice isoflavones, and Glycyrrhizin C each. Only 1 component was reported by more than two articles (Figure 10).
Other flavones.There were eight papers that reported nine components of other flavones and their structures.Among them, there were 2 studies on licocoumarone. The components listed in Table 8S were reported only once.Only 1 component was reported by more than two articles(Figure 11).
Terpenes and saponins
Several papers reported 167 components of terpenoids and saponins and their structures. Among them, there were 93 studies on glycyrrhizic acid, 31 articles on glycyrrhetinic acid, 18 articles on β-sitosterol, 7 articles on uralsaponin B, betulinic acid and daucosterol, 4 articles on licorice-saponin E2 and licorice-saponin G2, 3 articles on 3-oxo-glycyrrhetic acid, caryophyllene and 22β-acetoxyl-glycyrrhizin, 2 articles on 22β-acetoxylglycyrrhaldehyde, α-cadinol, β-sitosterol acetate,germacrene, stigmasterol, methylglycyrrhetate,glycyrrhetic acid acetate, glyeurysaponin,licorice-saponin A3, licorice-saponin B2, betulinic acid methylester, squalene, methylester of macedonic acid,macedonic acid, oleanolic acid, uralsaponin A,11-deoxoglycyrrhetic acid, and 24-hydroxyglabrolide. A total of 29 components were reported by two or more articles. The remaining 138 components were reported only once (Table 9S).
The most commonly reported were triterpenoids and β-sitosterol. The triterpenoid skeleton is generally 3β-hydroxy pentacyclic triterpene olefins, including 11-Oxo-olean-12-en-29/30-oic acids (Figure 12 A, B),Olean-12-ene-29/30-oic acids (Figure 12C),Olean-11,13(18)-diene-29/30-oic acids (Figure 12D),lupanes (Figure 12E), and β-sitosterol (Figure 12 F). The chemical framework of each component is shown in Figure 12. We only listed the components which were reported by two and more articles.
Coumarins
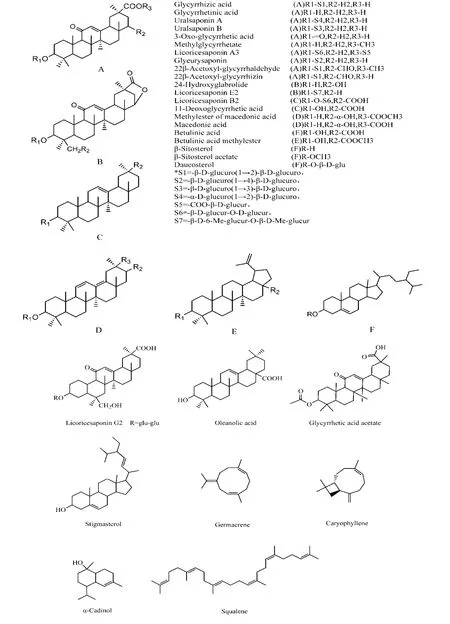
Figure 12 Chemical structures of main terpenes and saponins
There were 29 papers that reported 30 components of coumarins and their structures, of which 17 papers reported 12 components of 3-aryl coumarins, 16 articles reported 14 components of coumestan, and 4 articles reported 4 components of other coumarins. Licorice coumarin is divided into three categories according to the structure; the first category is 3-arylcoumarin, the second category is coumestan, and the third category is other coumarins.
3-Arylcoumarins.There were 17 reports involving 12 components of 3-Arylcoumarins and their structures, of which there were 8 articles on glycycoumarin, 2 articles on glycyrin, licopyranocoumarin, and licofuranocoumarin.The other 8 components were reported only once (Table 10S). A total 4 components were reported by two or more articles (Figure 13).
Coumestans.There were 14 components of coumestans with their structures reported in 16 articles, of which 4 components were reported by two and more articles,including glycyrol in 7 articles, isoglycyrol in 5 articles;5-O-methyl-glycyrol, and neoglycyrol in 2 articles; The other 10 compounds were reported only in one article listed in Table 11S. A total 4 components were reported by two and more articles (Figure 14).
Other coumarins.There were 4 components of other coumarins with their structures reported in 4 articles.Each component is reported by only one study. These components are: 1-methoxyphyllol, 6,7-dihydroxycoumarin, 7-methoxycoumarin, and glycyrrhizol B.
