Preparation of High Viscosity PAO from Mixed Alpha-Olefins over Metallocene Catalyst
Jiang Hongbo; Xu Xinlei; Hong Xianzhong; Song Shangfei
(1. Research Institute of Petroleum Processing, East China University of Science and Technology, Shanghai 200237; 2. Shanghai Naco Lubrication Technology Co., Ltd., Shanghai)
Abstract: Polymerization of mixed alpha-olefins originating from the Fischer-Tropsch synthesis catalyzed by the metallocene catalyst system, rac-Et(1-Ind)2ZrCl2/Al(iBu)3/[Me2NHPh]+ [B(C6F5)4]-, was studied. The effects of the Zr/olefin mole ratio, Al/Zr mole ratio, reaction temperature, and reaction time on the viscosity and molecular weight of the product were investigated. The conversion under optimized conditions reached 97.3%. The product structure was characterized by 13C NMR spectrometry and 1H NMR spectrometry, and the conversion of olefins with different carbon numbers under different conditions was determined by GC analysis. The polymer obtained under optimized conditions has a high viscosity index of 262 with a narrow molecular weight distribution of 1.95, which is a desired component for lubricating base oil.
Key words: poly alpha-olefin; metallocene; mixed alpha-olefins; lubricating base oil; polymerization
1 Introduction
Poly alpha-olefin (PAO) is widely used as base oil for the preparation of synthetic lubricating oils. Compared with petroleum-based base oils, the main advantages of PAO are high viscosity index, low pour point, good oxidation resistance, low volatility, and high flash point[1].The traditional catalysts such as the Lewis acid catalyst(BF3), and the titanium-based catalyst are mainly used in the production of low-viscosity PAO, and there are some defects such as low catalytic efficiency and large dosage of catalysts adopted[2]. The metallocene catalyst has high catalytic activity and single active sites, and the polymer obtained has a regular structure, a high polymerization degree and a narrow molecular weight distribution(MWD, which is the ratio of weight-average molar mass to number-average molar mass), which can be used as high-grade lubricating oil in various complicated working conditions[3].
As pure 1-decene used for the production of PAO is imported, the production cost of the domestic PAO is high. A possible alternative to pure 1-decene is the alphaolefins separated from the products of Fischer-Tropsch(F-T) synthesis. The mixed hydrocarbons produced by the high-temperature F-T synthesis process mainly consist ofn-paraffins and linear alpha-olefins. This characteristic feature indicates that the carbon number distribution of the mixture is continuous, and the separation ofn-paraffins and alpha-olefins can hardly be realized by traditional distillation. After cutting an appropriate fraction by distillation and removing the oxygenates, the mixed alphaolefins containingn-paraffins from the F-T synthesis can be used as the feedstock for the production of PAO. There are many patents and literature about the manufacture of PAO from alpha-olefin obtained via oligomerization of ethylene. However, there are few reports on the synthesis of high-viscosity PAO that is polymerized from the mixed F-T alpha-olefins in the presence of the metallocene catalyst.
2 Experimental
2.1 Materials
Mixed alpha-olefins emanated from the F-T synthesis were purified by passing through the water and oxygen scavenger columns, with the mixture composition covering: 17.3% of 1-nonene, 7.6% of nonane,29.0% of 1-decene, 9.8% of decane, 26.9%of 1-hendecene, and 9.4% of undecane. Triisobutylaluminum (Al(iBu)3) with a concentration of 1.1 mol/L of toluene solution was purchased from the Adamas Reagent, Ltd.N,N-Dimethylanilinium tetrakis(pentafluorophenyl)borate ([Me2NHPh]+[B(C6F5)4]-) was prepared according to the synthesis procedure described in the literature[4]. The metallocene catalyst rac-ethylenebis(1-indenyl)zirconium dichloride (rac-Et(1-Ind)2ZrCl2)was synthesized by means of the published methods with a slight modification[5]. All reactions involving either air or moisture sensitive compounds were conducted in highpurity nitrogen atmosphere according to the Schlenk line techniques.
2.2 Polymerization procedure
Polymerization was performed in a 1-L glass reactor,equipped with a stainless steel stirrer and a jacket filled with heat conducting oil for temperature control.The metallocene catalytic system including rac-Et(1-Ind)2ZrCl2, [Me2NHPh]+[B(C6F5)4]-, and Al(iBu)3was dissolved in 30 mL of toluene. Reagents were added to the reactor in the following order: At first the mixed alpha-olefins from the F-T synthesis were fed into the reactor, followed by the addition of a toluene solution of Al(iBu)3(serving as the scavenger), and then the toluene solution of metallocene catalytic system was added dropwise into the reactor in 20 min. Samples were taken at specified intervals of reaction in order to analyze the conversion of alpha-olefins with different carbon numbers. The reaction was carried out for 120 min and then was terminated by the addition of ethanol containing 5% of HCl. The products were repeatedly washed with water, prior to being subject to vacuum distillation at 200 °C to a constant weight, and were finally routed through a diatomite filter to obtain the pure polymer.
