Effect of Oleic Acid Tris-(2-hydroxyethyl) isocyanurate Phosphate Ester on Biodegradability and Tribological Performance of Mineral Lubricating Oil
Ding Jianhua; Fang Jianhua; Chen Boshui; Zhang Nan; Fan Xingyu; Zheng Zhe
(Department of Oil, Army Logistics University, Chongqing 401311)
Abstract: The oil solubility of synthetic oleic acid tris-(2-hydroxyethyl) isocyanurate phosphate ester (abbreviated as OHTP hereinafter) and its influence on the biodegradability and tribological performance of 400SN mineral oil were investigated on a tester and a four-ball tribotester, respectively, for fast evaluating the biodegradability of lubricants. Furthermore, the morphologies and tribochemical species of the worn surfaces lubricated by OHTP-doped oil were studied by scanning electron microscope (SEM) and X-ray photoelectron spectroscope (XPS). The results indicated that OHTP possessed good oil solubility and could improve obviously the biodegradability, the extreme pressure properties, the anti-wear properties and friction-reducing properties of the 400SN mineral oil. The analytical results of XPS spectra showed that the composite boundary lubrication films were mainly composed of absorbed films and tribochemical species such as FePO4, Fe3(PO4)2,Fe2O3 and Fe3O4, which contributed to improving the tribological performances.
Key words: additive, biodegradation, friction, wear, extreme pressure property
1 Introduction
As we know, the environment is seriously polluted by mineral lubricants on account of their low-biodegradable nature and inherent eco-toxicity[1-3]. It has been estimated that millions of tons of lubricants directly enter the environment because of spillages, emissions, machinery failure, leaks and careless disposal[4-5]. In recent years,with the ever growing public attention and awareness on environmental protection, it is imperative to develop lubricants that can show good compatibility with environment[6-7]. Nowadays, many base oils such as synthetic esters or vegetable oils are specifically prepared and have been applied practically in the formulation of biodegradable lubricants, thanks to their low toxicity,excellent biodegradability, and excellent tribological performance coupled with suitable additives[8-12].
It has been known that the petroleum-based lubricants,which are one of the most important and most widely used lubricants, will go on playing an important role in the future lubrication applications. Unfortunately, the petroleum-based lubricants are unreadily biodegradable.Although mineral base stocks have so far never been proposed as the base oils of biodegradable lubricant,the enhancement of their biodegradability is important,significant, practical and indispensable[13-15].
Researchers have indicated that many compounds containing nitrogen and phosphorus can effectively promote the biodegradation of hydrocarbons in the remediation of petroleum polluted water or soil[16-18]. On the other hand, nitrogenous heterocyclic compounds have been proven to possess good tribological performance[19-20].In the present paper, the oil solubility of synthetic oleic acid tris-(2-hydroxyethyl) isocyanurate phosphate ester and its impact on biodegradability and tribological properties of mineral oil was preliminarily studied.
2 Experimental
2.1 Materials
All experimental reagents purchased from the Chengdu Kelong Chemical Reagent Company were of analytically pure grade. A non-polarized paraffinic lubricating base oil(400SN) was provided by the Shenzhen Lubricating Oil Industry Company, with its kinematic viscosity at 40 °C equating to 84.93 mm2/s.
2.2 Preparation of additive
The synthesis pathways and molecular structures of oleic acid tris-(2-hydroxyethyl) isocyanurate phosphate ester(denoted as OHTP) are outlined in Figure 1. The brief synthesis process is demonstrated as follows: 30 mL of toluene functioning as the solvent and water carrying agent, 13.05 g (0.05 mol) of tris-(2-hydroxyethyl)isocyanurate, 28.2 g (0.1 mol) of oleic acid and the catalyst were added into a 500 mL three-neck flask equipped with a thermometer, a reflux condenser, a deanstark trap for water separation, and a stirrer. The reaction was carried out under refluxing and stirring at 150 °C until no obvious water production could be observed.Then the reactor was cooled down to about 40 °C and then phosphorus pentoxide (P2O5) was in batches added into the reactor. The mixture was heated to 80 °C and was subject to reaction under stirring for 4 h, and then a moderate amount of water was added into the reactor to take part in the reaction under stirring for 2 h in order to hydrolyze a small amount of polyphosphoester reactionproduct. After being cooled down, the mixture was subject to distillation to remove toluene under reduced pressure. Oleic acid tris-(2-hydroxyethyl) isocyanurate phosphate ester (OHTP) was then obtained.
The product was characterized by Fourier transform infrared (FT-IR) spectroscopy. The FT-IR spectra of OHTP are given in Figure 2. The significant features of OHTP are bands corresponding to the CH3/CH2stretching at 2 960―2 850 cm-1, the triazine ring at 1 456 cm-1and 763 cm-1, the C=O in triazine at 1 693 cm-1,the C=O in ester group at 1 739 cm-1, the P=O at 1 300―1 105 cm-1, the P-O-C at 1 042 cm-1. The results of FT-IR analysis suggest that OHTP has been synthesized successfully.
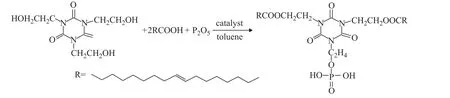
Figure 1 The pathway for synthesis of OHTP