Aliphatic compounds
There were 206 components of aliphatic compounds with their structures reported in 27 articles. They mainly comprised 84 types of alkanes, 22 types of olefins, 28 types of acids, 9 types of aldehydes, 24 types of esters, 19 types of ketones, and 20 types of alcohols. A total 14 components were reported by three and more articles.Among them, there were 6 studies on heptadecane,octadecane, nonadecane, eicosane, 5 studies on linoleic acid and hexadecane, 4 studies on hexacosane, and 3 studies on hexanoic acid, hexadecanoic acid, pentadecane,2,6,10,14-tetramethyl-hexadecane, octadecane, octane,and 1-octadecene. The chemical structures of 14 main components were shown in Figure 15. There were 40 components reported by 2 studies (Table 12S), and 152 components reported by 1 study (Table 13S).
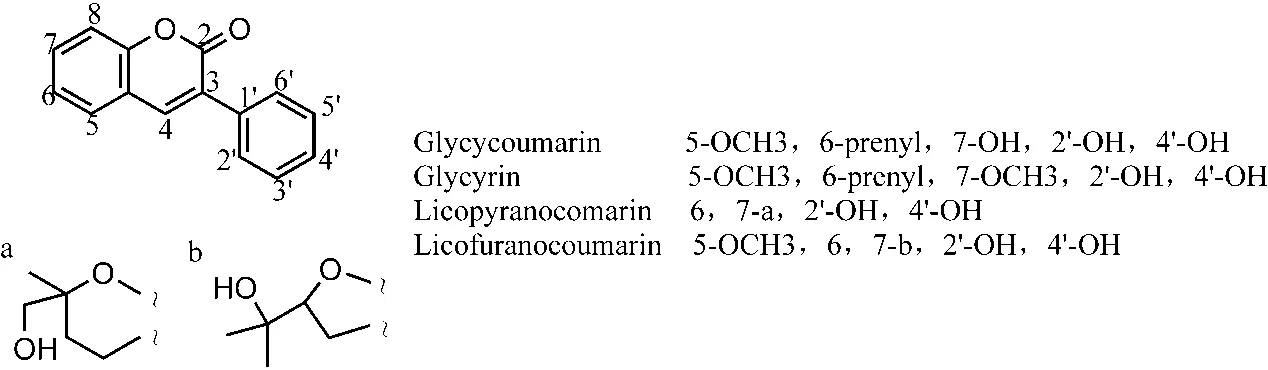
Figure 13 Chemical structures of main aliphatic compounds

Figure 14 Chemical structures of main coumestans
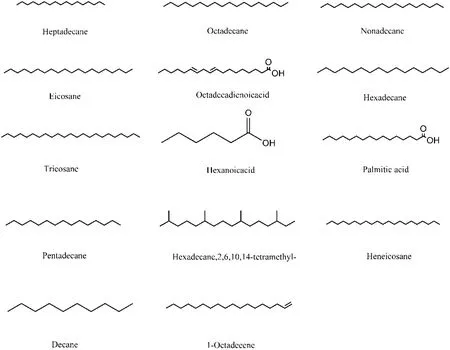
Figure 15 Chemical structures of main coumestans
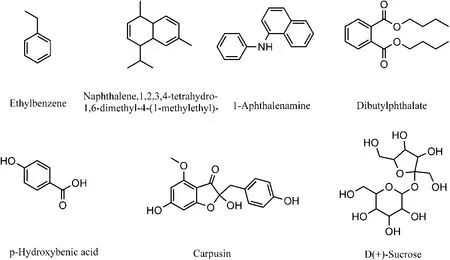
Figure 16 Chemical structures of main aromatic compounds and other compounds
Among the 206 constituents of the aliphatic compounds, there were n-alkanes containing from 5 to 8,11 to 23, 25 to 30, 32, 34 to 36 carbons and from 1, 5 to 10, 12 to 14, 16, 18, 19, 21 carbon-carbon chains and cyclic carbons. The alcohols include: 1, 4, 6, 15, 18, 22,27 carbon positive alcohols and with a substituent containing 2, 4, 6, 8, 16, 18 carbon chain and ring carbon alcohol. The aldehydes included 6, 7, 9, 11 to 15 carbon atoms, and a carbon chain containing 16 carbon atoms with substituents. The acids included 2, 4, 6, 7, 8, 9, 10,11, 12, 14, 15, 16, 18, 20, 22, 24, 26 carbonic acid and the presence of substituents with 2, 18, 30 carbon chains and ring-forming carbon acids. Ketones included 3, 7, 9, 11,14, 15 carbon-containing ketones and 5, 6, 8, 10, 11, 15 carbon chains and ketones with ring-forming carbon.Esters included 2, 3, 6, 8, 14, 16, 18, 22, 24 carbon-containing acid, and methyl ester, ethyl ester,propyl ester, butyl ester combination.