2.3 Characterization
The kinematic viscosity (Kv) of the polymer samples was measured according to the standard method ASTM D445.The viscosity index (VI) values were calculated according to the standard method ASTM D2270. The weightaverage molar mass (Mw) and number-average molar mass (Mn) analyses were examined on a Viscotek gel permeation chromatograph (GPC), in which polystyrene was used as the internal standard. The1H NMR (400 MHz) spectra and the13C NMR (400 MHz) spectra were recorded in CDCl3at 25 °C using a Bruker AVANCEIII-400 spectrometer, which were calibrated relative to the solvent peak in reference to the tetramethylsilane standard. Conversion of alpha-olefins with different carbon numbers was measured on an Agilent 7890B gas chromatograph equipped with an Agilent DB-1HT chromatographic column operating at 350 °C. The concrete operating procedure stipulated that 0.5 mL of sample should be taken at each moment, to which 2 mL of ethanol must be immediately added. The sample after being fully mixed was left to stand for 12 h, and then the supernatant was taken for analysis by GC using the nonreactiven-paraffins coupled with mixed alpha-olefins obtained from the F-T synthesis as the internal standard.
3 Results and Discussion
3.1 Influence of reaction conditions on polymerization of mixed alpha-olefins
Table 1 lists 13 sets of experimental data involving the influence of different reaction conditions on the polymer properties. Experiments numbered 1 to 5 show the influence of Zr/olefin mole ratio on polymer properties,with other reaction conditions covering: a dosage of 500 mL of mixed alpha-olefins withn-paraffins emanated from the F-T synthesis, an Al/Zr mole ratio of 120, a reaction temperature of 80 °C, a reaction time of 120 min, and a B/Zr mole ratio of 1.2. The results indicated that with the increase of the Zr/olefin mole ratio, the conversion of the mixed alpha-olefins at first increased and then gradually leveled off. When the catalyst dosage decreased, the number of active sites in the reaction system was less,and the conversion was almost proportional to the catalyst dosage[6], so there was a large amount of mixed alphaolefins which did not participate in the reaction. When the catalyst dosage increased and the reaction conversion was about to reach the end point, the concentration of mixed alpha-olefins was very low, so further increasing the catalyst dosage could not significantly change the conversion rate. It was found that the kinematic viscosity and molecular weight of polymers decreased with an increasing catalyst dosage, since higher catalyst dosage could produce more active sites and therefore a unit active site could be in contact with fewer alpha-olefin molecules which would produce a relatively short polymer chain.Therefore, a Zr/olefin mole ratio of 4×10−5was selected in further polymerization reactions.
Experiments numbered 6 to 9 show the influence of Al/Zr mole ratio on the polymer properties, with other reaction conditions being the same as those of experiment No. 3.The results indicated that the conversion, kinematic viscosity and molecular weight firstly increased and then decreased as the Al/Zr mole ratio increased. Generally,Al(iBu)3played an important role in the polymerization of alpha-olefins, such as alkylating the metallocene catalysts,removing a small amount of impurities from the solvent or mixed alpha-olefins, functioning as a chain transfer reagent, and eliminating alkyl radical from the Zr metal atom to form an alkyl cation with 14 electrons which were stabilized by the non-coordinating anion (B[C6F5]4-)[7-8].Therefore, when the mole ratio of Al/Zr was lower than 120, the concentration of Al(iBu)3was low, and its effects on removing impurities and alkylation were not fully realized, so the number of active sites was insufficient,and the conversion, kinematic viscosity and molecular weight would increase with an increasing Al/Zr mole ratio. When the Al/Zr mole ratio was more than 120,excessive Al(iBu)3could take part in the complexation reaction with active species, resulting in deactivation of catalyst and a decreased conversion rate. At the same time, the remaining Al(iBu)3acted as a chain transfer agent, which could promote the transfer of the polymerization chain to Al, so the viscosity and molecular weight of the polymers decreased. The mole ratio of Al/Zr should be about 120 for carrying out the polymerization reaction.