Figure 2 FT-IR spectra of OHTP
2.3 Oil solubility
The oil solubility of OHTP was evaluated by the method described in the reference[21], and was tested as follows.The additive was mixed with the 400SN base oil at a fixed concentration and the solution was stirred at 40 °C until the additive was completely dissolved. Then the solution was sealed and allowed to stand at the indoor temperature for several days. If there was no any precipitate, the additive was regarded as soluble in the base stock. Figure 3 shows photos of the 400SN base oil without and with 2.0% of OHTP,respectively. We can see from Figure 3 that although the color of the solution gets slightly darker, OHTP is well-dissolved in the 400SN mineral oil, because it is attributed to the inlet of long-chain groups of oleic acids, indicating that OHTP possesses excellent oil-solubility in the 400SN mineral oil.

Figure 3 Photos of 400SN base oil without and with 2.0%of OHTP, respectively
2.4 Biodegradation test
In order to evaluate the influence of OHTP on biodegradation of mineral base oil, different mass fraction of OHTP, namely, 0, 0.5%, 1.0%, 1.5%, and 2.0%, respectively, was added to the 400SN base oil.The biodegradability of the formulated oils was tested by means of a fast method created by our research group and described in the reference[22]. In brief, the test of lubricant biodegradability by means of this method was determined by the reference substance oleic acid and the parallel biodegradation reactions of the lubricant. The carbon dioxide created by test sample was measured every two days under formulated conditions, and after 12 days of biodegradation, the accumulated amount of CO2generated from oleic acid and the tested lubricant was measured, respectively. The biodegradability index(BDI), a comparative parameter of the percentage ratio of the amount of CO2produced by the single tested lubricant to that produced by oleic acid, was used to determine the biodegradability of the lubricant. The higher the accumulated amount of CO2and the BDI values, the better the biodegradability of the lubricant. Figure 4 displays the diagrammatic drawing of biodegradation test.
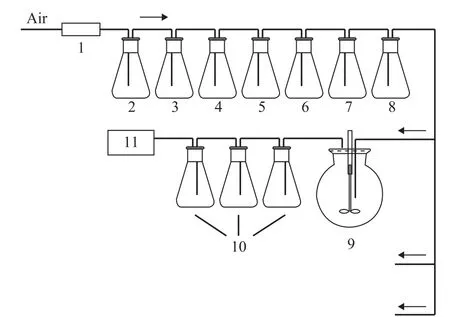
Figure 4 The diagrammatic drawing of biodegradation test
2.5 Tribological performance
The tribological performance of OHTP in the 400SN mineral base oil was evaluated on a four-ball tribotester following the procedures of GB/T 3142―1982, which was a Chinese standard method for determining the friction and wear properties of lubricants. The four-ball tribotester was composed of a rotating ball that glided on three fixed balls under chosen loads. The steel balls used in the present test were made of GCr15 standard steel balls with a diameter of 12.7 mm, a hardness of 59―61 HRC, and a surface roughnessof Ra 0.040 μm. The wear scar diameters (WSD), friction coefficients, the maximum non-seizure loads (PB), and the welding loads (PD) were evaluated in the present test. The WSD and friction coefficient were conducted under a load of 392 N and at a rotary speed of 1 500 r/min for 30 min. The duration of testingPBandPDwas 10 seconds under selected loads.
2.6 Surface analysis
The steel balls which were lubricated by the 400SN base oil and the formulated oil containing 1.0% of OHTP were tested under a load of 392 N and a rotary speed of 1 500 r/min for 30 min, and then they were ultrasonically cleansed with petroleum ether for 10 min. The surface morphology of the steel balls was observed by a scanning electron microscope(SEM). The chemical features of the typical elements on the worn surfaces were analyzed by an ESCALab250 X-ray photoelectron spectroscope (XPS), with Al Kα radiation used as the excitation source and the contaminated carbon(C1s: 284.8 eV) selected as the reference.
3 Results and Discussion
3.1 Biodegradable properties
The Variation of BDI with different mass fraction of OHTP and variation of the accumulated amount of CO2with degradation time are shown in Figure 5 and Figure 6,respectively. It can be observed from Figure 5 and Figure 6 that OHTP, incorporated into the 400SN mineral oil at different contents, can to a great extent improve the BDI and accumulated amount of CO2, especially at a content of 2%, indicating that OHTP could obviously enhance the biodegradation of base stock. Studies have shown that many nitrogen and phosphorus-containing compounds can effectively promote the biodegradation of hydrocarbons in the remediation of petroleum polluted water or soil[16-18].Promotion of biodegradation of mineral lubricating oil by OHTP is thus evidentially possible. Furthermore, the surface active OHTP might also decrease the interfacial tension of the oil and water, increase their interfacial area and thus promote lubricant to be metabolized by microorganisms.