Among them, there were 23 kinds of substituted compounds having carbon chains of 6 carbon, and 14 kinds of substituted compounds having carbon chains of 5 carbons and 18 carbons, respectively. There were 12 kinds of substituted compounds having 8 carbon chains.There were 11 kinds of substituted compounds in which the carbon chain contained 7 carbons. There were 10 kinds of substituted compounds having carbon chains containing 16 carbons.
Aromatic compounds and other compounds
There were 35 components of aromatic compounds with their structures reported in 14 articles. Among them, there were 3 studies on ethylbenzene, naphthalene,1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methyl-ethyl)-, and 1-aphthalenamine, and 2 articles on dibutylphthalate and p-hydroxybenzoic acid. The other 30 compounds were reported only in one article listed in Table 14S. A total 5 components were reported by two or more articles(Figure 16).
There were 17 papers reporting the other 14 components and their structures, of which there were three articles on D (+)-sucrose and 2 articles on carpusin.The other 12 compounds were reported only in one article,and are listed in Table 14S. A total of two components were reported by two and more articles (Figure 16).
Discussion
Our study brings together more than 250 studies on the components of GC and categorizes them to sort out all the ingredients that have been isolated and reported so far.We found that flavonoids and terpenes/saponins are the most active components. Among them, the flavonoids comprise mainly isoflavones such as formononetin and ononin, flavanones such as liquiritin and liquiritigenin,flavones such as 4’,7-Dihydroxy-flavone and liquiritinapioside, and chalcones such as isoliquiritigenin and isoliquiritin. The terpenes/saponins comprise tricyclic triterpenes such as glycyrrhizic acid and glycyrrhetinic acid. In addition, GC also contains a small number of components of coumarins.
China Pharmacopoeia 2015 edition has the requirements for determination the content of GC, that is,determination of the content of liquiritin (dihydrogen flavonoids) and glycyrrhizic acid (tricyclic triterpenoids).We can see that the two active ingredients on the role of GC have a huge impact. Here we mainly discuss reports on flavonoids such as liquiritin, liquiritigenin,4’,7-Dihydroxy-flavone, liquiritinapioside, formononetin,ononin, glabridin, isoliquiritin, isoliquiritigenin, and licochalcone A and terpenoids/saponins such as glycyrrhizic acid, glycyrrhetinic acid, β-sitosterol, and betulinic acid.
Licoflavones
Our research showed that among all constituents of GC,the flavanones are the most reported. Among the flavanones, liquiritin is the most reported, followed by liquiritigenin. Liquiritigenin is an aglycone of liquiritin,which mainly has an anti-cancer effect. Liquiritin has antidepressant [4], neuroprotective [5], myocardial cell-protective [6], and detoxification effects, along with many other therapeutic properties [7].
The most reported species of isoflavones was formononetin, followed by ononin. Formononetin is a weak phytoestrogen [8], which is a major ingredient of pratensein. It has anti-inflammatory [9], anti-cancer[10-12], and anti-tumor activities, as well as anti-osteoporosis [13] and anti-atherosclerotic [14]properties.
Among the chalcones, the composition of isoliquiritigenin was the most reported, followed by isoliquiritin. Isoliquiritigenin is an aglycone of isoliquiritin, with anti-cancer [15], anti-inflammatory [16],anti-oxidative [17], anti-HIV [18], and antispasmodic effects [19,20]. It also has a protective effect on the brain[21], liver [22], lung [23], gastrointestinal region [24].Isoliquiritigenin is an aldose reductase inhibitor, which can significantly prevent the occurrence of diabetic complications [25]. Licochalcone A is a type of chalcone constituent specific for the genus Glycyrrhiza glabra, and has anti-inflammatory, anti-cancer, anti-tumor,antibacterial, antimalarial, and antiparasitic effects [26].