Experiments numbered 10 to 13 show the influence of temperature on polymer properties, with other reaction conditions being the same as those of the experiment No. 3. The results indicated that the conversion firstly increased and then decreased, because the increase of temperature was beneficial to the formation of active sites and the diffusion of molecules, but higher temperature was harmful to this reaction which could destroy the stability of metallocene catalyst. On the other hand, the activation energy for catalyst deactivation was higher than that for chain propagation, which implied that the conversion in a certain polymerization time would drop from a maximum value as the temperature continued to increase[9]. The kinematic viscosity and molecular weight of the product decreased continuously with an increasing temperature. Since the activation energy of chain transfer was higher than that of chain propagation,the chain transfer rate would increase faster than the chain propagation rate when the temperature increased, so the viscosity and molecular weight of the polymer decreased.The ideal temperature for polymerization should be 80 °C.

Table 1 Influence of different reaction conditions on polymer properties
Table 2 lists 7 sets of experimental data, which were obtained from experiment No. 3. The samples were collected at 7 different moments and analyzed separately.The results indicated that the conversion firstly increased and then tended to be stable, and it was well understood that the remaining olefins would participate in the reaction as the time increased. The weight-average molar mass was 18 736 after 20 minutes, and decreased slightly as the reaction time increased. The rate of olefins inserted into the metallocene catalyst was very high and the degree of polymerization of final product could be achieved in a very short time. In this experiment, in an attempt to prevent polymerization reaction from being out of control, the method for dropwise addition of the catalyst system solution was adopted and the feeding time was 20 minutes, so that at the beginning of the reaction,the unit active site could be kept in contact with more olefin molecules, which would be easier to form a longer polymer chain.almost the same conversion at each moment although the overall conversion increased with an increasing reaction time. It implied that the catalytic activity of metallocene catalyst system had the same selectivity for mixed alpha-olefins with similar carbon numbers taking part in this polymerization process. Therefore with regard to the polymerization of mixed alpha-olefins obtained from the F-T synthesis, the three kinds of alpha-olefins with different carbon numbers could be regarded as a whole. Simpler assumptions could be made in studying the macroscopic kinetics and thermodynamics of the polymerization reaction, which was conductive to the industrial scale-up.

Table 2 Influence of reaction time on polymer properties
3.2 Characterization of PAOs
The polymers were analyzed by1H NMR in order to get the information on the end groups. Figure 2 shows an expanded scale of the region of higher chemical shift in order to indicate the suitable assignments. There were three signals of end groups, including the tri-substituted vinylidene (δ= 5.32), vinyl (δ= 5.05), and vinylidene(δ= 4.68). The tri-substituted vinylidene was obtained by the rearrangement of polymer chain, which was then terminated by β-H elimination. The vinyl end group was obtained by β-alkyl elimination, and the vinylidene end group was obtained by β-H elimination and chain transfer to monomer[10-11]. The amount of tri-substituted vinylidene was the biggest among these three end groups,which indicated that the rearrangement reaction occurred more frequently during this polymerization reaction. The most possible reason was that the end group from the rearrangement reaction was more stable than the polymer chain itself, and the rearrangement reaction was more likely to occur than β-H elimination reaction, when the polymer chain grew to a certain length.
Figure 3 shows the13C NMR spectra of the polymer, in which the peaks at the chemical shift between 11 and 17 verified the CH3groups, and the peaks at between 18 and 44 represented CH and CH2groups in the polymer. The branching ratio is an universally accepted methodology for understanding the extent of branching in a material and it can be calculated by the method recorded in the report[12]. The result of this polymerization reaction showed a very low branching ratio of 0.123, which indicated that the polymer had an extremely regular
Figure 1 shows the conversion of olefins with different carbon numbers, and) in mixed alpha-olefins formed during the F-T synthesis under different reaction conditions. The reaction conditions of Figures numbered a, b, c, d, e and f were corresponding to the experiments numbered 1, 5, 6, 9, 10 and 13, respectively. The results indicated that although the Zr/olefin mole ratio (“a” and“b”), the Al/Zr mole ratio (“c” and “d”) and the reaction temperature (“e” and “f”) varied and the content of olefins with different carbon numbers was different,while those olefins with different carbon numbers had structure, although the alpha-olefins from F-T synthesis were a mixture.