Figure 5 Variation of BDI with different mass fraction of OHTP

Figure 6 Variation of the accumulated amount of CO2 with degradation time
3.2 Anti-wear performance
Figure 7 shows the WSDs varying with different contents of OHTP in the 400 SN mineral base oil under a rotary speed of 1 500 r/min and a load of 392 N for 30 min. It can be seen from Figure 7 that addition of OHTP into the 400SN mineral oil could improve the anti-wear performance of the base oil by offering lower WSDs as compared to the neat mineral oil, showing that OHTP possessed excellent anti-wear property in the 400SN mineral base stock. The WSDs decreased dramatically from 0.64 mm to 0.42 mm when the content of OHTP increased to 1.0% mainly owing to the protective films formed from a series of tribochemical reactions in the friction process. These protective films could hold back direct contact of the two rubbing surfaces because of their soft character and low shear strength[20]. With the OHTP content increasing to 1.0%―2.0%, the WSDs began to increase slowly on account of corrosive wear of OHTP.
Figure 8 displays the effect of different loads (namely,196 N, 294 N, 392 N, and 490 N, respectively,) on WSDs of steel ball lubricated by base oil and by base oil containing 1.0% of OHTP under a rotary speed of 1 500 r/min for 30 min. As shown in Figure 8, OHTP exhibited excellent anti-wear performance in mineral base oil at an applied load ranging from 196 N to 490 N as compared with the base oil. On the one hand, the protective films were able to be generated under low load (196 N). On the other hand, the protective films were tight, stable and not easily to be taken away from the rubbing surface under high load because of the N-containing heterocyclic structure[23].

Figure 7 Variation of WSDs with different mass fraction of OHTP

Figure 8 Variation of WSDs under different loads
3.3 Friction-reducing performance
Figure 9 gives the variation of friction coefficient of base oil and the oil containing 1.0% of OHTP with the test duration under a rotary speed of 1 500 r/min and a load of 392 N. It can be seen from Figure 9 clearly that the formulated oil could provide lower friction coefficient than the neat base oil. Meanwhile, in the whole test duration of 30 min, the friction coefficient of formulated oil containing 1.0% of OHTP fluctuated more slightly than the neat oil. The results suggested that OHTP could facilitate significantly the friction-reducing performance of the 400SN mineral oil.

Figure 9 Variation of friction coefficient with test duration by using base oil with or without OHTP
3.4 Extreme pressure performance
The extreme pressure property is characterized by the maximum non-seizure load (PBvalue) and the welding load (PDvalue), with the results shown in Figure 10.It can be seen from Figure 10 that thePBvalues andPDvalues of test oils all increased with an increasing concentration of OHTP ranging from 0.5% to 2.0%.Compared with the base oil, thePBvalue of the oil containing OHTP was boosted from 495 N to 1 069 N and thePDvalues of the oil containing OHTP was increased from 1 236 N to 2 100 N. The results indicated that OHTP could improve the extreme pressure performance of 400SN oil.