In addition, 4’7-dihydroxy-flavone and liquiritinapioside are the most reported flavonoids, but there are still very few reports about their pharmacological effects. Glabridin is a isoflavan-specific component of the genus Glycyrrhiza glabra 1, which has antioxidant [27], anti-atherosclerosis, hypolipidemic,blood pressure-lowering [28], anti-inflammatory, and tyrosine-inhibiting effects, as well as whitening [29],neuroprotective and memory-enhancing properties [30].The whitening mechanism of glabridin involves the inhibition of tyrosine activity, which prevents the binding of the substrate and tyrosinase, ultimately inhibiting the synthesis of melanin [29].
Licoflavones mainly display anti-inflammatory,anti-oxidant, anti-aging, anti-viral, anti-tumor, sedative,analgesic, enzyme-inhibiting effects. Its anti-cancer mechanism involves the inhibition of cell cycle,destruction of DNA, and induction of apoptosis [15]. Its anti-inflammatory mechanism involves the inhibition of cyclooxygenase activity, thereby inhibiting the production of arachidonic acid [16]. Its anti-HIV mechanism involves inhibiting the production of HIV reverse transcriptase and protease [17]. Its antioxidant mechanism involves the elimination of free radicals and superoxide radicals, via the termination of a free radical chain reaction.
Glycyrrhiza terpenoids/saponins
Glycyrrhizic acid is the most reported glycyrrhiza terpenoid compound, followed by glycyrrhetinic acid.Glycyrrhetinic acid is the aglycone of glycyrrhizic acid; it mainly has anti-inflammatory, liver protective, anti-tumor,and anti-fibrotic effect. Its anti-inflammatory effect involves the inhibition of lipoxygenase and phospholipase A2 [6]; its hepatoprotective effect is achieved through the increased mobility of protein family 1 BOX-A binding,reduction of protein family 1 content, while increase the role of interleukin-6,10 [31, 32]. Its antitumor effect involves the inhibition of the proliferation of cancer cells by inhibiting the nucleotide reductase and decreasing the activity of DNA synthesis rate-limiting enzymes [33]. Its anti-fibrosis effect is achieved by reducing the synthesis of collagen type I and III [34]; Glycyrrhizic acid is also a steroid hormone metabolic enzyme inhibitor, which can improve endogenous and exogenous corticosteroid activity [35]. Glycyrrhizic acid is clinically used for a variety of acute and chronic hepatitis or liver injury [36],as an anti-cancer agent [37], for anti-AIDS treatment [38],and for modulating immunity [39].
Studies on the constituents of other triterpenes were lesser in number than those on glycyrrhizin and glycyrrhetinic acid. More than 5 studies have been reported on β-sitosterol, betulinic acid, uralsaponin B, and daucosterol; there were very few reports about uralsaponin B. Daucosterol is the 3-O-glucoside of sitosterol; daucosterol, β-sitosterol [40], and betulinic acid [41] have anti-inflammatory, anti-oxidative,tumor-inhibitory, and immunoregulatory effects, and these three components are widely distributed in vegetables, seeds, and herbs.
Aliphatic and aromatic
In addition to flavonoids, terpenoids, saponins and coumarins, aliphatic and aromatic also form a proportion of the components of GC. In fatty acids, linoleic acid and palmitic acid have been frequently reported. Palmitic acid is the main ingredient of palm oil, which can delay the process of alcoholic liver disease and reduce serum cholesterol levels; linoleic acid is the main component of sesame oil; both linoleic acid and its methyl esters have anti-inflammatory effects. It acts by reducing the levels of IL-1β, TNF-α, and NO at the inflammatory site. Aromatic compounds were mostly reported in only one study, and no pharmacological reports of these compounds were reported, and therefore this has not been discussed here.
Conclusion
A total of 252 reports on GC components were collected.In total, 653 components of GC were sorted and classified and their structures were drawn. They included 10 subspecies of flavonoids, terpenoids/saponins, 3 subclasses of coumarins, aliphatic compounds, aromatic compounds, and other compounds. The results showed that the most studied components were flavonoids(liquiritin and liquiritigenin as the representatives),followed by terpenoids/saponins (represented by the pentacyclic triterpene glycyrrhizic acid and glycyrrhetinic acid). We speculate that these two components have anti-inflammatory, antioxidant, anti-viral, anti-tumor, and other pharmacological effects; they also possess spleen tonifying, Qi-replenishing, heat-clearing, cough- and emergency pain-relieving, and expectorant effects.Therefore, we will use these components of GC for further research to explore the molecular mechanisms underlying the effects of the components of GC.