Figure 1 Conversion of olefins with different carbon numbers (, and) in mixed alpha-olefins from F-T synthesis under different reaction conditions
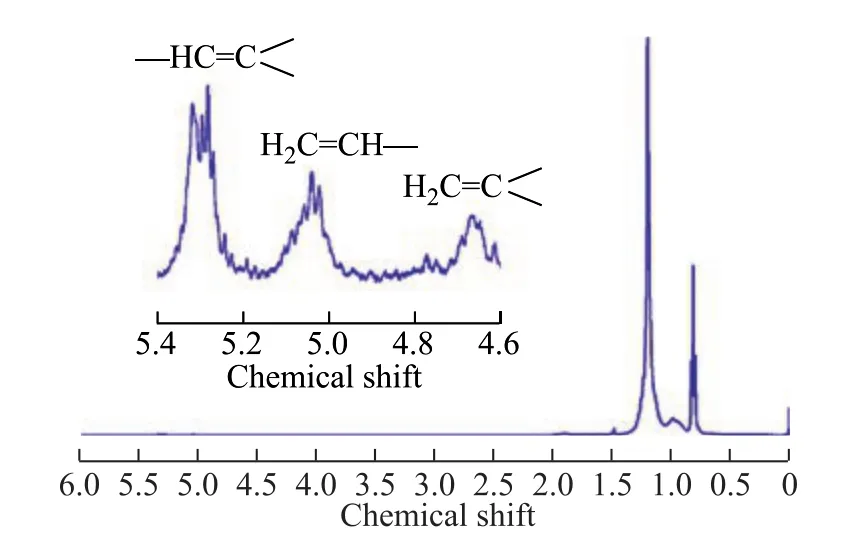
Figure 2 1H NMR spectra of poly(mixed alpha-olefins from the F-T synthesis)

Figure 3 13C NMR spectrum of poly(mixed alpha-olefins from F-T synthesis)
3.3 Comparison of the properties of products obtained in the presence of metallocene catalytic system and Lewis acid catalyst
The Lewis acid such as BF3was often used as a catalyst for alpha-olefin polymerization. Wang, et al.[13]reported a process of 1-decene oligomerization using BF3/alcohol catalyst in which the alcohol acted as an initiator. The reaction conditions covered: an 1-decene/alcohol molar ratio of 100:1,a reaction temperature of 30 °C, and a system pressure of 0.4 MPa. The test results showed that the viscosity index of the product reached 132, with its pour point equating to -60 °C. Its viscosity-temperature property was not as good as that of the polymer formed in the presence of metallocene catalytic system, which was determined by the mechanism of reaction. At first BF3reacted with alcohol to form a coordination complex, then the coordination complex attacked the olefins to produce active carbenium ions, and subsequently the chain propagation could occur,whereas the degree of polymerization could enhance the rearrangement reaction of carbenium ions, which could easily occur and would be accompanied with isomerization of polymers, resulting in the formation of numerous branched chains and a low pour point of the polymer. While the metallocene catalyst was a catalyst characteristic of single active center, the alpha-olefins were inserted into the active centers with the same pattern, so that the polymer had a tactic side-chain structure, resulting in a very high VI value.
4 Conclusions
(1) The optimized conditions for polymerization of mixed alpha-olefins obtained from the F-T synthesis catalyzed by the metallocene catalyst system, rac-Et(1-Ind)2ZrCl2/Al(iBu)3/[Me2NHPh]+[B(C6F5)4]-, covered:a Zr/olefin mole ratio of 4×10−5, an Al/Zr mole ratio of 120, a reaction temperature of 80 °C, and a reaction time of 120 min. The polymer obtained under optimized conditions had a high viscosity index of 262 and a narrow molecular weight distribution of 1.95, which was a desired component for lubricating base oil.
(2) Different carbon numbers, and) of mixed alpha-olefins emanated from the F-T synthesis had almost the same conversion at each moment which indicated that the catalytic activity of metallocene had the same selectivity for mixed alpha-olefins with similar carbon numbers formed during this polymerization process.
(3) The1H NMR spectra of PAO products illustrated that the main chain termination was caused by the β-H elimination after rearrangement reaction. The13C NMR spectra showed that the polymer obtained from mixed alpha-olefins emanated from the F-T synthesis had an extremely regular structure.
- 中国炼油与石油化工的其它文章
- Denitrification of Coal Tar Diesel Fraction by Phosphate Imidazolium Based Polymeric Ionic Liquids
- Development and Application of Hydrocracking Catalysts RHC-1 /RHC-5 for Maximizing High Quality Chemical Raw Materials Yield
- Study of Isothermal Equilibrium, Kinetics and Thermodynamics of Adsorptive Desulfurization on Synthesized CuIYIIIY Zeolite
- Direct Conversion of Glucose to 5-Hydroxymethylfurfural over Zirconium Phosphate Catalyst in a Biphasic System
- Mass Transfer Characteristics of H2 and CO in Mimicked F-T Slurry Bubble Column Reactor
- An Approach for Preparation of Excellent Antiwear PTFE Nanocomposites by Filling As-prepared Carbon Nanotubes/Nanorods (CNT/CNR) Mixed Nano-Carbon Material