Figure 10 Variation of PB or PD values with different mass fraction of OHTP
3.5 SEM analysis
Figure 11 shows the SEM morphology of the worn surfaces of steel balls lubricated by the 400SN base oil and the oil containing 1.0% of OHTP under a load of 392 N and a rotary speed of 1 500 r/min for 30 min. From Figure 11 we can obviously see that the WSD of the worn surface of steel ball lubricated with the formulated oil was smaller than that lubricated by the neat base oil, and the worn surface obtained from the formulated oil with 1.0%of OHTP was smoother and shallower than that achieved by the oil without OHTP. Furthermore, the slight corrosive phenomenon (Area A in Figure 11(b)) appearing on the worn surface lubricated by the formulated oil with 1.0% of OHTP was ascribed to the corrosive wear of the elemental phosphorus in OHTP. The results of SEM morphological analysis were in agreement with the experimental results of friction and wear tests mentioned in Sections 3.2 and 3.3.

Figure 11 SEM images of the worn surfaces lubricated with base oil and OHTP-doped oil
3.6 XPS analysis

Figure 12 XPS spectra of typical elements on worn surfaces lubricated with base oil and OHTP-doped oil
The XPS spectra of worn surfaces of steel balls lubricated with the 400SN base oil and the oil doped with 1.0% of OHTP under a rotary speed of 1 500 r/min and a load of 392 N for 30 min are displayed in Figure 12. The XPS spectrum of C1s suggests a peak in the binding energy range of 284.5—288.4 eV, which is attributed to organic species of C-C, C=O, COO- and indicates that OHTP is absorbed on the friction surface of steel ball. The peak of O1s at the binding energy of 531.8 eV and 529.9 eV may be attributed to phosphate and iron oxide, respectively. The peak of Fe2p at the binding energy of 710.6 eV suggests that the iron is oxidized into Fe2O3or Fe3O4. The peak of N1s around the binding energy of 400.0 eV may be ascribed to the nitrogen-containing compounds. In the spectrum of P2p, the peak at the binding energy of 133.2 eV may be attributed to FePO4or Fe3(PO4)2, indicating that OHTP can react tribochemically with the friction surface.The analytical results of XPS spectra show that a complex boundary lubrication film has been formed on the friction surface due to the adsorption of OHTP on the worn surface and the existence of complicated tribochemical reactions,which can play an important role in the friction-reducing and anti-wear abilities of OHTP in the 400SN mineral oil.
4 Conclusions
According to the results shown above, the conclusions are drawn as follows.
(1) Oleic acid tris-(2-hydroxyethyl) isocyanurate phosphate ester (denoted as OHTP) has been synthesized and characterized by FT-IR spectroscopy, indicating to its good oil-solubility in the 400SN base stock.
(2) The BDI and accumulated amounts of CO2of the formulated oil with OHTP were much higher than those obtained without OHTP. The addition of OHTP to some extent could promote biodegradation of unreadily biodegradable mineral lubricating oil.
(3) The friction coefficients and wear scar diameters of steel balls lubricated by the OHTP doped 400SN mineral oil were smaller than those achieved by base oil without OHTP. ThePBandPDvalues of steel balls lubricated with the OHTP doped 400SN base oil were higher than those achieved without OHTP. The addition of OHTP could to a great extent improve the anti-wear and friction-reducing properties and extreme pressure abilities of the 400SN mineral oil.
(4) The complicated boundary lubrication films formed on the worn surface lubricated by the OHTP doped 400SN mineral oil were mainly made up of organic adsorbate of OHTP and tribochemical species such as FePO4, Fe3(PO4)2,Fe2O3and Fe3O4. The complex boundary lubrication films might be the reasons illustrating that OHTP could improve the tribological properties of mineral oil.
Acknowledgments:The authors are grateful to the financial support from the National Defense Science Technology Foundation (Project No.3604003), the National Natural Science Foundation of China (Project No.51375491), the Natural Science Foundation of Chongqing (Project No. CSTC,2014JCYJAA50021), and the Natural Science Foundation of Chongqing (Project No. cstc2017jcyjAX0058).
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