1. Committee of Pharmacopoeia of China.Pharmacopoeia of people’s republic of China 2015 edition. Beijing: The Medicine Science and Technology of China Press 2015.
2. Hu K, Yang ZH, Liu XH, et al. Synthesis and antitumor activities of liquiritigenin. Chin J Synth Chem 2010, 18: 513-516b.
3. Chen J, Liu TY, Yu M, et al. Effects of liquiritigenin on growth, apoptosis and autophagy of human breast carcinoma MCF-7 cells. Chin J Gen Surg 2013, 22:1466-1470.
4. Li HF. Study of Liquintin to treat depression on pharmacodynamics and its possible antidepressant mechanisms. Beijing Univ Chin Med, 2008.
5. Liu RT, Bian GX, Zhou LB, et al. Neuroprotective effects of liquiritin and its inhibitory actions on cholinesterase activity. Chin J New Drugs 2008, 17:574-581.
6. Feng Y, Wu WF, Wei JH, et al. The extraction and purification methods and pharmacological research progress of glycyrrhizic acid and liquiritin. Renshen Res 2012, 3: 46-50.
7. Ding XS, Dai DZ. Proliferation inhibiting on human gastric carcinoma BGC-823 cell by barbering.Pharmacol Clin Chin Mater Med 2002, 18: 12-13.
8. Jie FY, Xue CK, Shen K, et al. Effect of formononetin on the expression of estrogen receptor β in aortic of castrated rat. China Pharm 2011, 14:1250-1252.
9. Wang Y, Tao Y, Wang XY, et al. Formononetinn inhibits Th2 type allergic contact dermatitis in mice.Chin J Exp Tradit Med Formulae 2016, 22: 88-91.
10. Zhang L, Ren ZH, Xue YF et al. Effect of formoononetin on endostatin, VEGF, MMP-2 and bFGFin hydrothorax and serum and tumor biomarkers of elderly patients with advanced lung cancer. Chin J Biochem Pharmaceuticals 2015, 35:154-157.
11. Sheng JY, Chen HF. Effects of formononetin combined with Akt inhibitor MK2206 on proliferation and apoptosis on different subtypes of breast cancer cell lines. Chin J Cancer Prev Treat 2015, 22: 998-1003.
12. Zhou RJ, Chen HF, YM, et al. Effects of formononetin proliferation and cell cycle of different subtypes of breast cancer cell lines. Cancer Res Prev Treat 2012, 39: 1051-1055.
13. Zhou GQ, Wang MG, Chen KP, et al. Protective effect of formononetin on hypoxic injuries of esteoblasts in vitro. Zhong Yao Tong Bao 2011, 28:671-677.
14. Zhao P, Yang Y, Xu Y, et al. Effect of formononetin on abnormal lipid metabolism and atheroscierosis.Chin J New Drugs 2009, 18: 925-929.
15. Zhang MF, Shen YQ. Anticancer activities of crude extracts of licorice and its flavonoids. Drugs Clinic 2013, 38: 99-104.
16. Yang XL, Liu D, Bian K, et al. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae Radix et Rhizoma and its ingredients.China J Chin Materia Medica 2013, 38: 99-104.
17. Mourboul ABLISE, Anwar MAMET, Rena KASM.Advance in study on the preparation techniques and pharmacological actions of isoliquiritigenin. Chin J Modern Appl Pharm 2009, 26: 277-280.
18. Haraguchi H, Ishikawa H, Mizutani K, et al.Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorg Med Chem 1998, 6: 339-347.
19. Kim DC, Choi SY, Kim SH, et al. Isoliquiritigenin selectively inhibits H(2) histamine receptor signaling.Mol Pharmacol 2006, 70: 493-500.
20. Jing WA, Ao YY. Metallothionein mediates cardioprotection of isoliquiritigenin against ischemia-reperfusion through JAK2/STAT3 activation. Acta Pharmacol Sin 2006, 11: 1431-1437.
21. Hou YY, Yang Y, Yao Y, et al. Neuroprotection of glycyrrhizin against ischemic vascular dementia in Vivo and glutamate-induced damage in vitro. Chin Herb Med 2010, 2: 125-130.
22. Zhang DH, Ye LL, Cheng H, et al. Cytoprotective effect and its mechanisms of isoliquiritigenin on acetaminophen induced acute injury of hepatocytes.Chin J Clin Pharmacol Ther 2008, 13: 293-298.
23. Liu B, Wen QS, Zhang JR, et al. Antiasthmatic effect of isoliquiritigenin and its mechanism. Chin J Clin Pharm 2007, 16: 348-352.
24. Sato Y, He JX, Nagai H, et al. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine.Biol Pharm Bull 2007, 30: 145-149.
25. Masato T, Kaoru A, Toshikazu N, et al. Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharm 1992, 212: 87-92.
26. Yang LW, Song XB, Zhang LJ, et al. Progress in preparation methods and pharmacological effects of licochalcone A. J Liaoning Univ Tradit Chin Med 2013, 15: 85-87.
27. Mourboul ABLISE, Rena KASMU, MA SY, et al.Isolation and antioxidant property of Glabridin. Nat Prod Res Dev 2007, 19: 675-677.
28. Aoki F, Nakagawa K, Kitano M. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J Am Coll Nutr 2007,26: 209-218.
29. Luo CY, Mu CH, Wang YJ, et al. Study on inhibition of tyrosinase and antioxidant activity by glabridin in vitro. Zhong Yao Cai 2010, 33: 1776-1780.
30. Yu XQ, Xue CC, Zhou ZW, et al. In vitro and in vivo neuroprotective effect and mechanisms of glabridin,a major active isoflavan from Glycyrrhiza glabra(licorice). Life Sci 2008, 82: 68-78.
31. Committee of experts of clinical application of glycyrrhizin preparation in treatment of liver disease.Expert consensus on clinical application of glycyrrhizin preparation in treatment of liver disease.J Clin Hepatol 2016, 32: 844-852.
32. Feng C, Wang H, Yao C, et al. Diammonium glycyrrhizinate, a component of traditional Chinese medicine Gan-Cao, prevents murine T-cell-mediated fulminant hepatitis in IL-10- and IL-6-dependent manners. Int Immunopharmcol 2007, 10: 1292-1298.
33. Wang B, Wang YX, Zhao XH, et al. Research progress of the major components and the pharmacological effect of Glycyrrhiza uralensis fisch.J Jilin Med 2013, 34: 215-218.
34. Wang JY, Liu WT, Hu MY, et al. Inhibitory effect of glycyrrhizin on the expression of typeⅠand Ⅲprocollagen mRNA in fibroblasts. Chin J Dig 1997,17: 60-61.
35. Zhang MF, Shen YQ. Advances in studies on mineralocorticoid-like effect of glycyrrhizic acid and its aglycone glycyrrhetic acid. Drugs Clin 2011, 26:448-452.
36. Mao HY, Kang T, Yao L, et al. Efficacy of glycyrrhizin in patients with chronic severe hepatitis B: a meta-analysis. J Clin Hepatol 2015, 31: 63-67.
37. Zhang YB, Fei HX, Zhong LL, et al. The effects of diammonium glycyrrhizinate on BxPC-3 cell line of pancreatic cancer. Chin J Gerontol 2016, 36:523-525.
38. Nakashima H, Matsui T, Yoshida O, et al. A new anti-human immunodeficiency virus substance glycyrrhizin with reverse transcriptase- inbibitory activity bychemical modification. Jpn J Cancer Res 1987, 78: 767-771.
39. Yao L, Liu YL. The research progress of glycyrrhizic acid and its congeners in treatment of autoimmune hepatitis. Me Recapitulate 2014, 20: 2336-2338.
40. Xiao ZB, Liu XL, Cheng RQ, et al. Influence of β-sitosterol on gastric mucosal side effect induced by aspirin and its pharmacological functions. Chin J Exp Tradit Med Formulae 2016, 22: 148-152.
41. Yi JE, Wu J, Wen LX, et al. Research progress on pharmacological activities of betulinic acid. Chin Tradit Herb Drugs 2014, 45: 2118-2124.
 Traditional Medicine Research2018年2期
Traditional Medicine Research2018年2期
- Traditional Medicine Research的其它文章
- History of recurrent miscarriage in traditional Chinese medicine literature
- Clinical evidence and potential mechanisms of Chinese medicines for the treatment of diabetic retinopathy
- Pharmacological intervention of traditional Chinese medicine for the quality of life in patients with colorectal cancer: a systematic review and meta-analysis
- Meta-analysis of neostigmine injections given at the Zusanli (ST 36) acupoint in the treatment of postpartum urinary retention
